Open Access Article
Open Access ArticleWater-soluble perylenediimides: design concepts and biological applications
Mengmeng
Sun
a,
Klaus
Müllen
b and
Meizhen
Yin
*a
aState Key Laboratory of Chemical Resource Engineering, Key Laboratory of Carbon Fiber and Functional Polymers of Ministry of Education, Beijing Laboratory of Biomedical Materials, Beijing University of Chemical Technology, 100029, Beijing, China. E-mail: yinmz@mail.buct.edu.cn
bMax-Planck Institut für Polymerforschung, Ackermannweg 10, 55128, Mainz, Germany
First published on 21st January 2016
Abstract
Water-soluble perylenediimides (PDIs) with high fluorescence intensity, photostability and biocompatibility have been successfully prepared and applied in the biological field. In this tutorial review, we briefly focus on the synthetic strategies for the preparation of water-soluble PDIs by incorporating ionic or non-ionic substituents with multiple polar groups into the bay-region, imide- or ortho-positions of PDIs. These ionic/non-ionic substituents can suppress π–π aggregation and shield the inner perylene chromophores, thus contributing to the water solubility which is essential for biological applications. The optical properties, absorption and emission maxima above 500 nm, minimize the autofluorescence background of cells and provide access to imaging in living cells. The biological applications of water-soluble PDIs are discussed from simple (basic) to complex (advanced) processes, including biosensing in vitro studies, imaging and gene/drug delivering in living cells, tissues and the whole body. The promising future of designed multi-functional water-soluble PDIs will be highlighted in this review.
Key learning points(1) The chemical structure affects the physical and optical characteristics.(2) Hydrophilic substituents (cationic, anionic or non-ionic polar groups) improve the water solubility. (3) Fluorescence-based techniques for biosensing and bioimaging. (4) Nanocarriers for gene/drug delivery. (5) In vitro and in vivo assays (living cells, tissues and the whole body). |
1. Introduction
Fluorescence-based techniques have been widely applied in the visualization of biological components and processes due to their high selectivity and sensitivity. Successful fluorophores for biosensing or bioimaging should possess some unique properties, such as high water-solubility, fluorescence intensity, photostability and biocompatibility. As an attractive class of chromophores, perylene-3,4,9,10-tetracarboxylic acid diimides (perylene diimides, PDIs) have been widely used as active components of field-effect transistors (OFETs)1 and organic light-emitting diodes (OLEDs),2 and as dye sensitizers in solar cells3,4 because of their high extinction coefficients, high fluorescence quantum yields (FQYs), a broad color range, as well as outstanding chemical, thermal, and photochemical stability in organic solvents. In particular, the absorption and emission maxima of PDIs are above 500 nm, which minimize the autofluorescence background of living cells. However, PDIs exhibit poor water solubility, very weak fluorescence in aqueous solution, and the tendency to form aggregates due to intrinsic π–π stacking interactions between perylene backbones.5,6 This creates a major challenge for the application of PDIs in the biological and medicinal fields. Great efforts have been made to increase the water solubility of PDIs by introducing hydrophilic groups in the bay-region or at the imide- and ortho-positions.Ionic groups such as cationic ammonium salts,7–9 anionic carboxylic acids,10 sulfonic acids11 and phosphonic acids12 have been directly incorporated into perylene chromophores to generate water-soluble PDIs. Another powerful strategy to enhance water solubility is the attachment of non-ionic substituents with multiple polar groups, such as poly(ethylene glycol) (PEG), polyglycerol (PG) dendrons, and dendritic carbohydrate derivatives, in the bay region or at the imide positions of PDIs. These approaches effectively obstruct the aggregation of perylene cores, and the functionalized PDIs exhibit strong fluorescence and high FQYs in aqueous solution.
In this tutorial review, we briefly present synthetic strategies for the preparation of water-soluble PDIs by incorporating ionic or non-ionic substituents with multiple polar groups into the bay-region, imide- or ortho-positions of PDIs. These ionic/non-ionic substituents can suppress the π–π stacking of inner perylene chromophores, and also contribute to the biocompatibility. The optical properties and biological applications of water-soluble PDIs are discussed with a special focus on their biosensing and bioimaging applications in living cells, tissues, and the whole body. Moreover, benefiting from the positive charges or cavities provided by dendrimers, PDIs can be used as nanocarriers for gene/drug delivery and achieve gene therapy or chemotherapy.
2. Design of water-soluble perylenediimide chromophores
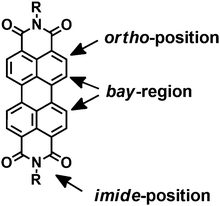
In general, PDIs are chemically modified in the bay-region, imide- or ortho-positions of PDIs (Fig. 1). The substituents at the imide-positions are primarily used to tune the solubility of PDIs in various solvents while minimally altering the optical and electronic properties. The resulting derivatives retain the green-yellow emission of PDIs and their high FQYs. In contrast, modifications in the bay-region of PDIs result in a twist of the two naphthalene rings, which can tune the spectroscopic properties and yield a significant red shift in the absorption maximum with an increased Stokes shift. This has attracted great interest in biological and medicinal research because it can minimize phototoxicity and interference from biological auto-fluorescence. Selective functionalization in the ortho-positions can successfully tune the optical and electronic properties without affecting the planarity of the perylene core. Moreover, the solid state emission and solubility in organic solvents are significantly enhanced compared to the unsubstituted PDIs.13–16 Typically, ionic or non-ionic functions with multiple polar groups such as –SO3H, –OH, –NH2, –NHR, –COOH, –O– are introduced into modified PDIs to enhance the water-solubility, because these groups can form hydrogen bonds between the polar pendant groups and the water molecules. In addition, the polar substituents attenuate intermolecular π–π aggregation of PDIs due to steric hindrance or electrostatic repulsion forces.92.1 Anionic substituents
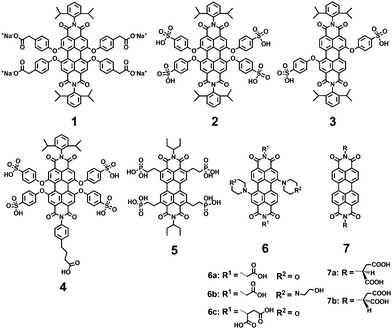
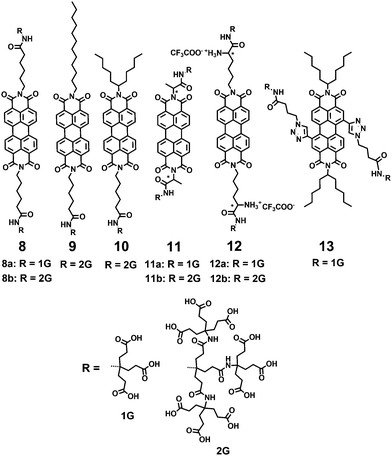
Anionic substituents, such as carboxylic, sulfonic and phosphonic acids and their corresponding salts, are the most widely used to improve the water-solubility of PDIs. Müllen and coworkers9,12,17 reported a series of water-soluble PDIs with anionic substituents in the bay-regions and imide-positions (Fig. 2). Compound 1, containing four carboxylic acid sodium salts attached in the bay-region, was prepared by reacting the parent acid with sodium hydroxide. The carboxylates improve the water solubility, while the chromophore with four carboxylic acid substituents is not soluble in water. Compound 2, containing four sulfonic acids in the bay-region, had a water solubility of 8.0 × 10−2 mol L−1. With a decreasing number of hydrophilic groups, from four down to two, the water solubility and FQY of 3 decreased (FQY = 12%). The monofunctional PDI 4 showed similar water solubility to the symmetric ionic PDIs 2. In addition, 4 can be further functionalized due to the presence of a carboxylic acid handle at the imide-positions of PDI. The FQY of 2 is 58%, displaying a reduction of 42% compared to PDI chromophores in organic solvents (FQY ≈ 100%). The reduction of the FQY in polar solvents was explained by aggregation, vibrational relaxation and photoinduced electron-transfer (PET). Compound 1 bearing carboxylate groups at the terminus of a short alkyl spacer displays a low FQY of 7%, due to lipophilic and vibrational relaxation resulting from the alkyl spacers. The FQY of 4 is slightly lower (49%) than that of 2, because imide substitution affects the fluorescence of PDIs in water. Compound 5 with phosphonic acids in ortho-positions is capable of carrying negative charges at biologically relevant pH values, with enhanced resistance to aggregation and fluorescence quenching. The FQY of 5 is 77% in water. Because of the water-solubility and high FQY, 5 was impregnated into HeLa cells for successful labeling. Akkaya and coworkers synthesized several water-soluble green PDI dyes 6(a–c) by introducing dialkylamino groups into the bay-region.18 Under red light excitation, 6c was shown to be efficient generators of singlet oxygen and displayed significant light-induced cytotoxic effects on living cells. A pair of chiral PDIs 7(a–b) with aspartic acid (L or D) at the imide-positions was prepared by Malik and coworkers.19 After the pH was decreased to 4.00, these two enantiomers formed a reddish-brown hydrogel with reddish color under UV light. Dried 7a or 7b gel formed well-defined left- or right-handed helical fibers of 20 nm diameter with an average pitch length of 20 nm. The perylene core forced 7a or 7b to coil until thick filaments were formed. These fibers maintained the original helical of the subunit and were stabilized by π–π stacking of the perylene core and hydrogen bonding interactions of the carboxyl groups.Newkome and coworkers described the synthesis of 1 → 3 C-branched polyamide dendrons (named Newkome-type dendrons) firstly.20 The terminal carboxylic acids were easily hydrolyzed starting from peripheral esters, and consequently enhanced solubility of hydrophobic groups in aqueous media. Moreover, the peripheral carboxylic acids served as active sites for growing dendrons or coupling with biologically active species for medical applications. Hirsch and coworkers21–23 synthesized a series of water-soluble symmetric and asymmetric, chiral and achiral PDIs by incorporating first-generation (1G) and second-generation (2G) Newkome-type dendrons at the imide-positions (8–12) or in the bay-regions (13) (Fig. 3). The polyamide dendrons act as hydrophilic substituents to enhance water solubility and also exert an aggregation-inhibiting effect because of their bulkiness and anionic character, which can be easily generated in a buffer solution at pH 7.2. The symmetric 1G dendronized dye 8a forms aggregates more easily than its 2G-counterpart 8b. The perylene core in 8b is effectively shielded due to the increased steric demand of the dendron units and electrostatic repulsion, as most of the 18 carboxy groups are deprotonated at neutral pH. The asymmetric derivative 9 reveals pronounced aggregation properties due to the presence of both a 2G-dendron and a flexible and non-bulky dodecyl substituent, and forms regularly shaped micelles in water (pH = 7.2) with a mean diameter of 16 nm. However, its 1G-analogue is not soluble in water, because the presence of just one 1G-dendron does not provide sufficient overall hydrophilicity. Asymmetric PDIs 10 with 2G-dendron are water soluble; however, their 1G-counterpart, bearing only three acid groups, is insoluble in water. The symmetric and chiral PDIs 11 and 12 are connected with 1G or 2G Newkome-type dendrimers by the amino acids alanine and lysine, respectively. The formation of chiral superstructures of 11a was found to be pH- and concentration-dependent. Compound 13 is amphiphilic PDIs with 1G-dendron in the bay-regions. The symmetric 1G-dendronized dyes 8a, 11a and 12a form aggregates more easily than their 2G-counterparts 8b, 11b and 12b, due to the bulkiness of dendritic carboxylates.
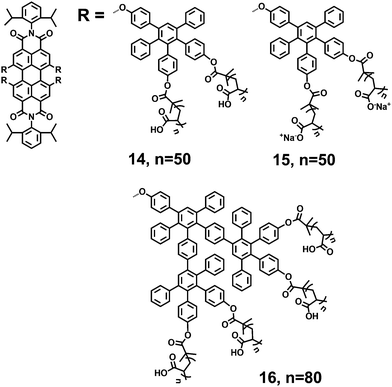
Müllen and coworkers described a method for the rapid construction of branched polyphenylene dendrimers by Diels–Alder cycloaddition in 1997.24 Three-dimensional polyphenylene dendrimers are shape-persistent and out-of-plane twisted phenyl components. Yin et al.25–27 reported types of negatively charged fluorescent core–shell macromolecules (14, 15 and 16) consisting of a central PDI chromophore, surrounded by hydrophobic polyphenylene dendrimers and outer flexible polymers (Fig. 4). Compared with the above polyamide dendrons, the polyphenylene dendrimers are bulkier and more rigid, which can more effectively suppress aggregation and shield the inner perylene chromophores. The outer polymer shells with multiple carboxylic acids, introduced by atom transfer radical polymerization (ATRP), contribute to the water solubility and charge densities of the core–shell macromolecules. PDIs 14 and 15 are 1G polyphenylene dendrimers, bearing eight arms with multiple carboxylic acids and corresponding sodium salts. 16 is the 2G analogue, bearing sixteen arms with multiple carboxylic acids. PDIs 14, 15 and 16 exhibit water solubilities of >10 g L−1 in aqueous media. The FQYs of 14 and 16 are 13% and 22% in water, respectively. PDI 14 (n = 55) forms unimolecular fluorescent polymeric micelles (UFPM) in aqueous solution.28 The size and the fluorescence intensity of UFPM are pH-sensitive due to the polyelectrolytic nature of the outer polymer shell.
2.2 Cationic substituents
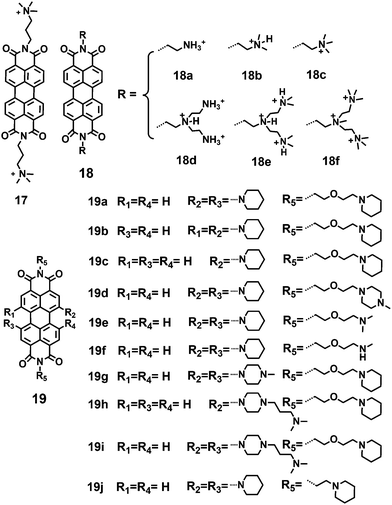
Hydrophobic dyes can be generally converted into water-soluble dyes by adding positively charged substituents. Nitrogen-containing functional groups often carry positive charges, and for this reason, cationic protonated amines (primary, secondary, tertiary and quaternary ammonium salts) are widely used to improve the water-solubility of PDIs.A cationic PDI 17 with two positive charges was reported as a fluorescent probe by Yu (Fig. 5).29 Its considerable water solubility (>30 mM) was provided by the two positive charges. Due to the repulsive interactions between the electrostatic charges, the tendency of PDI to aggregate decreased. Thus, 17 existed in both an aggregated form and a free monomeric form in aqueous solution. Yin and coworkers30 reported a series of water-soluble symmetric cationic PDIs 18(a–f), which inhibit cancer cell growth as DNA intercalators. These PDIs contain a fluorescent perylene core and various protonated amines at the imide positions. Their nano-size and planar structure allow intercalation into double-stranded DNA base pairs. The water solubility is greatly increased due to the positive charges afforded by these protonated amines. PDIs 18c and 18f containing quaternary ammonium cations show higher water solubility (>10 g L−1 in water) than 18a and 18d carrying primary ammonium salts (≤2 g L−1 in water). The FQYs of 18(a–f) are 1.1%, 22.8%, 26.2%, 4.7%, 5.6% and 25.3%, respectively. PDI 18b exhibits low cytotoxicity with a cell viability of 90% to non-cancer cells. PDIs 18b, 18c, and 18e can specifically accumulate in the cell nuclei of animal tissues by intercalation and electrostatic interaction with DNA. A series of PDIs 19 with different N-cyclic substituents in the bay-region and different side chains at the imide-position have been reported by Franceschin and coworkers.31 Their respective water-soluble hydrochlorides were prepared by precipitation with diethyl ether from an acidic (HCl) solution of methanol.
 | ||
| Fig. 5 Imide or bay substituted-PDIs 17, 18(a–f) and 19(a–j) with primary, secondary, tertiary or quaternized ammonium salts and tertiary amines. | ||
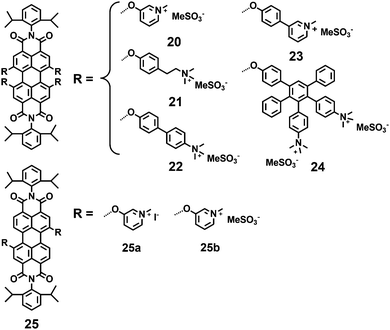
Müllen and coworkers9,17 also reported a series of water-soluble positively charged PDIs (20–25) by quaternization of dimethylamino or pyridinium-substitutes in the bay-regions of PDI (Fig. 6). Chromophore 20 possesses the highest solubility in water (8.0 × 10−2 mol L−1), while 25 with two hydrophilic groups exhibits low water solubility. The hydrophilic groups directly located on the phenyl ring (20) lead to a high FQY (66%), while those located at the terminus of a short alkyl chain (21) lead to a low FQY of 14%. The introduction of additional phenylene rings (22 and 23) produces highly lipophilic compounds with very limited water solubility and results in low FQYs in water (11%, 23%). The bulky and rigid dendritic polyphenylene dendrimer branches can effectively suppress aggregation and shield the inner chromophores. However, polyphenylene dendrimer 24 displays only weak FQY (15%), which is mainly attributed to energy loss through vibrational relaxation.32 PDI 25b with two positively charged pyridinium substituents exhibits sufficient water solubility and high FQY (98%), due to the suppressing of PET processes by a minimum number of functional groups.
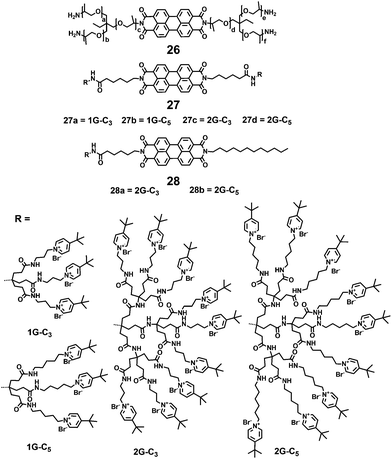
Montalti and coworkers synthesized a fluorescent polyetheramine 26 by a microwave assisted reaction (Fig. 7).33 PDI 26 can disperse in water and self-assemble into non-fluorescent nanoparticles. Hirsch and coworkers34 synthesized a series of symmetric and asymmetric dendronized polycationic PDIs, 27 and 28, by using amide coupling reactions of pyridinium salt head groups with terminal carboxylic groups of PDIs 8 and 9. The water solubility of 27 and 28 is provided by the positively charged pyridinium salts at the periphery of these PDIs and is independent of pH. Compared with 27a and 27b containing small 1G dendrons, all the 2G dendronized PDIs (27c, 27d, 28a and 28b) exhibit a pronounced increase in water-solubility, owing to the steric hindrance of larger dendrons in perylene stacking and aggregation. The FQYs of 27c, 27d, 28a and 28b are 63%, 79%, 68% and 69% in water, respectively. In contrast, the fluorescence of 27a and 27b is clearly quenched in water with low FQYs (7% and 0.11%). This observation is explained by a radiation free decay from the excited state due to the close proximity of aggregated molecules.34
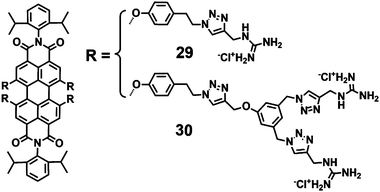
A class of highly photostable and water-soluble guanidinium-dendronized PDIs (29–30) were synthesized by utilizing the copper-catalyzed azide–alkyne cycloaddition (“click”) reaction (Fig. 8).35 These dendronized guanidinium moieties were introduced to suppress the aggregation of the central PDI chromophore in water. Also the electrostatic repulsions of positive guanidinium moieties will further minimize the aggregation. Due to the planar conformation and high basicity of guanidine (pKa = 13.5), the guanidinium moieties can form strong ion pairs with oxoanions, ensuring protonation and positive charge over a wide pH range. PDI 29, bearing four guanidinium end groups, only dissolved in hot water and exhibited nearly no fluorescence. In contrast, 30, containing eight analogues, showed increased water solubility with a FQY of 39%. Such differences are ascribed mainly to the lower number of guanidinium moieties, which cannot provide sufficient inhibition of aggregation and overall hydrophilicity. The moderate FQY for 30 is mainly due to substituents in the bay-regions of PDIs, which decreases the planarity of the aromatic scaffold. The cell viability of 30 treated cells was higher than 90% at concentrations of 5 mM, indicating the low toxicity of 30.
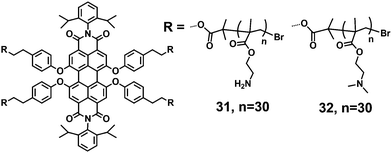
Two water-soluble PDI-cored star polycations, 31 and 32, with two different amines in the bay region of PDI, were synthesized by ATRP (Fig. 9).36 The star polycations consist of a fluorescent central PDI and outer polymer arms of poly(2-aminoethyl methacrylate) (PAEMA, 31) or poly(2-(dimethylamino)ethyl methacrylate) (PDMAEMA, 32). PAEMA or PDMAEMA provides water solubility, due to the presence of polar groups such as –NH2, –OCO– and –N(R)2, which can form hydrogen bonds with water molecules. The linear concentration-dependent absorption of 31 and 32 is observed in aqueous solutions, suggesting that both 31 and 32 remain in a single-molecule state over the whole concentration range studied (7.26–28.9 μM). In addition, the maximum emission peak is located at 622 nm, thus 31 and 32 with good FQYs (14% and 6%) are well-suited for single-molecule fluorescence detection and fluorescence tracing and imaging in live cell experiments. The high zeta potentials of 31 and 32 (37.8 and 56.5) provide the possibility to complex with DNA via electrostatic interactions.
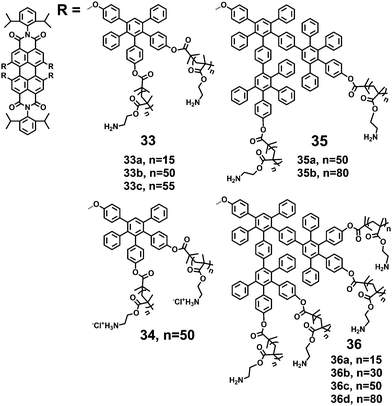
Yin et al.25,27,37 synthesized several water soluble fluorescent core–shell macromolecules (33–36) surrounded by polyphenylenes dendrimer grafted with positively charged polymer chains (Fig. 10). The architectures consist of a fluorescent central PDI surrounded by 1G and 2G polyphenylene dendrimers and an outer polymer shell with multiple amine groups. The rigid polyphenylene dendrimer shell prevents the PDI chromophore from aggregation in water, and the outer polymer shell with multiple amine groups provides water solubility (>10 g L−1) with FQYs varying from 7.6% to 16.8%. The absorption maxima exhibit bathochromic shifts for those macromolecules with a higher number of amino groups (36b, 36c, 36d). This trend is also observed in emission spectra: emission intensities increase and emission maxima shift bathochromically with increasing numbers of repeating units.
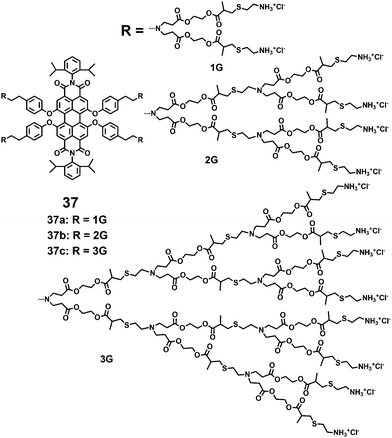
A series of water-soluble PDI-cored cationic dendrimers, 37(a–c), with three generations synthesized via a “click” reaction were reported by Yin and coworkers (Fig. 11).38 The central fluorescent PDI chromophore allows the tracing of cell uptake via fluorescence microscopy. The peripheral primary amines provide positive charges upon a final treatment with hydrochloric acid. The outer cationic dendrimers noticeably suppress the aggregation of the encapsulated PDI chromophores due to steric hindrance and electrostatic repulsion forces. The peripheral primary amines and multiple polar groups (–OCO– and –S–) enhance the water solubility. All the dendrimers show a water solubility of above 10 g L−1 and high photostability. The absorbance, fluorescence intensities and FQYs of 37(a–c) (9%, 12% and 25%) in water increase with higher dendron generation. The cell viabilities of 37(a–c) were all above 90% at concentrations of 6 mM, showing that the dendrimers exhibit low cytotoxicity.
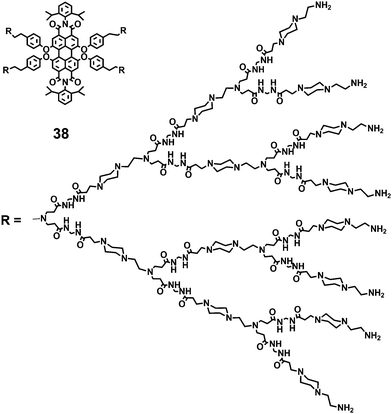
Apart from the advantages in water solubility and photostability, 37(a–c) have some shortcomings such as the instability of the outer dendritic polyester units under basic conditions, which have to be treated with diluted hydrochloric acid to yield their ammonium salts. Subsequently, Yin and coworkers39 developed the water soluble PDI-cored cationic dendrimer 38 by incorporating the poly(amido amine) (PAmAm) dendritic units into the bay-regions of PDIs (Fig. 12). The increased water solubility (>20 g L−1) and chemical stability are resulting from the more polar and stable building blocks –CONH– in the PAmAm dendritic units, together with the polar groups –N(R)2. The linear concentration-dependent absorbance implies that 38 exists in a typically monomeric form in the concentration range of 0.2 μM to 2.0 μM. The PAmAm shell provides steric bulkiness with electrostatic repulsion forces that notably suppress aggregation of the central PDI chromophore in aqueous solution. The FQY of 38 is 18% in water. The cell viabilities of 38 treated cells are higher than 92% at all the concentrations studied (1–4 μM), indicating that 38 has low cell toxicity.
2.3 Non-ionic substituents
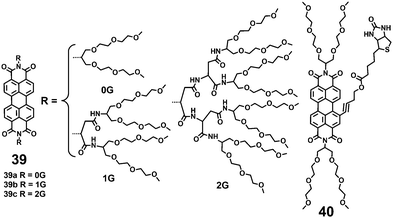
Compared with the ionic hydrophilic groups, the non-ionic hydrophilic groups possess the unique advantages of pH and ionic strength insensitivities in biological buffer systems. The non-ionic hydrophilic groups also minimize undesired nonspecific ionic interactions that may occur with membranes and other charged biomolecules. Widely used non-ionic substituents, which were introduced to improve the water-solubility of PDIs, are poly(ethylene glycol) (PEG), polyglycerol (PG) dendrons, and dendritic carbohydrate derivatives. These non-ionic bulky substituents provide an effective steric hindrance that weakens the π–π interactions between the PDI backbones. Furthermore, the non-ionic substituents consist of multiple polar groups such as ether and hydroxyl, which can form hydrogen bonds with water molecules and lead to water solubility.A series of PDI-cored PEG dendrimers 39(a–c) were synthesized (Fig. 13).40 These PDIs consist of a PDI fluorescent core, branched oligo(glutamic acid)s, and PEG chains. The branched oligo (glutamic acid)s function as scaffolds to suppress the aggregation of the central PDI. Dye 39a bearing 0G dendrons exists in an aggregated state over the whole accessible concentration range (1 × 10−6 to 1 × 10−4 M) evidenced by a broad absorption spectrum with a hypochromically shifted absorption maximum and weak fluorescence (4%). With higher dendron generation (39b and 39c), the steric hindrance increases, leading to improved hydrophilicity of PDIs. Moreover, the FQYs of 39b and 39c also increase in water (65% and 93%), because the PDI fluorophores are efficient emitters in water as their aggregation is completely suppressed. PDIs 39(a–c) show very low cytotoxicity (over 90% cell viability) using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) cell-viability assay, because PEG chains prevent the dyes from interacting nonspecifically with the extracellular proteins. In further studies, the authors prepared a bay substituted PDI 40 with a biotin ligand.41 Compound 40 can form self-assembled nanostructures with high stability in aqueous solution, leading to complete quenching of fluorescence.
Haag and coworkers42–44 synthesized a series of neutral, water-soluble PG-dendronized PDIs with high FQYs (Fig. 14). The 1G dendronized PDI 41a exhibits the typical absorption spectra of the aggregated form over the whole accessible concentration range (1 × 10−6 to 1 × 10−4 M), as the 1G dendron at the imide position is apparently too small to shield the perylene core and suppress aggregation. With increased PG dendron generation, aggregation is inhibited. The observed absorption spectra of 41c and 41d bearing 3G and 4G dendrons arise from PDI monomers over the whole concentration range because these dendrons are sufficiently large to shield the π surfaces of the PDI. The FQYs of 41(a–d) also reveal a continuous increase from 33% for 1G substituted 41a up to 99% for 4G substituted 41d with increasing dendron generation, due to the strong dendritic effect. Optical studies and dynamic light scattering reveal that 42 forms spherical micelles with a narrow size distribution and very low FQY (1%) in water, due to the aggregation of the PDI core. MTT assay shows that 42 is completely nontoxic to cells even at concentrations of 10−5 M. Upon gradually increasing the temperature from 298 K to 368 K, 43a displays a transition from an aggregated state into monomeric species, as proven by the typical characteristics in temperature-dependent fluorescence measurements and 1H NMR spectroscopy. Notably, the FQY of 43a is still low (1.1%) even at high temperatures. The quenched fluorescence can be explained by the PET mechanism from the phenoxy groups to the PDI core. The 3G-dendronized 43b maintains monomeric species over the whole accessible concentration, but is almost nonfluorescent in water (FQY = 0.2%). The low FQY is again related to PET from the electron-rich pyrrolidine to the electron-poor PDI.
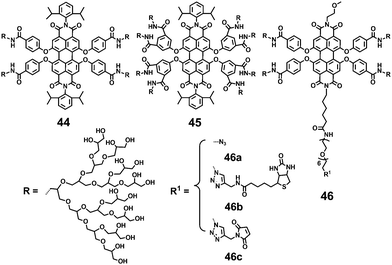
The introduction of functional groups in the bay-region of PDIs results in a significant red shift of the absorption maximum and an increased Stokes shift, minimizing phototoxicity and interference from biological auto-fluorescence. Zimmerman and coworkers39 developed several neutral water-soluble PDIs (44, 45 and 46) that are bay-substituted by amide coupling of the PDI cores with amine-functionalized PG dendrons (Fig. 15).45 The PG dendrons provide steric bulkiness necessary to minimize π–π interactions of the perylene cores. Compounds 46b and 46c were obtained by the click reactions of monofunctional 46a with alkyne-functionalized biotin and maleimide. All these neutral PDIs are water-soluble and highly fluorescent in water with FQYs ranging from 57% to 83%. The difference can be attributed to the varying degrees of encapsulation and solubilization of PDIs within the PG dendritic shell. The bulkier and more substituted 45 exhibits the highest FQY of 83%, due to the more effective encapsulation and solubilization of the PDI core by eight dendrons carrying a total of 128 peripheral hydroxyl groups. In further studies, intramolecular crosslinking of the dendronized and hyperbranched PG, which is substituted in the bay-region of PDI, was reported.46,47 The crosslinking results in a significant reduction in the FQY, due to the decrease of hydroxyl end-groups causing lower aqueous solubility.
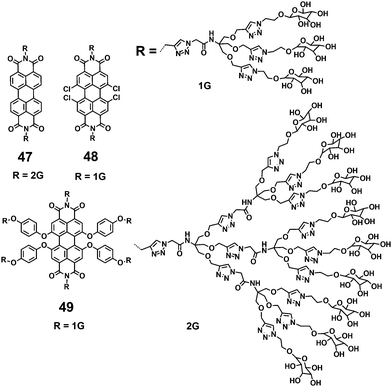
Water soluble PDIs (47–49) modified by glycodendrimers at the imide-positions or in the bay-region have been synthesized through the click reaction and subsequent removal of protecting groups (Fig. 16).48–50 Upon a gradual increase of concentration and a decrease of temperature, the UV-vis spectra of 47 and 48 display the typical spectral characteristics of monomeric to aggregated state, indicating that the π–π aggregation of these PDIs is concentration- and temperature-dependent. The concentration-dependent aggregation of 46 and 48 also affects the FQYs: at a low concentration of 5.0 × 10−6 M, the FQYs of 47 and 48 are 59.9% and 54%, respectively. With increasing concentration, the fluorescence emission is gradually quenched, accompanied by a bathochromic shift because of aggregation. Notable, 49 bearing glycodendrimers both at the imide-positions and in bay-regions was barely detectable by fluorescence because of the intramolecular electronic transfer process from the electron-rich substituents (triazole group) to the PDI backbone. The MTT experiment reveals that the cell viability of 47 treated cells is 110% at the concentration of 50 μg mL−1, indicating that 47 is noncytotoxic to living cells.
3. Biological applications of water-soluble perylenediimides
Water-soluble PDIs have been successfully applied in biological studies, because of their high fluorescence intensity and photostability, biocompatibility, and absorption and emission maxima above 500 nm to minimize the autofluorescence background of cells. Charged components are widely present in cells and tissues. For example, negatively charged extracellular matrices support and connect the organization structure, and regulate the physiological activity of the organization and cell; negatively charged membranes provide a relatively stable internal environment for cell activities and regulate the cellular uptake and efflux of molecules. In addition, positively charged histone proteins and negatively charged DNA form a DNA–protein complex for storing the genetic material. The ionic PDIs allow interactions with natural polyelectrolytes such as DNA or nuclear proteins through electrostatic interactions.Non-ionic PEG, PG dendrons and dendritic carbohydrate derivatives have been widely used in biological studies. PEG is highly water soluble, biocompatible and nontoxic, and it shows no immunogenicity and possesses antifouling properties. In addition, PEG can prolong the circulation time in blood due to the resistance of nonspecific protein adhesion/denaturing. Therefore, it has been approved for medical use by the Food and Drug Administration (FDA). Hydrophilic PG dendrons show similar properties, such as highly biocompatible and minimal nonspecific interaction. Glycodendrimers exhibit the potential to recognize carbohydrate–protein (lectin) interactions involved in key biological processes, such as cell recognition, differentiation and adhesion.48 The biological applications of water-soluble PDIs are summarized below from simple (basic) to complex (advanced) processes, including imaging studies of living cells, tissues and the whole body.
3.1 In vitro studies
Due to the intrinsic fluorescence properties, the cationic or glycodendronized PDIs can be used as reporters for the specific and sensitive detection of biological molecules. The interactions between functional PDIs and biomolecules provide the foundation for further studies in living cells, tissues and the whole body.Fluorescent nanotubes fabricated by oppositely charged 34 and 15 were reported as DNA biosensors by Yin et al.27 Positively charged 34 was deposited at surfaces and interacted with negatively charged DNA through electrostatic forces (Fig. 17A). Compared with the nanotube alone, the nanotube/DNA complex showed an increase in normalized fluorescence intensity because the hydrophilic DNA structures improved the protection of the central PDI chromophore in the aqueous solution. Subsequently, Yin and coworkers developed a time-resolved, ultrasensitive and selective DNA sensor by combining fluorescence detection with energy transfer using the negatively charged 16 as the donor molecule and Cy5 labeled DNA targets as the acceptor molecule (Fig. 17B).51 Upon excitation at 543 nm, excitation energy was transferred from the donor to the acceptor. Due to the high molar ratio of the donor and the acceptor, the fluorescence signal was greatly increased, achieving the ultrasensitive and selective detection of DNA.
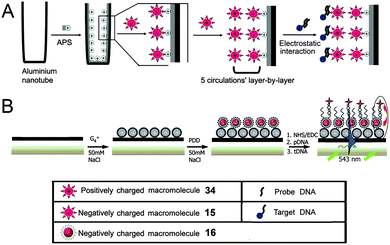 | ||
| Fig. 17 (A) Schematic diagram describing the formation of fluorescent nanotubes by layer-by-layer deposition of 15 and 34. Reproduced with permission from ref. 27. Copyright 2011, John Wiley and Sons. (B) Energy transfer between the 16 donor and the Cy5 acceptor. Reproduced with permission from ref. 51. Copyright 2011, John Wiley and Sons. | ||
PDI-cored glycodendrimers 48 and 49 showed selective binding to Concanavalin A (Con A) due to the carbohydrate–lectin interaction of the peripheral mannose moieties and Con A (a model lectin).48,49 Fluorescence spectroscopy and circular dichroism (CD) measurements were performed to investigate the nature of binding. As described in Fig. 18A, with increasing concentrations of Con A, the fluorescence emission of 48 exhibits a progressive decrease. The PDI backbones enter the hydrophobic region of Con A, via hydrogen bonds and hydrophobic interactions, leading to progressive quenching of fluorescence. Upon the addition of 49, the CD signal of the Con A, generated from the secondary-structure of β-sheet rich proteins, exhibits a progressive decrease and a bathochromic shift (Fig. 18B). The loss of the secondary structure due to the formation of cross-linked complexes induces the decreased intensity of the CD signal. The observed decrease of fluorescence emission intensity and CD signal intensity demonstrated the interactions of Con A with 48 or 49.
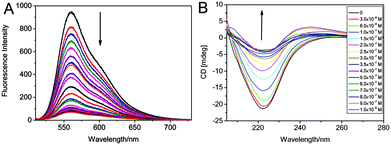 | ||
| Fig. 18 (A) Fluorescence spectra of 48 upon the addition of Con A in PBS. Reproduced with permission from ref. 49. Copyright 2013, Royal Society of Chemistry. (B) CD spectra of Con A in PBS upon the addition of 49. Reproduced with permission from ref. 48. Copyright 2013, Elsevier. | ||
A fluorescence turn-on approach for the sensitive and selective detection of proteins based on a nucleic acid aptamer and probe 17 is reported.29 In aqueous solution, PDI 17 exists in both aggregated and monomeric forms, thus strong fluorescence can be detected. When a negatively charged nucleic acid aptamer is added to the aqueous solution, PDI 17 rapidly binds to the aptamer via strong electrostatic interactions between the dye monomer/aggregates and the aptamer. When the monomer of 17 aggregates, a significant decrease in the fluorescence intensity is observed. The lysozyme shows high affinity toward the aptamer. Upon the addition of lysozyme, 17 aggregates are replaced by the lysozyme. As a result, the monomer of 17 is released, and the fluorescence signal is turn-on (Fig. 19).
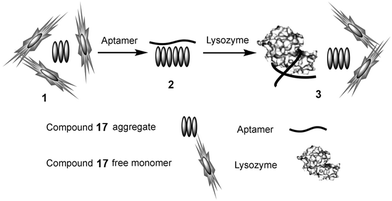 | ||
| Fig. 19 Schematic diagram for the selective detection of lysozyme. (1) Coexistence of the aggregates and monomer of 17 in aqueous solution; (2) binding of the aptamer to 17 aggregates; (3) 17 is replaced by lysozyme, thus its monomer is released and the fluorescence is turn-on. Reproduced with permission from ref. 29. Copyright 2010, John Wiley and Sons. | ||
3.2 Live cell studies
Thanks to high selectivity and sensitivity, fluorescence bioimaging has been widely used in biological research because it offers a unique approach to visualize and manipulate the morphological details of cells and biological processes. Water soluble PDIs with high fluorescence intensity and biocompatibility have thus attracted great attention in bioimaging. Applications of water-soluble PDIs in live cell studies fall into three broad categories: the dye (i) target-specific label receptors produced by bacteria, (ii) are taken up into living cells via endocytic routes and accumulated in the cellular cytoplasm, plasma membrane, and small membrane-bound structures, or (iii) carry DNA into the cell through electrostatic forces, which can be applied to gene delivery and bioimaging.Zimmerman's group45 reported a PG-dendronized PDI 46b containing a single biotin used in target-specific fluorescence bioimaging. Biotinylated PDI 46b could be immobilized on a PEG-passivated surface through biotin–neutravidin linkages with bright fluorescence, while only negligible binding of 46b was observed without pre-incubated neutravidin, indicating that immobilization was indeed highly specific. The target-specific fluorescence bioimaging was performed in living bacterial cells. Escherichia coli (E. coli) produced biotinylated λ receptors on the extracellular surface. The cells were pre-incubated with streptavidin. Fig. 20 shows that the bright fluorescent spots produced by 46b appear only at the surfaces of the cells. Furthermore, the spots present a helical pattern along the long axis of the cell in some bacteria, which is consistent with the arrangement of the proteins on the bacterial surface. The above observation demonstrates that 46b can be used as a highly target-specific label in bioimaging. This approach provides a new design for biolabeling by coupling a monofunctional fluorescent core to a multivalent periphery containing cellular receptors, therapeutic agents, targeting groups, or ligands.
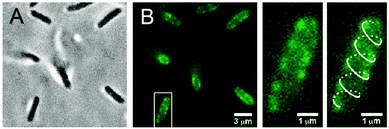 | ||
| Fig. 20 (A) Bright-field and (B) fluorescence images of E. coli cells labeled with 46b. Reproduced with permission from ref. 45. Copyright 2011, American Chemical Society. | ||
A guanidinium-dendronized PDI 30 was reported for the cellular cytoplasm imaging of HeLa cells (Fig. 21A).35 The acquired images indicated that 30 was efficiently taken up into HeLa cells and accumulated in the cellular cytoplasm. Fluorescent, positively charged dendritic star polymers (33, 35 and 36) were able to cross the cell membrane and enter living cells, whereas negatively charged dendritic star polymers (14 and 16) bearing carboxylic acids did not show any tendency to enter the cells.25 For example, a confocal microscopy image of 33a (red), which demonstrates penetration into the cells, is shown in Fig. 21B. The authors reported that the PDIs bearing a higher density of polymer chains with fewer amino groups underwent faster cell entry; however, PDIs with high numbers of amino groups led to considerable cytotoxicity.
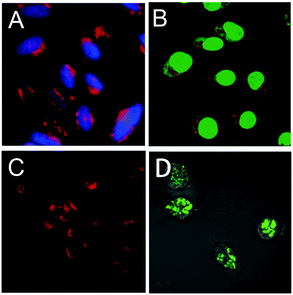 | ||
| Fig. 21 (A) Fluorescent microscopy images of HeLa cells stained with 30 (red) and cell nuclei stained with DAPI (blue). Reproduced with permission from ref. 35. Copyright 2013, Royal Society of Chemistry. (B) Penetration of 33a (red) into ECV-304 cells stained using a green fluorescence cell tracker. Reproduced with permission from ref. 25. Copyright 2008, American Chemical Society. (C) Fluorescence images of HeLa cells stained with 39c. Reproduced with permission from ref. 40. Copyright 2011, Royal Society of Chemistry. (D) Confocal microscopic images of murine macrophage cells after incubation with 47. Reproduced with permission from ref. 50. Copyright 2014, John Wiley and Sons. | ||
Besides the above cationic PDI, two non-ionic PDIs, 39c and 47, were also used for live-cell imaging.40,50Fig. 21C reveals that 39c is efficiently internalized by live-cells and accumulated in the cytoplasm. Based on the successful demonstration of binding interactions between 48 and 49 with Con A, the interaction was further investigated via confocal microscopy in macrophage cells. These cellular uptakes are mannose receptor-mediated and the cells possess selective binding with mannosylated derivatives. The green fluorescence of 47 inside the cell (Fig. 21D) suggested that the endocytosis mechanism led to vesicular (endosomal) localization. Control experiments indicated no obvious fluorescence by using another water-soluble PDI with cyclodextrin conjugates, suggesting that 47 possessed selective binding interactions with the surface mannose receptor of the macrophage cells.
An amphiphilic PG-dendronized PDI 42 was reported as a membrane marker.44 For highly specific membrane labeling, two alkyl chains were used as membrane anchoring groups and the length of the aliphatic chains was chosen to ensure that the overall length of the hydrophobic part matches the thickness of one lipid bilayer leaflet. In water, chromophore 42 formed micelleular aggregates and was nearly nonfluorescent. However, when 42 was incorporated into a lipophilic environment, such as inside biological membranes, the micelles disaggregated and the fluorescence of the perylene core was restored (Fig. 22). Cellular uptake studies documented that 42 was accumulated in the plasma membrane and in small vesicular structures such as Golgi, suggesting that it is taken up into living cells via endocytosis. Furthermore, the stained intracellular structures arose from a passive diffusion of 42 through the plasma membrane, as no colocalization of 42 and BODIPY TR methyl ester, which efficiently stains membranes of intracellular organelles, was shown with the exception of Golgi. PG-dendronized PDI 42 is thus an ideal label to anchor and track PG bound bioactive compounds in cellular membranes.
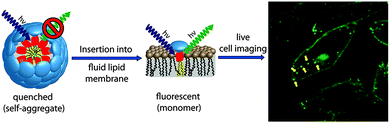 | ||
| Fig. 22 Schematic diagram of the self-quenched amphiphilic dye 42 lighting up in the fluid lipid membrane and the confocal microscopy image of cells incubated with 42. Reproduced with permission from ref. 44. Copyright 2013, American Chemical Society. | ||
PDI-cored cationic dendrimers 37(a–c) and star polymers 31–32 were used for gene delivery and bioimaging by Yin and coworkers.36,38 By using fluorescence microscopy, the cellular uptake experiments demonstrated that all the cationic PDIs were internalized into cells. The cell viabilities of 37(a–c) and 31–32 are all above 90%, indicating that the cationic PDIs exhibit low cytotoxicity. All the positively charged 37(a–c), 31 and 32 could act as DNA carriers because of the electrostatic forces with negatively charged DNA. The fluorescence images of 37c/DNA and 32/DNA complexes internalized into cells upon incubation (Fig. 23) displayed that 37c (A–A′′) and 32 (B–B′′) were able to deliver DNA into cells. The gene transfection efficacies of 37(a–c) decrease in the following order: 37a > 37b > 37c. This observation can be attributed to larger outer branches and more positive charges. The cationic dendrimers and star polymers are explored as a general tool for in vivo gene transfection.
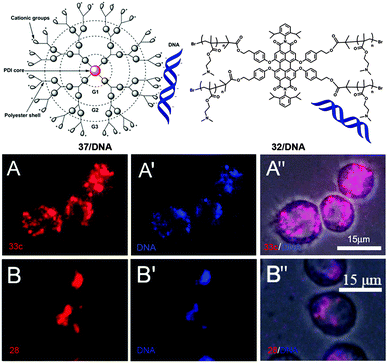 | ||
| Fig. 23 Fluorescence images of the 37c/DNA complex (A–A′′) and 32/DNA (B–B′′) internalizing into cells after incubation. A and B are fluorescence images of 37c and 32 (red), respectively. A′ and B′ are fluorescence images of CXR Reference Dye labeled DNA (blue). Reproduced with permission from ref. 38. Copyright 2013, Royal Society of Chemistry. Reproduced with permission from ref. 36. Copyright 2014, American Chemical Society. | ||
Some water-soluble PDIs have unique self-assembly properties, which have been used in biological research. PDI 40 with a biotin ligand in the bay-region was used as an “off–on” fluorescent probe for detecting and tracking proteins, which was driven by assembly–disassembly behavior.41 In aqueous solution, compound 40 self-assembles into nanostructures with complete quenching of fluorescence. After the addition of the target protein, the self-assembled nanostructures are disassembled since the probes bind to the targeted protein, resulting in a strong fluorescence signal. In the presence of avidin, 40 can be internalized by living cells (Fig. 24A and A′). Under physiological conditions, 26 behaves as a fluorogenic probe in biological cells, yielding a dosage-dependent green or red fluorescence.33Saccharomyces cerevisiae yeast cells were incubated with two concentrations of 26 in PBS (1 × 10−6 M and 1 × 10−5 M). The fluorescence microscopy images of the two samples are clearly different. Green emission can be detected in the yeast cells at the low concentration of 26 (1 × 10−6 M, Fig. 24B), while a red emission centered at about 630 nm was detected at the high concentration (1 × 10−5 M, Fig. 24B′). Particularly, under strong visible light, the fluorescence of some specific cellular compartments can be turned from red to yellow and then to green, due to the photo-tuning process of 26. The chiral PDI 7a can be internalized by living cells, since fluorescence is observed in the cytoplasm.19 Taking advantage of the aggregation of 7ain vitro, the pH of different regions of the cellular environment can be estimated. In the lambda scanning image (excitation wavelength = 488 nm, Fig. 24C), the co-existence of aggregated (640 nm) and monomeric emissions (550 nm and 590 nm) is observed in spot A, indicating that the pHs of these areas are below 4.5. In contrast, only monomeric emissions (580 nm) are observed in spot B, indicating that the pH is above 4.5.
 | ||
| Fig. 24 (A) Bright-field and (A′) fluorescence images of HeLa cells after co-incubation with 40 and avidin. Reproduced with permission from ref. 41. Copyright 2013, Royal Society of Chemistry. Fluorescence images of yeast suspensions for the concentration of 26 (B) 1 × 10−6 M and (B′) 1 × 10−5 M. Reproduced with permission from ref. 33. Copyright 2013, Royal Society of Chemistry. (C) Lambda scan image and (C′) the corresponding emission intensity vs. wavelength plots. Reproduced with permission from ref. 19. Copyright 2012, Royal Society of Chemistry. | ||
N-Cyclic bay-substituted PDIs 19(a–j) were reported as G-quadruplex ligands, causing DNA damage in transformed cells.31 Modifications of the ending groups or the length of side chains at the imide-positions did not result in major changes of the affinity to the G-quadruplex formed by the human telomeric sequence. In contrast, the number or the nature of the groups in the bay-region has a large influence on the affinity. Transformed human fibroblasts (BJ-EHLT) were incubated with 19c. Deconvolution microscopy images revealed that some of the damaged foci containing phosphorylated 53BP1 (a DNA-damage response factor) were induced by 19c co-localized with TRF1 (an effective interphase telomeres marker), forming the so-called telomere-dysfunction induced foci (TIFs) (Fig. 25). The G-quadruplex binding compound 19c can act as a telomere inhibitor and exhibit its potential as a selective antineoplastic drug.
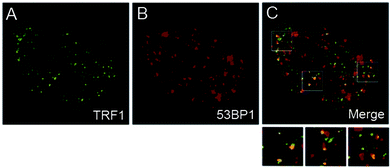 | ||
| Fig. 25 Transformed (BJ-EHLT) human fibroblasts were treated with 19c, fixed, and processed for IF by using antibodies against 53PB1. Representative images of IF using a Leica Deconvolution microscope. Enlarged views are below the merged images. Reproduced with permission from ref. 31. Copyright 2011, American Chemical Society. | ||
3.3 Tissue imaging and body studies
Tissue imaging is usually achieved by fluorescence labeling, which is the process of covalently or non-covalently attaching a reactive derivative of the fluorophore to a functional group in the target molecule. The most commonly labeled objects are antibodies, proteins, amino acids, and peptides. Electrostatic interaction is very common in tissue imaging because there are many electrolytes in biological bodies.Although the negatively charged dendritic star polymer 14 did not enter the cells,25 it could specifically label the cell nucleus by binding to positively charged positively charged nuclear proteins in fixed tissues.26 Fixed Drosophila larval tissues were used to demonstrate the binding interaction of 14 and the nuclear proteins. As described in Fig. 26, 14 staining (red) shows no overlap with CD8-GFP (cell–membrane marker, green, Fig. 26A) or with anti-α-Tubulin antibodies (cytoskeletal marker, green, Fig. 26B). In Fig. 26C and D, 14 staining is in accordance with DAPI (DNA dye, blue, Fig. 26C′′) and demonstrates a significant co-localization with histone H4 (green, Fig. 26D′′). Compound 14, bearing multiple –COOH groups, was demonstrated to bind specifically and directly with positively charged histones, resulting in the labeling of the cell nucleus. Compound 14 offers an attractive alternative to conventional expensive antibodies or chromophores displaying broad emission spectra such as DAPI.
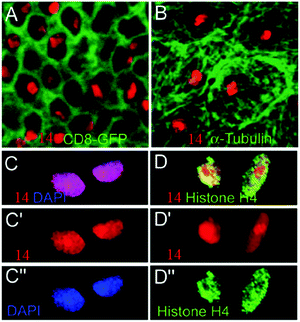 | ||
| Fig. 26 Confocal images of Drosophila larval tissues stained by 14. Double staining of 14 (red) with (A) CD8-GFP (green), (B) anti-α-Tubulin (green). (C–C′′) DAPI (blue), and (D–D′′) histone H4 (green). C′, C′′ and D′, D′′ are single channels. C and D are merged channels. Reproduced with permission from ref. 26. Copyright 2008, John Wiley and Sons. | ||
The positively charged dendritic star polymer 33b was able to enter and label living cells.25 In 2008, Yin et al.37 proposed a new application of 33b to directly stain the extracellular matrix (ECM) (Fig. 27A) network at high resolution and achieve ECM network visualization firstly. The positively charged chromophore 33b was strongly bound to the negatively charged constituents of ECM in both fixed and living tissue via electrostatic forces. The confocal images of ECM staining with 33b (red, Fig. 27C) and Vkg-GFP (green, Fig. 27B) in living trachea epithelium are presented in Fig. 27. By using high resolution confocal microscopy, 33b staining almost completely overlapped with Vkg-GFP (Fig. 27D), indicating that 33b gave the same specificity as Vkg-GFP for the ECM micronetwork structure. The ECM staining with 33b was much faster than antibody staining procedures, which may require several days to complete. Spectral analysis reveals a significantly increased emission intensity of 33b after binding to ECM components, supporting the direct binding between negatively charged ECM components and 33b. All these observations support that 33b is an efficient and specific ECM labeling agent.
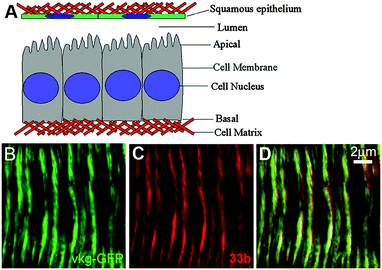 | ||
| Fig. 27 Schematic illustration of the extracellular matrix in a small central section of the Drosophila wing disc epithelium (A). Confocal images of ECM staining with 33b (red, C), Vkg-GFP (green, B) in living tracheal epithelium and merged channels (D). Reproduced with permission from ref. 37. Copyright 2008, American Chemical Society. | ||
In addition to the applications in the detection and imaging of biological molecules, some water-soluble PDIs can be used as gene/drug carriers or DNA intercalators together with simultaneous imaging in plants, insects and mice. Thus, these PDIs can be applied in the fields of diagnosis and therapy, which have attracted great attention in recent years. PDI-cored cationic dendrimers 37(a–c) have been successfully applied to deliver DNA into animal cells in vitro by Yin and coworkers.38
Apart from the above application, the authors have applied 37b as non-viral gene-vectors in plants using Arabidopsis as a model plant.52 Fluorescence images displayed that 37b was able to pass through the cell wall and deliver DNA efficiently throughout the root tissues, because 37b and DNA were detected in both the root cap and hair. The 10-day-old seedlings were treated with 37b/dsRNA by dropping the complex solution to the seedling root. Two genes, Shoot Meristemless (STM) and WEREWOLF (WER), which are essential for shoot tip and root development respectively, were chosen as target genes. 37b/dsSTM-treated plants exhibit a smaller shoot tip compared with the control, which is treated with H2O/dsRNA (Fig. 28(A and B)). The root morphology after H2O/dsWER and 37b/dsWER-treatment is given in Fig. 28(C and D). 37b/dsWER-treated plants produced more lateral roots compared with the control. These observations reveal that 37b can efficiently deliver dsRNA into plant cells and cause gene silencing with systemic phenotypes. Dendrimer 37b provides a new platform for gene transfection and systemic gene silence of important developmental genes in plants.
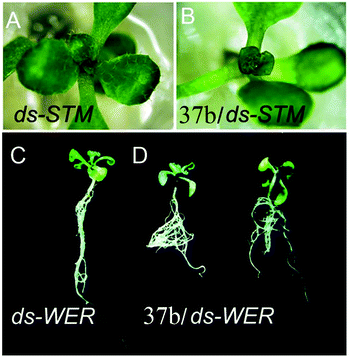 | ||
| Fig. 28 Shoot morphological images of (A) H2O/dsSTM-treated (control) and (B) 37b/dsSTM-treated plants. Root morphology of (C) H2O/dsWER-treated (control) and (D) 37b/dsWER-treated roots. Reproduced with permission from ref. 52. Copyright 2014, Royal Society of Chemistry. | ||
Yin and coworkers53 first reported that the cationic and PDI-cored dendritic star polymer 33a was able to deliver dsRNA to kill insect pests in oral feeding experiments. To kill insect pests, such as the corn borer, CHT10–dsRNA was synthesized to silence a key developmental gene and GFP–dsRNA was used as control. Fig. 29A presents a schematic diagram of a longitudinal section of the insect with red lines indicating the spreading directions of the gene-vectors. As demonstrated in Fig. 29B, 33a/CHT10–dsRNA fed larvae are clearly smaller than 33a/GFP–dsRNA fed ones. Moreover, all the 33a/CHT10–dsRNA-fed larvae died due to defective growth and molting. The in vivo experiments provided firm evidence that dsRNA was delivered into insect cells and efficiently silenced the target gene. Subsequently, the authors reported another gene vectors 37b, which also exhibited high gene transfection and gene interference performance in vivo via oral feeding.54 After three days of oral feeding, 37b (red) is observed in the mid-gut and penetrated into the gut cells (Fig. 29C and C′). This non-viral gene delivery system opens a new avenue for the control of insect pests without using chemical insecticides, which could cause environmental pollution.
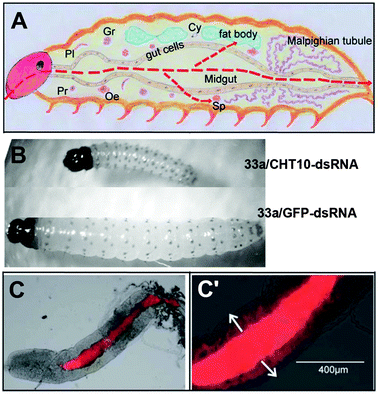 | ||
| Fig. 29 (A) Schematic diagram for longitudinal section of an insect larva. Reproduced with permission from ref. 54. Copyright 2014, Royal Society of Chemistry. (B) Corn borer larvae fed with 33a/CHT10–dsRNA and 33a/GFP–dsRNA. Reproduced with permission from ref. 53. Copyright 2013, John Wiley and Sons. (C) Fluorescence images of dissected larval gut after three days oral feeding of 37b. (C′) part of enlarged (C), red: 37b. Reproduced with permission from ref. 54. Copyright 2014, Royal Society of Chemistry. | ||
Besides the above applications in gene delivery, 37b was also used as a hydrophobic drug carrier by Yin et al. due to the cavities provided by outer dendritic polyester units.55 The dendrimer-based nanocarriers could efficiently deliver the drug into the living cells and largely increase the cytotoxicity of the drug. The larvae fed with 37b/thiamethoxam complexes possess a smaller size than that of the controls fed with 37b/H2O (Fig. 30). Furthermore, after five day feeding with 37b/thiamethoxam complexes, more than 90% larvae died. The in vivo experiments indicate that the drug toxicity was greatly enhanced with the help of 37b-mediated cellular internalization.
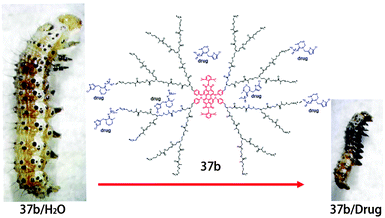 | ||
| Fig. 30 The structure of 37b/drug complexes and the digital photographs of larvae fed with diet containing 37b/H2O and 37b/drug complexes. Reproduced with permission from ref. 55. Copyright 2014, Royal Society of Chemistry. | ||
Yin and coworkers8 reported a perylene-based DNA intercalator 18b that could efficiently localize at the cell nucleus and inhibit cancer cell and tumor growth. The small cationic groups are incorporated in the imide-positions of PDIs to generate water solubility and maintain the planar structure of 18b, which provides the potential for use as a DNA intercalator. Spectral analyses show that 18b specifically accumulates in the cell nuclei by strong interaction with DNA. Chromophore 18b shows a stronger suppression effect to cancer cells, which is attributed to the effective DNA intercalation and nuclear accumulation that leads to the inhibition of cancer cells. However, 18b possesses a lower cytotoxicity to non-cancer cells, because of the much higher DNA replication rate of cancer cells than that of normal cells. Furthermore, with 18b intercalation, DNA replication of cancer cells is inhibited more strongly than that of normal cells, which leads to the higher toxicity to cancer cells. Mice with HCT116 tumors were used as a model to investigate the antitumor activity of 18b. The body weights of the mice were not changed after 10 days of injection with 18b. In addition, the tumor sizes are significantly smaller than those of the control (Fig. 31). The DNA intercalator 18b with low toxicity is a potential anti-cancer drug for the suppression of cancer cells and tumors. Because of DNA intercalation, these types of PDIs could be developed as potential drugs for suppressing cancer cells and tumors.
 | ||
| Fig. 31 Antitumor activity of PBDI was evaluated in vivo. (A) Mice injected with 18b (15 mg kg−1 per injection). In the control group, mice were injected with the same volume of stroke-physiological saline solution (SPSS). (B) Tumor size and weight of dissected tumors from (A). Reproduced with permission from ref. 8. Copyright 2014, John Wiley and Sons. | ||
4. Conclusions and outlook
This review summarizes the design and synthesis of water-soluble PDIs as well as their biological applications. The synthetic strategies leading to water-soluble PDIs include the introduction of ionic or non-ionic substituents with multiple polar groups in the bay-region, imide- or ortho-positions of PDIs. The biological applications include simple to complex studies: in vitro studies, live cell studies, tissue and body imaging. Due to the fluorescent nature of the central PDI fluorophore, which allows for the tracing of cellular uptake via fluorescence microscopy, biological applications can be visualized using optical spectroscopy, energy transfer imaging, and other bioimaging techniques. Nevertheless, due to undesirable self-aggregation, the low FQY of PDI fluorophores in aqueous solution and low cytotoxic selectivity toward cancer cells, the synthesis of multi-functional water-soluble PDIs with useful applications in biological fields is still a topic of ongoing research. Despite these challenges, a promising future of water-soluble PDIs can still be predicted. Looking ahead, water-soluble PDIs with multiple functions will find a wide range of applications such as simultaneous imaging with high specificity, diagnosis with high accuracy, and therapy with a few side effects. The combination of fluorescence techniques and nanotechnology in biological applications will remain an active research area in the future, which can be supported by facile synthetic routes to multi-functional and water-soluble PDIs with high biological specificity.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21174012, 51221002 and 21574009), the Beijing Natural Science Foundation (2142026), the Innovation and Promotion Project of Beijing University of Chemical Technology, and the Fundamental Research Funds for the Central Universities (YS201402).Notes and references
- C. Huang, S. Barlow and S. R. Marder, J. Org. Chem., 2011, 76, 2386–2407 CrossRef CAS PubMed.
- J. Qu, J. Zhang, A. C. Grimsdale, K. Müllen, F. Jaiser, X. Yang and D. Neher, Macromolecules, 2004, 37, 8297–8306 CrossRef CAS.
- L. Schmidt Mende, A. Fechtenkötter, K. Müllen, E. Moons, R. Friend and J. MacKenzie, Science, 2001, 293, 1119–1122 CrossRef CAS PubMed.
- C. Li and H. Wonneberger, Adv. Mater., 2012, 24, 613–636 CrossRef CAS PubMed.
- H. Langhals, W. Jona, F. Einsiedl and S. Wohnlich, Adv. Mater., 1998, 10, 1022–1024 CrossRef CAS.
- D. Görl, X. Zhang and F. Würthner, Angew. Chem., Int. Ed., 2012, 51, 6328–6348 CrossRef PubMed.
- K. Liu, Z. Xu, M. Yin, W. Yang, B. He, W. Wei and J. Shen, J. Mater. Chem. B, 2014, 2, 2093–2096 RSC.
- Z. Xu, K. Guo, J. Yu, H. Sun, J. Tang, J. Shen, K. Müllen, W. Yang and M. Yin, Small, 2014, 10, 4087–4092 CAS.
- C. Kohl, T. Weil, J. Qu and K. Müllen, Chem. – Eur. J., 2004, 10, 5297–5310 CrossRef CAS PubMed.
- L. Zhong, F. Xing, W. Shi, L. Yan, L. Xie and S. Zhu, ACS Appl. Mater. Interfaces, 2013, 5, 3401–3407 CAS.
- K. Peneva, G. Mihov, F. Nolde, S. Rocha, J. I. Hotta, K. Braeckmans, J. Hofkens, H. Uji-i, A. Herrmann and K. Müllen, Angew. Chem., Int. Ed., 2008, 47, 3372–3375 CrossRef CAS PubMed.
- G. Battagliarin, M. Davies, S. Mackowiak, C. Li and K. Müllen, ChemPhysChem, 2012, 13, 923–926 CrossRef CAS PubMed.
- S. Nakazono, Y. Imazaki, H. Yoo, J. Yang, T. Sasamori, N. Tokitoh, T. Cedric, H. Kageyama, D. Kim and H. Shinokubo, Chem. – Eur. J., 2009, 15, 7530–7533 CrossRef CAS PubMed.
- S. Nakazono, S. Easwaramoorthi, D. Kim, H. Shinokubo and A. Osuka, Org. Lett., 2009, 11, 5426–5429 CrossRef CAS PubMed.
- G. Battagliarin, C. Li, V. Enkelmann and K. Müllen, Org. Lett., 2011, 13, 3012–3015 CrossRef CAS PubMed.
- G. Battagliarin, Y. Zhao, C. Li and K. Müllen, Org. Lett., 2011, 13, 3399–3401 CrossRef CAS PubMed.
- J. Qu, C. Kohl, M. Pottek and K. Müllen, Angew. Chem., Int. Ed., 2004, 43, 1528–1531 CrossRef CAS PubMed.
- F. Yukruk, A. L. Dogan, H. Canpinar, D. Guc and E. U. Akkaya, Org. Lett., 2005, 7, 2885–2887 CrossRef CAS PubMed.
- P. K. Sukul, P. K. Singh, S. K. Maji and S. Malik, J. Mater. Chem. B, 2013, 1, 153–156 RSC.
- G. R. Newkome, K. K. Kotta and C. N. Moorefield, J. Org. Chem., 2005, 70, 4893–4896 CrossRef CAS PubMed.
- C. D. Schmidt, C. Böttcher and A. Hirsch, Eur. J. Org. Chem., 2007, 5497–5505 CrossRef CAS.
- C. D. Schmidt, C. Böttcher and A. Hirsch, Eur. J. Org. Chem., 2009, 5337–5349 CrossRef CAS.
- C. D. Schmidt, N. Lang, N. Jux and A. Hirsch, Chem. – Eur. J., 2011, 17, 5289–5299 CrossRef CAS PubMed.
- F. Morgenroth, E. Reuther and K. Müllen, Angew. Chem., Int. Ed. Engl., 1997, 36, 631–634 CrossRef CAS.
- M. Yin, C. R. Kuhlmann, K. Sorokina, C. Li, G. Mihov, E. Pietrowski, K. Koynov, M. Klapper, H. J. Luhmann and T. Weil, Biomacromolecules, 2008, 9, 1381–1389 CrossRef CAS PubMed.
- M. Yin, J. Shen, R. Gropeanu, G. O. Pflugfelder, T. Weil and K. Müllen, Small, 2008, 4, 894–898 CrossRef CAS PubMed.
- M. Yin, C. Feng, J. Shen, Y. Yu, Z. Xu, W. Yang, W. Knoll and K. Müllen, small, 2011, 7, 1629–1634 CrossRef CAS PubMed.
- S. You, Q. Cai, K. Müllen, W. Yang and M. Yin, Chem. Commun., 2014, 50, 823–825 RSC.
- B. Wang and C. Yu, Angew. Chem., Int. Ed., 2010, 49, 1485–1488 CrossRef CAS PubMed.
- Z. Xu, W. Cheng, K. Guo, J. Yu, J. Shen, J. Tang, W. Yang and M. Yin, ACS Appl. Mater. Interfaces, 2015, 7, 9784–9791 CAS.
- V. Casagrande, E. Salvati, A. Alvino, A. Bianco, A. Ciammaichella, C. D'Angelo, L. Ginnari-Satriani, A. M. Serrilli, S. Iachettini, C. Leonetti, S. Neidle, G. Ortaggi, M. Porru, A. Rizzo, M. Franceschin and A. Biroccio, J. Med. Chem., 2011, 54, 1140–1156 CrossRef CAS PubMed.
- M. Lor, J. Thielemans, L. Viaene, M. Cotlet, J. Hofkens, T. Weil, C. Hampel, K. Müllen, J. W. Verhoeven and M. Van der Auweraer, J. Am. Chem. Soc., 2002, 124, 9918–9925 CrossRef CAS PubMed.
- M. Montalti, G. Battistelli, A. Cantelli and D. Genovese, Chem. Commun., 2014, 50, 5326–5329 RSC.
- J. Schönamsgruber, B. Schade, R. Kirschbaum, J. Li, W. Bauer, C. Böttcher, T. Drewello and A. Hirsch, Eur. J. Org. Chem., 2012, 6179–6186 CrossRef.
- J. Zhou, J. Zhang, Y. Lai, Z. Zhou, Y. Zhao, H. Wang and Z. Wang, New J. Chem., 2013, 37, 2983–2986 RSC.
- S. You, Q. Cai, Y. Zheng, B. He, J. Shen, W. Yang and M. Yin, ACS Appl. Mater. Interfaces, 2014, 6, 16327–16334 CAS.
- M. Yin, J. Shen, G. O. Pflugfelder and K. Müllen, J. Am. Chem. Soc., 2008, 130, 7806–7807 CrossRef CAS PubMed.
- Z. Xu, B. He, J. Shen, W. Yang and M. Yin, Chem. Commun., 2013, 49, 3646–3648 RSC.
- Z. Xu, B. He, W. Wei, K. Liu, M. Yin, W. Yang and J. Shen, J. Mater. Chem. B, 2014, 2, 3079–3086 RSC.
- B. Gao, H. Li, H. Liu, L. Zhang, Q. Bai and X. Ba, Chem. Commun., 2011, 47, 3894–3896 RSC.
- F. Bo, B. Gao, W. Duan, H. Li, H. Liu and Q. Bai, RSC Adv., 2013, 3, 17007–17010 RSC.
- T. Heek, C. Fasting, C. Rest, X. Zhang, F. Würthner and R. Haag, Chem. Commun., 2010, 46, 1884–1886 RSC.
- T. Heek, F. Würthner and R. Haag, Chem. – Eur. J., 2013, 19, 10911–10921 CrossRef CAS PubMed.
- T. Heek, J. R. Nikolaus, R. Schwarzer, C. Fasting, P. Welker, K. Licha, A. Herrmann and R. Haag, Bioconjugate Chem., 2013, 24, 153–158 CrossRef CAS PubMed.
- S. K. Yang, X. Shi, S. Park, S. Doganay, T. Ha and S. C. Zimmerman, J. Am. Chem. Soc., 2011, 133, 9964–9967 CrossRef CAS PubMed.
- S. K. Yang and S. C. Zimmerman, Adv. Funct. Mater., 2012, 22, 3023–3028 CrossRef CAS PubMed.
- A. T. Zill, K. Licha, R. Haag and S. C. Zimmerman, New J. Chem., 2012, 36, 419–427 RSC.
- K. Wang, Y. Wang, J. Li, H. An, L. Zhang, J. Zhang and X. Li, Bioorg. Med. Chem. Lett., 2013, 23, 480–483 CrossRef CAS PubMed.
- K. Wang, H. An, F. Qian, Y. Wang, J. Zhang and X. Li, RSC Adv., 2013, 3, 23190–23196 RSC.
- K. Wang, H. An, R. Rong, Z. Cao and X. Li, Macromol. Rapid Commun., 2014, 35, 727–734 CrossRef CAS PubMed.
- C. L. Feng, M. Yin, D. Zhang, S. Zhu, A. M. Caminade, J. P. Majoral and K. Müllen, Macromol. Rapid Commun., 2011, 32, 679–683 CrossRef CAS PubMed.
- L. Jiang, L. Ding, B. He, J. Shen, Z. Xu, M. Yin and X. Zhang, Nanoscale, 2014, 6, 9965–9969 RSC.
- B. He, Y. Chu, M. Yin, K. Müllen, C. An and J. Shen, Adv. Mater., 2013, 25, 4580–4584 CrossRef CAS PubMed.
- D. Shen, F. Zhou, Z. Xu, B. He, M. Li, J. Shen, M. Yin and C. An, J. Mater. Chem. B, 2014, 2, 4653–4659 RSC.
- X. Liu, B. He, Z. Xu, M. Yin, W. Yang, H. Zhang, J. Cao and J. Shen, Nanoscale, 2015, 7, 445–449 RSC.
| This journal is © The Royal Society of Chemistry 2016 |