Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Smart fluorescent probes for imaging macrophage activity†
Antonio
Fernández
and
Marc
Vendrell
*
MRC Centre for Inflammation Research, Queen's Medical Research Institute, The University of Edinburgh, EH16 4TJ Edinburgh, UK. E-mail: mvendrel@staffmail.ed.ac.uk; Tel: +44 (0)131 242 6685
First published on 11th January 2016
Abstract
Macrophages are multi-functional immune cells with key roles in host defense and tissue remodelling. The broad array of macrophage functions has prompted the development of very diverse smart fluorescent architectures, rationally designed to elicit a fluorescent signal only after target engagement. This tutorial review covers recent advances in the design, synthesis and application of smart fluorescent probes for imaging macrophage cellular activity, ranging from small fluorophores to peptide-based probes with internally quenched fluorescent pairs or activity-based reactive groups.
Key learning points1. Recent advances in chemical strategies to optimise small reactive fluorophores targeting intracellular macrophage activity.2. The power of chemical architectures with enhanced fluorescence amplification (e.g. quenched polymers, FRET peptides) and their associated challenges. 3. Activity-based probes as a chemical strategy to fine-tune enzyme specificity. 4. Diversity-oriented fluorophores as an alternative to conventional rational design for the discovery of macrophage-specific fluorophores. 5. Perspectives and challenges of in vivo imaging and theranostic approaches for macrophage-associated disorders. |
1. Introduction
1.1. The ‘grand challenge’ of developing chemical tools for imaging cellular activity in real time: smart fluorescent probes
Optical imaging offers exceptional opportunities to visualise and quantify multiple dynamic events in living cells with high molecular resolution. Recent advances in the translation and resolution power of fluorescence imaging, together with our increasing knowledge on the development of fluorescent probes, have opened the possibility of generating bespoke reagents to image cellular activity in real time. Compounds emitting fluorescence only after activation by specific signalling molecules or proteins are ideal for this purpose, exhibiting high signal-to-noise ratios –thus, high sensitivity – and enabling in situ imaging without any washing steps. Such fluorescent architectures are usually known as activatable or smart fluorophores, since they are rationally designed to elicit a fluorescent signal only after target engagement. The construction of smart fluorophores is a multidisciplinary exercise that involves organic chemistry to design suitable chemical structures with ‘off–on’ properties, and chemical biology to validate the probes and their molecular targets in relevant models. Smart fluorescent probes for imaging cellular activity offer a whole range of possibilities in biological and medical research, from the interrogation of biological processes in vivo to the visualisation of differential patterns between healthy and disease states with a direct application in diagnostics and drug discovery.Macrophages are multi-functional immune cells with key roles in host defense and tissue remodelling, and have been widely studied as a model for imaging cellular activity. Imaging the activity of macrophages is not straightforward since they exhibit relatively plastic phenotypes, with enzyme and metabolic activity profiles depending on their microenvironment. However, this diversification has facilitated the development of chemical architectures to assess macrophage activity at different levels (e.g. enzymatic activity, redox states, phagocytosis). In this article, we will review the advances over the last 10 years in the design, synthesis and application of smart fluorescent probes for imaging the activity of macrophages. Our review specifically covers the development of activatable fluorescent architectures but does not include macrophage imaging probes based on genetically-encoded reporters, non-activatable fluorophores1 or nanomaterials, which have been reviewed elsewhere.2
1.2. Macrophages: a case-study for imaging cellular activity
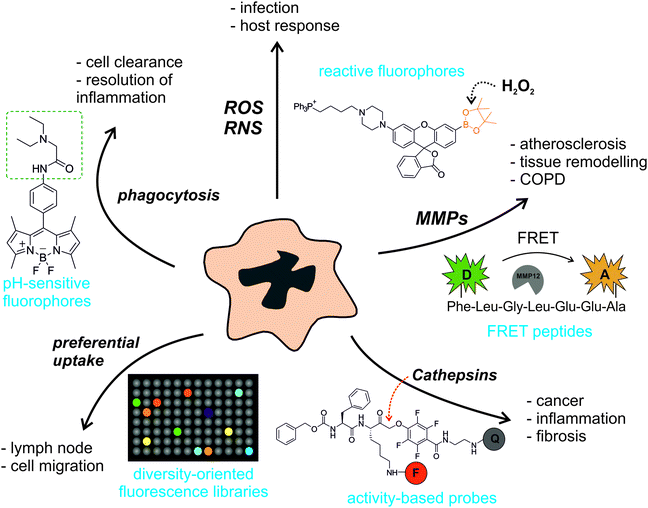
Macrophages display a broad range of cellular functions, and their activity has been associated with many diverse biological processes. They are phagocytic cells that recycle and clear cells during inflammatory processes, and remove pathogens upon infection. Macrophages also play a critical role in tissue repair and remodelling, as well as in the orchestration of the host response to infectious diseases through secretion of cytokines, enzymes and reactive oxygen species (ROS), the downside of which is a major role in fibrotic scarring of damaged tissues.3 There is evidence that macrophages are essential in the tumour microenvironment, actively participating in tumour growth, metastasis and progression to malignancy, and the number and phenotype of macrophages also influence the fate of atherosclerotic plaques in cardiovascular diseases and neurodegenerative disorders. All these properties make macrophage activity a relevant target for diagnostic and therapeutic exploitation, which has prompted the development of suitable imaging probes in the last few years.We have structured our review according to different molecular targets in macrophages that are employed to trigger the emission of smart fluorophores. Notably, these targets not only define the biological context in which the activity of the macrophages is imaged but also they pre-determine the main chemical features of the smart fluorescent probes to be employed, which can range from reactive small molecule fluorophores to intramolecular-quenched peptides or activity-based probes. We have grouped smart fluorescent probes for macrophages into two large families: (1) probes targeting intracellular activity (e.g. ROS, pH gradients, phagocytosis) (Section 2), and (2) probes monitoring enzymatic activity (e.g. matrix metalloproteases (MMPs), cathepsins) (Section 3). Finally, we have also incorporated a section on the preparation of fluorophores using diversity-oriented fluorescence libraries as an alternative strategy to discover unique structures for macrophage imaging (Section 4).
As illustrated in Fig. 1, the broad array of macrophage functions in healthy and pathological states has stimulated the preparation of chemical structures with various fluorescence amplification mechanisms. Smart probes based on small molecule fluorophores are ideal to target intracellular macrophage activity, which correlates with gradients of pH in phagocytic macrophages or high levels of ROS in infected areas. Their excellent cell permeability and rapid kinetics are essential to ensure the correct localisation of these probes and their ability to react with short-lived molecular species (e.g. ROS, reactive nitrogen species (RNS)). Larger structures are typically required to image enzymatic activity in macrophages, which is often associated to the remodelling of the extracellular matrix in inflammatory and cancerous disorders. Smart substrate-based probes exploit the proteolytic activity of these enzymes (e.g. MMPs, cathepsins) to elicit a fluorescent response upon recognition by the active enzyme. Their amplification mechanisms are either linked to the dequenching of a fluorophore or to the efficient Förster resonance energy transfer (FRET) between fluorescent donor and acceptor moieties. On the other hand, activity-based probes react with nucleophiles in the catalytic sites of enzymes (e.g. cysteines in the active site of cathepsins) to form irreversible bonds. Every family of enzymatic probes has advantages and shortcomings (e.g. fluorescence amplification, adaptability to proteomic analysis, kinetics); hence they must be fine-tuned to meet the requirements of the biological imaging studies. Finally, diversity-oriented fluorescence libraries are a good source of smart probes to image macrophages in biological or medical contexts where little is known about the molecular markers that are associated with macrophage activity.4 Their simplicity and direct applicability make them useful tools for translational imaging (e.g. intraoperative imaging, fluorescence-guided surgery).
2. Targeting intracellular macrophage activity
2.1. Reactive oxygen and nitrogen species
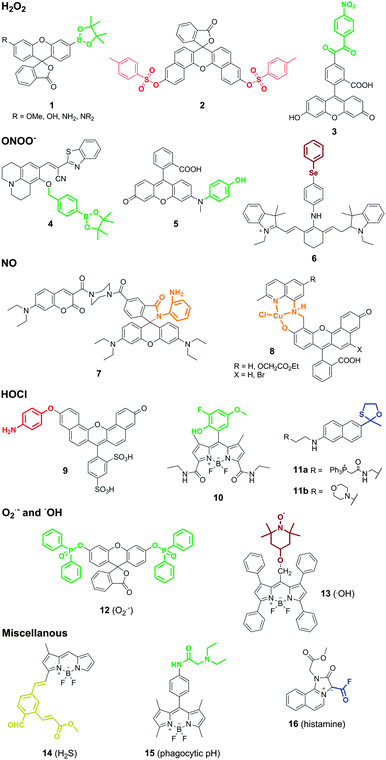
ROS and RNS are directly associated with many functions of macrophages. Macrophages produce and release ROS in response to phagocytosis of microbes or after stimulation by different agents in connection with bacterial killing, inflammation or tissue injury. Macrophages also produce ROS as a second messenger to regulate signalling pathways involved in cell proliferation, apoptosis and gene expression.Hydrogen peroxide (H2O2) is one of the most important endogenous ROS in macrophages. In phagocytic cells, the oxidase NOX2 is activated to produce superoxide (O2˙−), which undergoes dismutation to form H2O2 and later hypochlorous acid (HOCl). Owing to its short lifetime, the levels of H2O2 in macrophages are estimated to be in the lower nanomolar range, thus requiring highly sensitive probes for real-time imaging. Most of the smart probes for the visualisation of H2O2 in macrophages rely on small molecule fluorophores containing H2O2-reactive moieties. Boronates, disulfonates and benzoylcarbonyl groups have been employed for this purpose. The incorporation of these groups at specific positions of fluorescent scaffolds modulates their internal charge or electron transfer properties so that fluorescence is only elicited upon chemoselective cleavage with H2O2. The group of Chang has become one of the most prolific labs in the development of smart fluorophores for imaging H2O2 by exploiting the chemospecific boronate-to-phenol switch.5 Notably, the incorporation of reactive boronate groups has proven compatible with various fluorescent scaffolds with diverse spectral properties. An interesting example of this adaptability has been the preparation of a palette of boronate-based rhodol and fluorescein smart probes with high quantum yields and varying emission wavelengths (1, Fig. 2).6 Such versatility allowed to perform multiplexing experiments to simultaneously monitor the fluxes of H2O2 and HOCl in the phagosomes of stimulated macrophages. Disulfonate groups have been also used as reactive moieties for imaging the intracellular production of H2O2 in macrophages. Disulfonate probes based on naphthofluorescein, a scaffold with red-shifted spectral properties when compared to fluorescein, were reported as smart probes for H2O2 in mice peritoneal macrophages (2, Fig. 2). These compounds showed good selectivity for H2O2 over other ROS and very low detection limits, in the pM range.7 More recently, Nagano et al. prepared 5-(4-nitrobenzoyl)carbonylfluorescein (NBzF) as a highly sensitive probe for H2O2 regulated by donor-excited photoinduced electron transfer (d-PeT) with a remarkable 150-fold fluorescence increase after activation (3, Fig. 2).8 The corresponding diacetylated version, showing improved cell permeability, was used to visualise the production of H2O2 in activated murine macrophages. Subsequent studies have led to the adaptation of NBzF for SNAP-tag labelling, opening the possibility to visualise the production of H2O2 in specific subcellular compartments.9 Fluorescent scaffolds with enhanced spectral properties can provide additional benefits. Ratiometric probes, which are self-referenced and thus concentration-independent, have been derivatised with boronate groups to produce 1,8-naphthalimide-based imaging probes with blue-to-green shifts upon reaction with H2O2 and compatibility with two-photon imaging.10 The engineering of cyanine–xanthene probes with an optically tunable hydroxyl group has led to smart fluorescent architectures with suitable properties for in vivo imaging, due to their emission in the near-infrared region of the spectrum.11
The potential cross-reactivity of some of the reactive moieties and the short lifetime for most ROS/RNS hampers the selective targeting of these species. Nagano et al. observed cross-reactivity of their H2O2-sensitive benzoylcarbonyl probe NBzF with peroxynitrite (ONOO−),8 a RNS formed by the reaction between nitric oxide (NO) and O2˙− in inflammatory cells. Likewise, boronates have been employed to produce smart probes for imaging ONOO−. Kim et al. recently reported the synthesis of a boronate coumarin precursor with rapid intramolecular cyclisation and subsequent fluorescence activation upon reaction with ONOO− (4, Fig. 2).12In vitro characterisation assays indicated the preferential reactivity of the probe for ONOO−over other RNS and ROS (including H2O2), although the fact that cell imaging assays were performed in macrophages activated under generic stimulation conditions (e.g. LPS, IFNγ) does not rule out the possibility that other ROS/RNS could be detected using these boronate derivatives. The group of Yang developed rhodol-based reactive structures with intramolecular-quenching N-phenyl groups that triggered fluorescence emission upon reaction with ONOO− and subsequent N-dearylation. Initial attempts employed a trifluoromethyl ketone as the warhead reactive group. These probes were later optimised by screening of different N-phenylrhodol derivatives for selectivity towards ONOO− over other ROS (HOCl). The N-phenylrhodol derivative HKGreen-4 (5, Fig. 2) showed a remarkable amplification in ONOO− after two-electron oxidation and formation of an iminium ion that was further hydrolysed to release the highly fluorescent N-dearylated rhodol.13 HKGreen-4 was employed to image increased endogenous levels of ONOO− in Escherichia coli-challenged macrophages, and its selectivity was corroborated by blockage with nitric oxide synthase (iNOS) and NADPH oxidase (NOX) inhibitors. These probes will allow to image macrophage activity at infection sites as well as studying the bactericidal effects of ONOO− production.
One major limitation of smart fluorescent probes that rely on the conjugation of quenching reactive moieties to small molecule fluorophores is their irreversibility, which impedes continuous monitoring of ROS/RNS in real time. This limitation was recently overcome with the preparation of a reversible near-infrared tricarbocyanine smart probe (Cy-PSe) containing an organoselenium functional group for real-time monitoring of ONOO− in live macrophages (6, Fig. 2).14 The fluorescence emission of Cy-PSe remained low in the basal state due to PeT quenching but was activated through selective Se oxidation with ONOO− (23-fold fluorescence increase). Notably, the oxidised fluorescent structure reverted back to the ‘off’-state after glutathione reduction within 5 min. This unique family of probes might become a useful tool to monitor longitudinally the production and elimination of ROS in macrophages in a non-invasive manner.
Nitric oxide (NO) is an indispensable biological mediator produced by nitric oxide synthase (NOS2), whose expression in macrophages is regulated by cytokines and microbial products. Macrophages present high levels of NOS2 upon infection or inflammatory stimuli so that the endogenous release of NO produces a cytotoxic and protective response against infectious agents (e.g. viruses, bacteria, fungi). The development of smart fluorophores for imaging NO in macrophages still very much relates to the seminal work of Nagano and colleagues with the conjugation of o-diaminebenzene groups to fluorescent scaffolds.15 Nagano's approach relies on the formation of N2O3 from NO in aerobic conditions and its subsequent reaction with o-diamine groups – which behave as intramolecular PeT quenchers – to produce highly fluorescent electron-deficient triazoles. This strategy has proven compatible with all sorts of fluorescent structures (e.g. BODIPY, cyanine, rhodamine, fluorescein), including hybrid and ratiometric dyes. An interesting example was described by the group of Lin with one of the first reports of ratiometric imaging of endogenous NO in macrophages using a highly sensitive coumarin–rhodamine hybrid (LOD: 30 nM) (7, Fig. 2).16 The design of these probes, however, suffers from two limitations: (1) o-diaminoaromatic groups show cross-reactivity with dehydroascorbic acid (DHA), and (2) their activation relies on the formation of N2O3, implying that NO cannot be detected under hypoxic or anoxic conditions. Regarding the former, o-hydroxyamino groups have been employed to minimise the reactivity for DHA at the expense of increasing the LOD for NO to the low micromolar range.17 As for the latter, Lippard et al. described an alternative approach for sensing NO in macrophages based on the preparation of transition metal complexes with small fluorophores. Metal-fluorophore complexes exploit the close proximity of the fluorophore to the metal to quench the fluorescence emission until it reacts with NO. One elegant example of this strategy was described in the synthesis of seminaphthofluorescein Cu(II) complexes (8, Fig. 2) with red-shifted emission properties and their application to imaging endogenous NO in IFN-γ and LPS-stimulated macrophages.18
Hypochlorous acid (HOCl) is an oxidant molecule formed by the reaction between a chloride ion and hydrogen peroxide, which is catalysed by the enzyme myeloperoxidase (MPO). Like other ROS, HOCl is generated as part of the host immune protection to infection, but abnormal levels of MPO are also implicated in the pathogenesis of atherosclerosis and other inflammatory states. Libby et al. reported a sulfonaphthoaminophenyl fluorescein (SNAPF) for the selective detection of HOCl in MPO+ macrophages (9, Fig. 2).19 Monocytes and neutrophils are immune cells with high expression levels of MPO; however, only MPO+ macrophages can be found in atherosclerotic plaques, thus being a potential biomarker to predict risk for coronary events and myocardial infarction. Upon activation with HOCl, SNAPF displayed a remarkable fluorescence increase in the far red region of the spectrum. The red-shifted emitting properties of SNAPF are an important asset given the potential interference from autofluorescent elastin-rich arterial tissues. SNAPF was validated in vitro as well as in MPO+ stimulated macrophages, and provided one of the first reports of direct imaging of HOCl in tissue sections of human atherosclerotic plaques. Other fluorescent probes have been reported to image HOCl in macrophages using a relatively broad range of activatable groups and small fluorophores. These include non-fluorescent thiosemicarbazides as precursors of highly fluorescent oxadiazoles,20 or BODIPY-phenols with high fluorescence amplification upon oxidation to the corresponding quinones with reduced intramolecular PeT quenching. This latter family of probes has been subjected to optimisation studies to enhance the chemical stability by derivatization with halogen groups, leading to successful probes for imaging endogenous HOCl in THP-1 human macrophages (10, Fig. 2).21 Recently, the group of Chang has developed a new class of probes for imaging HOCl with subcellular resolution using two-photon microscopy.22 Two-photon microscopy provides high resolution with deep tissue penetration and minimal phototoxicity as it employs long excitation wavelengths. The authors derivatized acedan, a two-photon-compatible fluorophore, with an oxathiolane HOCl-reactive group as well as mitochondria and lysosome-targeting moieties (i.e. triphenylphosphine and morpholine, respectively) (11a and 11b, Fig. 2). The resulting probes were used for in vitro imaging of macrophage activity and in vivo imaging in relevant mouse models of inflammation. These smart probes will be important to study the trafficking and subcellular localisation of ROS and ROS-related enzymes (e.g. MPO) in vivo.
Superoxide (O2˙−) is one of the few ROS that can be detected with chemical architectures that undergo either oxidative or reductive activation mechanisms. Reduced fluorophores (e.g. hydroethidine, dihydrofluorescein, dihydrorhodamine) are widely used as commercially available reagents for general measurements of O2˙−. However, one of the main limitations of these reagents is their potential cross-reactivity with other ROS. Following the pioneering work of Itoh et al. in the preparation of probes for O2˙− relying on a non-redox mechanism,23 the group of Tang reported the synthesis of phosphinate-based architectures to image endogenous levels of O2˙− in macrophages.24 The design of these phosphinate-protected fluorophores relies on the nucleophilicity of O2˙− to ‘uncage’ the fluorescent core and release a bright fluorescent signal (12, Fig. 2). These probes were prepared in one single step by conjugation of diphenylphosphinic chloride to either fluorescein (for green emission) or naphthofluorescein (for red emission), and underwent chemical deprotection only after incubation with O2˙− but not other with other ROS. Their high selectivity allowed to visualise the endogenous production of O2˙− in peritoneal mouse macrophages after activation with phorbol myristate acetate (PMA), and their specificity was demonstrated by inhibition of the fluorescence signal upon treatment with Tiron, a non-enzymatic O2˙− scavenger.
Less usual chemical architectures have been employed for the detection of very reactive species, such as hydroxyl radicals (˙OH) with lifetimes in aqueous media in the range of nanoseconds. The detection of these intracellular species in macrophages is extremely challenging, not only because of their short lifetimes but also due to the potential interference from ONOO−, which has similar oxidation capacity. Tang et al. designed a smart fluorophore for ˙OH imaging based on a tetraphenyl-BODIPY derivative attached to the 4-hydroxy-TEMPO (4-hydroxy-2,2,6,6-tetramethyl-1-piperidinoxyl) moiety (13, Fig. 2).25 The authors exploited the high reactivity of the nitroxide group of TEMPO towards methyl radicals to form fluorescent methylated BODIPY derivatives in environments with high levels of ˙OH and DMSO (acting as the source of methyl radicals). This approach allowed imaging of ˙OH in stimulated macrophages with very low detection limits (LOD: 18 pM). However, the need for high concentrations of DMSO – in the milimolar range – might compromise the applicability of this strategy to visualise the release of ˙OH in vivo.
2.2. Other intracellular biomarkers and phagocytic activity
In addition to ROS and RNS, macrophages produce a wide range of metabolites that can be used as indicators of their intracellular activity. Hydrogen sulfide (H2S) is a signalling molecule that plays an important role in a vast number of physiological and pathological states. Macrophages, among other cells, produce H2S endogenously from cysteine with the involvement of cystathione β-synthase and cystathione γ-lyase enzymes. Smart fluorescent probes for H2S have been generated following similar strategies to the ones described above for ROS, mainly conjugating reactive moieties to small fluorophores at sensitive positions for electron or charge transfer. An internal charge transfer (ICT) BODIPY derivative was prepared by conjugation of the 1,3-dimethyl-BODIPY core to an aromatic aldehyde with an ortho-α,β-unsaturated acrylate methyl ester group (14, Fig. 2).26 While the meso position of the BODIPY scaffold is preferred to introduce PeT quenching moieties, this H2S-reactive moiety was conjugated to the position 3 of the BODIPY scaffold. This modification not only led to effective ICT but also red-shifted the fluorescence emission properties of the BODIPY scaffold. The resulting probe (ZS1) reacted with H2S to produce a five-membered hemithioacetal ring with remarkable fluorescence emission. ZS1 showed good selectivity for H2S over other biothiols and was used to monitor the changes in H2S levels in murine macrophages. Macrophages were stimulated with PMA to increase the production of ROS, which subsequently led to a reduction of the endogenous levels of H2S. Therefore, the decrease in the fluorescence emission of ZS1 (due to the decrease in H2S levels) was used as an indirect readout of the ROS activity in macrophages. Although the quantification of decreases in fluorescence emission is often more complex than for enhancement signals, these fluorescent probes might be used to study redox cycles between ROS-induced oxidative stress and H2S-mediated repair, either alone or in combination with some the above mentioned ROS-sensitive fluorophores. Other H2S-reactive moieties include dinitrophenyl ethers, which can be readily incorporated into coumarin-based scaffolds to produce ratiometric probes.27 Yuan et al. conjugated a H2S-sensitive coumarin scaffold to rhodamine, which favoured mitochondrial localisation and was used as an internal reference since its emission remained unchanged with H2S activation. This example highlights the benefits of ratiometric structures to minimise fluctuations in the readout due to variations in the concentration of the probes, cell confluency or the efficiency of the activation mechanism.Phagocytosis is one of the main functions of macrophages. Macrophages are professional phagocytes which help to clear necrotic cells, pathogens as well as other tissue components after injury. The clearance of apoptotic cells (e.g. neutrophils) has been also identified as a key step in the resolution of inflammatory processes. Imaging macrophage phagocytosis has been traditionally achieved with nanomaterials – generally compatible with molecular resonance imaging (MRI), such as iron oxide or gadolinium nanoparticles – that preferentially accumulate in macrophages. These nanomaterials are excellent tools for in vivo studies using MRI. Recent progress in optical-based modalities has enabled imaging the phagocytic cascade in macrophages using multimodal MRI-optical composites or novel smart fluorophores.
Vendrell and Lavilla et al. developed a synthetic strategy to prepare smart fluorophores for imaging phagocytic activity in macrophages.28 The authors optimised the adaptation of multicomponent reactions (i.e. convergent reactions where three or more starting materials react to form a new product under a unified mechanism) to the BODIPY scaffold to synthesise derivatives via Ugi, Passerini and Bienaymé–Blackburn–Groebcke reactions. Using this approach, a highly sensitive smart fluorophore (PhagoGreen) was generated to image phagosomal acidification in macrophages (15, Fig. 2). PhagoGreen has a pKa of 5.7, which is in accordance with the pH values observed in late phagosomes in macrophages, and showed a preferred localisation into intracellular vesicular structures, making it an ideal probe to monitor the pH changes associated to the maturation of the phagosomes. Notably, the authors proved the selective activation of PhagoGreen in zymosan-stimulated macrophages by treatment with bafilomycin A, an inhibitor of the vacuolar-type H+-ATPase that is essential for the acidification of the phagosomes. The high selectivity and sensitivity of PhagoGreen enabled its application for in vivo imaging of phagocytic macrophages in zebrafish in real time (Movies S1 and S2 in ESI†). The colocalisation of PhagoGreen with macrophage markers and the discrimination between early (non-acidic and non-fluorescent) phagosomes and late (acidic and highly fluorescent) phagosomes validate PhagoGreen as a versatile smart fluorescent probe for imaging phagocytic macrophage activity in vivo, and represents an optical alternative to MRI-active nanomaterials. Multicomponent reactions have been also employed for the preparation of other smart fluorescent probes for imaging macrophages. Of note is the development of Histamine Blue, a mesoionic acid fluoride with enhanced fluorescence emission upon reaction with histamine (16, Fig. 2).29 While limited to short excitation and emission wavelengths, Histamine Blue enabled direct imaging of intracellular histamine in macrophages that were treated with thapsigargin, a Ca2+ ATPase inhibitor that blocks the last stage of the autophagic process involving the fusion of autophagosomes and lysosomes.
3. Monitoring the enzymatic activity of macrophages
3.1. Quenched probes for imaging MMPs
MMPs constitute a family of zinc-dependent proteases, which can degrade most tissue components. In humans there are 23 different MMPs, whose activities are regulated at different levels, being directly associated with various physiological processes (e.g. wound repair, tissue remodelling) and pathologies (e.g. cancer, arthritis, atherosclerosis). In this section, we will specifically cover smart fluorescent probes for MMPs that can be employed to image the activity of macrophages (excluding other cell types, such as cancer cells or fibroblasts) in different biological contexts.Some of the first attempts for optically imaging MMPs were based on poly-L-lysine structures containing self-quenched fluorescent groups that were released upon MMP cleavage. This strategy was employed to generate smart near-infrared probes for in vivo visualisation of MMP-2 and MMP-9 activity in macrophages using mouse models of atherosclerosis.30 Near-infrared fluorophores offer important advantages for in vivo imaging due to minimised tissue autofluorescence and deeper penetration into tissue, which facilitates their use for whole-body fluorescence molecular tomography (FMT) and reflectance imaging studies. The authors modified the GGPRQITAG sequence (a peptide substrate for MMP-2 and MMP-9) with the near-infrared cyanine dye Cy5.5 (excitation: 675 nm, emission: 694 nm). The close proximity of the different Cy5.5 fluorophores within the polymeric structure resulted in a non-fluorescent basal state, which changed into a highly fluorescent response upon MMP cleavage and subsequent release of the fluorophores to the media. Using these probes, the authors observed high levels of MMP-2 and MMP-9 activity in the atherosclerotic aortas from apolipoprotein E-deficient (apoE−/−) mouse models, which have impaired clearing of plasma lipoproteins and develop atherosclerosis in a short time. Notably, the authors performed co-localisation experiments with macrophage-specific markers (Mac3) and confirmed that areas showing high MMP activity were rich in macrophages, whereas fibrous plaques with low MMP activity contained few macrophages. These smart fluorescent probes show potential as novel diagnostic tools to interrogate atheroma inflammation and identify patients with high risk of cardiovascular diseases.
One limitation of self-quenched polymers is their inherently large molecular weight. Whereas large macromolecules can be advantageous for tissue retention and enhanced contrast, their relatively long half-lives in circulation may be detrimental for translational imaging. As a result, there has been an interest in developing smaller probes that can be selectively activated by MMPs. Many of these probes are based on internally quenched peptides containing one specific MMP substrate peptide flanked by two chromophores, either a donor–acceptor fluorescent pair or a fluorophore–quencher pair. The main requirement for selecting an effective donor–acceptor fluorescent pair is the overlap of their spectral properties (i.e. emission donor matching excitation acceptor) for efficient FRET between the chromophores. These probes typically display higher quenching efficiencies than self-quenched polymers and also offer the possibility of reading ratiometric measurements when a donor–acceptor fluorescent pair is used. FRET quenched peptides represent the vast majority of fluorescent reagents for MMPs and, despite not being highly selective, they have been widely used as tracers for imaging MMP activity in macrophages. One exceptional example of the utility of these reagents was recently reported by Werb et al., who performed intravital imaging of tumour-associated macrophages in breast cancer mouse models.31 The authors employed the commercial probe MMPSense 680 to monitor the proteolytic activity of several MMPs (mainly 2, 9, 12 and 13) in the tumour microenvironment in real time. Notably, most of the MMP activity was observed in myeloid cells, which included macrophages and dendritic cells but not neutrophils. The treatment with anti-CSF1R antibody (i.e. an experimental anticancer therapy) delayed tumour growth and depleted dramatically MMPSense 680-labelled cells, indicating their active role in promoting angiogenesis, invasion, and metastasis. This study highlights the potential of combining smart fluorescent probes with high-resolution intravital imaging technologies to unveil key functions of macrophages in the tumour microenvironment and to advance in the validation of novel anticancer therapies.
Different chemical strategies have been explored to enhance the selectivity of smart fluorescent probes for MMPs. One revolutionary chemical design was reported by Tsien et al. with the preparation of activatable cell-penetrating peptides (ACPPs).32 These architectures consisted of polyarginine-based cell-penetrating peptides, whose cellular uptake was blocked by an inhibitory domain made up of negatively charged residues. ACPPs employ MMP-cleavable linkages between the two domains so the internalisation and subsequent detection of the cell-penetrating peptide is dependent on MMP activity. This concept has been extrapolated to engineering large macromolecules with high selectivity for activated macrophages, with the derivatisation of binding domains for scavenger receptors (i.e. cell surface-markers of macrophages in atherosclerotic lesions) using MMP-9 activatable linkers.33
Enhanced cellular targeting can be also achieved by incorporation of lipid motifs to quenched MMP-activatable peptides. The group of Schultz developed a ratiometric FRET probe for imaging MMP-12 activity in macrophages involved in pulmonary inflammation (Fig. 3).34 The authors derivatised the peptide sequence PLGLEEA (with good selectivity for human and mouse MMP-12) using different combinations of FRET fluorophore pairs (e.g. coumarin343/TAMRA (LaRee1), coumarin343/methoxycoumarin (LaRee5)) to obtain probes with detection limits for MMP-12 in the low nanomolar range. A remarkable feature of Schultz's design was the incorporation of a palmitic acid at the N-terminal group of the FRET peptides. The lipid moiety targeted the probe LaRee1 to the plasma membrane (where MMP-12 is mainly localised in activated macrophages) and facilitated the internalisation and intracellular accumulation of one of the fragments after the proteolytic cleavage, producing a memory effect on macrophages with MMP-12 pericellular activity. Imaging studies of LaRee1 in LPS-stimulated macrophages confirmed the MMP-12 activity not only by loss of FRET but also by accumulation of one of the fragments in internal membranes (Fig. 3 and Movies S3, S4 (ESI†)). LaRee1 was employed to monitor the proteolytic activity in bronchoalveolar lavage samples from mouse models of pulmonary inflammation and compared to untreated and Mmp12−/− mice. These assays identified alveolar macrophages as the main cell type showing MMP-12 activity at the plasma membrane in inflamed lung tissue, and confirmed the importance of their stimulation in several inflammatory lung diseases. The development of this family of smart probes represents an important step forward towards the validation of MMP activity as a biomarker for chronic obstructive pulmonary disease (COPD).
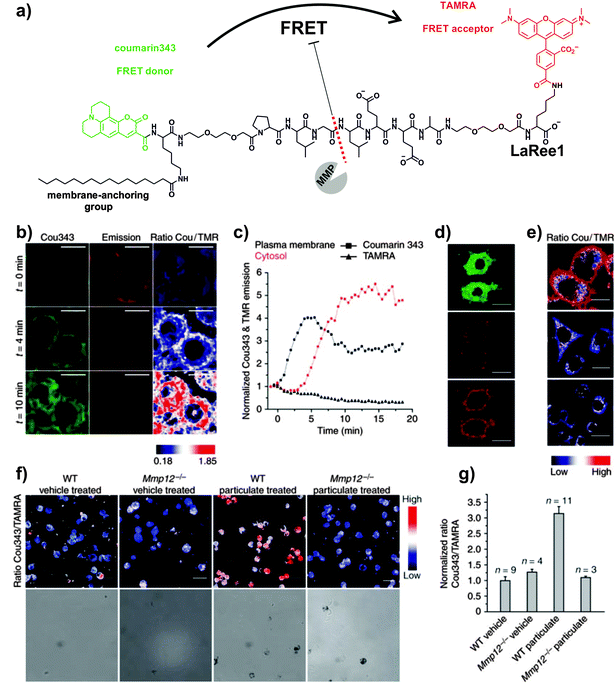 | ||
| Fig. 3 FRET probes for imaging MMP-12 activity on macrophages. (a) Chemical structure of the FRET probe LaRee1 incorporating coumarin343 as the donor and TAMRA as the acceptor. (b) Time-course fluorescence microscopy images of macrophages after treatment with LaRee1 and recombinant MMP-12: t = 0, coumarin343 fluorescence emission was quenched and TAMRA showed sensitized emission, t = 4 and 10 min: strong ratiometric change was observed and the green fluorescence shifted from the plasma membrane to the cell interior (scale bar: 10 μm). (c) Plots of coumarin343 and TAMRA fluorescence emission traces at the plasma membrane and in the cytosol over time. (d) After LaRee1 was added to LPS-stimulated macrophages, the emission ratio was accompanied by an increase of green fluorescence inside cells (top panel) and a loss of FRET signal emission (centre panel). Direct TAMRA excitation showed the predominant plasma membrane location of the remaining intact reporter and demonstrated extracellular cleavage (bottom panel) (scale bar: 20 μm). (e) MMP activity was found on LPS-stimulated macrophages (top panel) but not in unstimulated ones (centre panel) or those stimulated but also treated with the MMP inhibitor GM6001 (bottom panel). (f) LaRee1 images MMP12 activity from wild-type (WT) and Mmp12−/− mice that were challenged with particulate fraction or vehicle by intratracheal instillation. (g) Quantification of MMP-12 activity showed a significant increase in WT particulate-challenged mice compared to control or Mmp12−/− mice. Reproduced with permission from the Nature Publishing Group.34 | ||
3.2. Activity-based probes targeting cathepsins
Cysteine cathepsins constitute a family of 11 proteases with an essential role in the breakdown of proteins. Being macrophages key players in tissue modelling, many of their functions are directly related to the expression and/or secretion of cathepsins, which have been associated with several pathological diseases, including cancer, arthritis or COPD. Most cathepsins are activated at low pH; hence their activity is preferentially localised in the lysosomes, which are membrane-bound cellular organelles enclosing numerous enzymes for protein degradation.Analogous to the above mentioned MMP probes, co-polymers have been also adapted as smart fluorescent probes for imaging cathepsin activity. These structures are the basis of the commercially available ProSense probes, and represent powerful tools to visualise cathepsin activity in macrophages. Weissleder et al. reported the preparation of Cy5.5-labelled co-polymers including the sequence GHPGGPQKC as smart fluorescent probes with high selectivity for cathepsin K (CatK).35 These polymers were used to monitor the activity of CatK in vivo in the experimental murine atherosclerosis model apoE−/− (described above in Section 3.1). The authors observed high fluorescence signals in atherosclerotic plaques due to the increased CatK activity, which correlated with areas of elastin fibre disruption and high abundance of macrophages. Similar results were obtained in fresh carotid human atheroma specimens, asserting the potential of these probes for translational imaging.
Smart probes that emit fluorescence only after recognition by active cathepsins are advantageous because they provide a direct readout of the maturation of the proenzymes, helping to understand their function in healthy and disease states. To this end, activity-based probes (ABPs) have enabled direct imaging and profiling of cathepsin activity in vivo. ABPs are designed to be highly specific for the catalytically active form of the enzyme, and they typically consist of three components: (1) a chemical group or ‘warhead’ that reacts irreversibly with the nucleophile at the active site of the cathepsin, (2) a binding region for enzyme specificity, which can be of peptidic or non-peptidic nature, and (3) a reporter system (e.g. typically a fluorophore alone or a fluorophore–quencher pair) for direct visualisation of the tagged enzymes (Fig. 4).
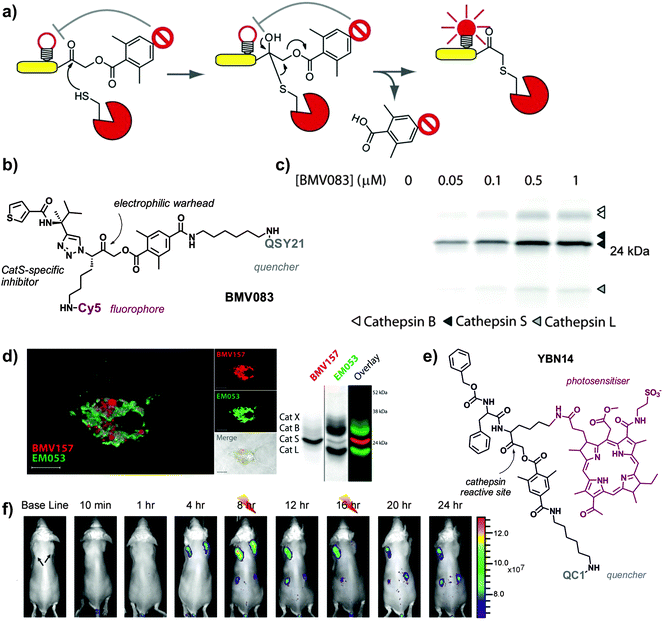 | ||
| Fig. 4 Activity-based smart fluorescent probes for imaging cathepsin activity. (a) Schematic representation of cathepsin activity-based probes, where the active cysteine at the catalytic site reacts with the electrophilic warhead to form an irreversible bond and dequench the fluorophore. (b) Chemical structure of BMV083, a CatS-directed smart probe. (c) Labelling profile of BMV083 in living human primary macrophages. Cells were exposed to the indicated concentrations of BMV083 for 3 h, before being harvested, washed, and lysed. Total protein was resolved on 15% SDS-PAGE, and fluorescently labeled proteins were visualised by in-gel fluorescence scanning. (a–c) Reproduced with permission from Cell Press.38 (d) Confocal microscopy images of mouse bone-marrow derived dendritic cells exposed to a red CatS-directed smart probe (BMV157) and a green pan-reactive cathepsin probe (EM053); biochemical characterisation of the dual probe system by SDS-PAGE in bone-marrow derived mouse macrophages. Reproduced with permission from the American Chemical Society.41 (e) Chemical structure of YBN14, a cathepsin specific activity-based theranostic agent. (f) 4T1 cancer cells were injected subcutaneously into two separate locations on the back of BALB/c mice. After tumours were established (marked by black arrows), YBN14 was injected intravenously and whole-body in vivo imaging was performed. After injecting YBN14 and start acquiring the first images, the right tumour was treated with light at 8 and 16 h (indicated with thunder cartoons) while the left tumour was kept in the dark, which resulted in a significant decrease of cathepsin activity. Reproduced with permission from Ivyspring.43 | ||
Over the last 10 years, the group of Bogyo has remarkably accelerated the development of ABPs for imaging cathepsin activity in macrophages. Initial designs of ABPs consisted of peptide acyloxymethylketones (AOMKs) as the electrophilic group to react with cysteine residues at the catalytic site, and Cy5 as the fluorescent reporter (the Cy5:QSY21 pair was used as a fluorophore:quencher pair for the preparation of internally quenched ABPs).36 This group of smart probes offer the advantage of monitoring the labelling of specific cathepsins by SDS-PAGE analysis, which allows to assign unequivocally the activity of different cathepsins to the fluorescence signals observed during imaging experiments. Cathepsin-targeted ABPs have been used in vivo to identify the cathepsins that are activated in macrophages during colitis (i.e. inflammation of the colon) and to dissect some of the signalling pathways involved.37 Fluorescent ABPs with high affinity for CatB, CatL and CatS were employed in experimental models of colitis using in vivo FMT imaging and subsequent proteomic characterisation. These studies revealed the activation of cathepsins (primarily CatS) in macrophages in the inflamed colon, and identified CatS as a mediator molecule for colonic pain. ABPs have accelerated the validation of cathepsin inhibitors as potential therapies for colitis and will become important tools for the early diagnosis of colonic disease via fluorescence endoscopy.
One of the main limitations of ABPs over substrate-based enzyme-activatable probes is the lack of amplification. Whereas one molecule of the target enzyme can cleave (and therefore activate) many substrate-based probes, the irreversible covalent binding between the reactive ABP and cysteine at the catalytic site results in the inactivation of the enzyme, thus limiting the amplification of the fluorescent signal. In order to compensate this shortcoming, the binding affinity and electrophilicity of the ‘warhead’ of ABPs need to be finely tuned. A second generation of non-peptidic ABPs with high affinity for CatS was generated using CatS-selective 1,4-disubstituted-1,2,3-triazole inhibitors developed by the group of Ellman as the main scaffold (Fig. 4).38 The authors replaced the norleucine group with a lysine residue that allowed the incorporation of a Cy5 fluorophore, and used the previously validated 2,6-dimethyl benzoic AOMK as the electrophilic warhead with a pendant QSY21 quencher for near-infrared fluorescence activation. The resulting non-peptidic ABPs maintained the potency of previous peptidic ABPs while showed enhanced selectivity for CatS in human monocyte-derived macrophages and bone-marrow derived mouse macrophages. High activity CatS levels have been reported for tumour-recruited macrophages, which contribute to tumour progression and are associated with poor prognosis in many cancers. In vivo imaging studies in breast cancer mouse models proved the utility of these quenched ABPs to image and report cathepsin activity in tumour-associated macrophages (validated as CD11b+ and macrophage mannose receptor (MMR)+ cells). These smart fluorescent probes are excellent tools to image and characterise subpopulations of macrophages in the tumour microenvironment, and have opened different research avenues to enhance prognosis in cancer patients.
Further modifications on ABPs have been aimed at optimising their properties for different applications. Zhang et al. developed trifunctional ABPs incorporating an octa-arginine cell-penetrating peptide to perform cathepsin proteomic profiling studies inside living macrophages.39 These ABPs contained one fluorophore (i.e. fluorescein) and one biotin motif, which facilitated the enrichment, purification and visualisation of the active enzymes in live cells to identify active cathepsins in their native environment with very high sensitivity. As a result, the authors were able to detect for the first time low abundant cathepsins (i.e. cathepsins F and O) in live macrophages.
Verdoes and Bogyo et al. have also systematically optimised the different components of their quenched ABPs to enhance their biodistribution and in vivo applicability.40 First, the incorporation of a sulfonated quenching moiety and exploration of different linkers between the electrophile and quencher groups resulted in an improvement of the water solubility of their ABPs. Furthermore, modifications in the electrophilic ‘warhead’ demonstrated the tunability of ABPs to target different enzyme populations. This is an advantage of ABPs over substrate-based architectures, as the binding/reactive moieties can be fine-tuned to be highly specific or non-specific for different subfamilies of proteases. In order to develop a pan-reactive cathepsin probe, the authors replaced the 2,6-dimethylbenzoic acid-derived AOMK with a 2,3,5,6-tetrafluoro-substituted phenoxymethyl ketone, which is smaller and displays greater reactivity against cysteine residues. The resulting ABPs exhibited enhanced serum stability and kinetics, near-infrared fluorescence emission properties and broad cathepsin reactivity (i.e. cathepsins B, L, S and X) in both mouse and human monocyte-derived macrophages. Non-invasive in vivo imaging of macrophage activity in breast cancer mouse models confirmed the primary function of these cathepsins in CD68+ tumour-associated macrophages. Pan-reactive ABPs have improved current diagnostic tools for conditions where multiple macrophage cathepsins are involved. These smart probes have also opened the possibility to perform multiplexed imaging experiments with complementary ABPs. A recent report demonstrates the potential of dual-color macrophage imaging by combining ABPs that are highly selective for CatS with pan-reactive probes (Fig. 4).41 This unique toolbox enabled the visualisation of CatS in relation to other cathepsins, and indicated some key differences in the subcellular localisation of CatS between macrophages and dendritic cells.
The rapid activation of quenched ABPs, which emit fluorescence only upon cleavage by cathepsins, renders high signal-to-noise ratios at very early time points compared to non-activatable versions or substrate-based probes. This is an essential feature when rapid readouts are required, such as pre-screening examinations or clinical interventions. Quenched ABPs have been recently employed in endoscopic imaging of the colon to visualise early lesions that remained undetectable by conventional colonoscopy. Early detection of colonic polyps can prevent most of colorectal cancer deaths; however, there is a need for imaging contrast agents that can improve current diagnostic methods. Bogyo et al. recently described the application of one of their near-infrared ABPs for enhanced detection of colorectal lesions using fluorescence-guided colonoscopy.42 Notably, the administration (either intravenously or intrarectally) of the quenched ABP probe strongly labelled tumour-associated inflammatory cells (mostly macrophages) within 1 h, rapidly discriminating polyps from healthy areas both in mice and human tissue. Altogether, these probes represent an important advancement for targeted colonoscopy and may help in the identification of at-risk patients as well as to improve the outcome of surgical interventions.
ABPs have been also adapted for theranostics (i.e. agents combining imaging and therapeutic capabilities). The group of Blum has recently developed new quenched ABPs incorporating bacteriochlorin derivatives as both fluorophores and photosensitizers (PS-qABPs, Fig. 4).43 These novel chemical structures not only enabled imaging of cathepsin activity in tumour-associated macrophages in vivo but also assessing the effects of selective macrophage ablation using photodynamic therapy (PDT). PDT relies on the irradiation of photosensitive molecules in the presence of oxygen to produce singlet oxygen and ROS that cause cell death. The targeted properties and irreversible binding of PS-qABP resulted in the release and accumulation of photosensitizers in tumour-associated macrophages after cathepsin cleavage. Further activation by treatment with light induced significant tumour shrinkage in an aggressive breast cancer mouse model. Blum's design overcomes some important limitations of conventional PDT agents, such as (1) off-target cytotoxicity, which is reduced since the photosensitizer is only activated in cells with high cathepsin activity, and (2) rapid clearance, which is overcome by the covalent binding of PS-qABP to the catalytic site of cathepsins. The combination of macrophage-specific theranostic probes with translational modalities for imaging and drug activation will open a new era for the study and treatment of macrophage-related disorders.
4. Alternative fluorescence amplification mechanisms for imaging macrophages: diversity-oriented fluorescence libraries
In the last few years, there has been an emerging interest in the preparation of diversity-oriented fluorescence libraries as an alternative strategy for the discovery of highly selective fluorescent probes. Diversity-oriented fluorescence libraries make use of combinatorial chemistry to produce large collections of small molecules with the potential to elicit an enhanced fluorescence response upon binding to a defined target (e.g. metabolites, proteins, cells, tissues).4 One of the main advantages of these libraries is their compatibility with a broad range of targets (from small molecules to cells) even when no prior molecular knowledge is available, making it an ideal approach for the discovery of probes to discriminate between closely related cell states or phenotypes (e.g. resident vs. recruited macrophages, M1 vs. M2 polarised-macrophages). Furthermore, when a new probe is identified, it does not need to be further modified as it already incorporates both binding and reporting motifs. On the other hand, diversity-oriented fluorescence libraries require the generation of vast collections of compounds with broad chemical diversity and highly efficient imaging screens to successfully discover new smart fluorophores.Chang et al. has pioneered the use of diversity-oriented fluorescence libraries for numerous applications. His group recently reported this strategy to discover microglia-specific fluorophores.44 Microglia are glial cells that represent the first line of immune defense in the central nervous system, behaving as the resident macrophages in the brain and spinal cord tissues. The activity of microglia is conventionally imaged using antibodies, which are limited in the acquisition of dynamic information as well as in cell permeability. In this work, the authors screened over 5000 small molecule fluorophores in the BV2 microglia cell line by high-throughput cell imaging to discover two BODIPY compounds with preferential uptake in microglia over other glial cells and minimal impact in their native function (Fig. 5). Further studies suggested mitochondrial protein cytochrome c oxidase subunit 2 as one of their potential intracellular targets. These cell permeable smart probes are powerful tools for monitoring microglia activity in real time and in culture conditions that are only compatible with live cell imaging (Movie S5 (ESI†)).
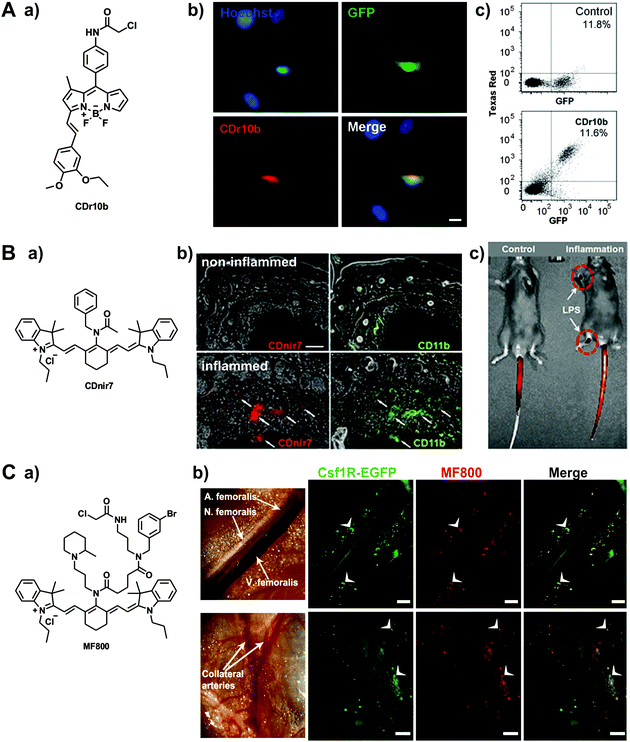 | ||
| Fig. 5 Diversity-oriented fluorescence libraries for the discovery of new macrophage-specific fluorophores. (A) a. Chemical structure of the microglia-specific probe CDr10b. b. Fluorescence microscopy images of GFP-expressing microglia from CX3CR1-GFP transgenic mice. Cells were stained with 500 nM of CDr10b (red) and Hoechst 33342 for nuclear counterstaining (blue). Scale bar: 10 μm. c. Flow cytometry analysis confirmed that CDr10b stained the GFP+ fraction of CX3CR1-GFP primary neural cells. Reproduced from ref. 44 with permission from The Royal Society of Chemistry. (B) a. Chemical structure of the macrophage-specific probe CDnir7. b. Whole-body in vivo imaging and CDnir7 detection in LPS-induced inflamed regions of mouse paws. c. Immunostaining analysis of CDnir7 (red) and the macrophage marker CD11b (green) from control tissue (top) and inflamed tissue (bottom). Scale bar: 50 μm. Reproduced from ref. 45 with permission from The Royal Society of Chemistry. (C) a. Chemical structure of the probe MF800. b. (top) Intravital fluorescence imaging along the femoral artery displays MF800-labeled cells (red), which co-localise with the genetically-labelled macrophages (green) in Csf1r-EGFP mice. Scale bars: 200 μm; (bottom) regions of collateral arteries with arteriogenesis show prominent cellular infiltration of MF800 + Csf1r + cells (white arrows), indicative of arterial growth and repair by macrophages. Scale bars: 1000 μm. Reproduced from PLOS One.46 | ||
An important advantage of diversity-oriented fluorescence libraries is their adaptability to most fluorophores, which allows to diversify chemical structures with specific spectral properties. This has been the case for the near-infrared tricarbocyanine core, which was adapted to a diversity-oriented approach to discover macrophage probes for in vivo imaging using FMT and multispectral optoacoustic tomography (MSOT).45 From an initial screen of around 200 near-infrared compounds, one probe (CDnir7) was selected for its preferential uptake in macrophages over other immune cells. Subsequent experiments validated the application of CDnir7 for in vivo imaging of macrophage influx in a mouse model of inflammation using FMT as well as MSOT (Fig. 5). CDnir7 showed good properties as a translational imaging agent, with rapid clearance (i.e. plasma half-life: 21 min) and a much faster response (less than 10 min) than most nanoparticle-based contrast imaging agents. Further studies have been directed towards the optimisation of macrophage-specific near-infrared fluorophores.46 A high-throughput flow cytometry screening was designed to examine near-infrared fluorophores in different human immune cell lines: MOLT-4 for lymphocytes, U-937 for monocytes, and U-937-DM for monocyte-derived macrophages. From a stringent primary cell-based imaging screen, one hit compound (MF800) was validated in vivo to visualise macrophage-rich areas of inflamed tissue. The authors also performed intravital imaging experiments in Csfr1-EGFP mice, where high correlation was observed between the signals of the transgenic green fluorescent macrophages and the fluorescence emission of MF800 (Fig. 5). Immunofluorescence characterisation of the MF800-labelled cells confirmed the expression of two macrophage surface markers (i.e. CD68 and CD169), which asserts the high selectivity of the MF800 architecture for macrophages.
The excellent safety profile (i.e. low toxicity, rapid clearance) of small molecule fluorescent architectures makes them optimal candidates for clinical translation as contrast agents in image-guided surgery. Intraoperative imaging allows to visualise areas of interest while the patient is undergoing surgery, helping the surgeon to keep healthy tissue undamaged. One important application of intraoperative imaging regards the identification and staging of sentinel lymph nodes, which can be reached by cancer cells from primary tumours in the early stages of metastatic disease. Most optical probes for lymph node visualisation rely on non-specific fluorophores that accumulate in the nodes because of their hydrodynamic range. Chang et al. devised a new intraoperative technology for lymph node staging based on macrophage-specific fluorophores.47 Since these smart probes showed a preferential uptake in macrophages compared to other cells in the constituting nodes, high fluorescence signals were observed in macrophage-rich lymph nodes with no or very minor metastasis while low signals were detected in nodes that had been replaced by cancer cells. This indirect readout represents a new strategy for enhanced surgical guidance and in situ detection of metastasis in lymph nodes.
5. Conclusions and outlook
5.1 Towards translational imaging
Recent advances in medical imaging have opened the possibility to monitor the progression of disease in patients in real time and with minimal invasiveness. The integration of smart fluorescent probes for macrophages into translational imaging technologies will provide a powerful experimental tool to revolutionise our understanding on the roles that macrophages play in vivo. Remarkable developments have been achieved in endoscope-based imaging modalities, which enable direct access to deep tissues (e.g. gastrointestinal tract, colon, lungs, among others) for high-resolution imaging. A recent example was reported by Lesur et al. with the application of smart fluorophores to monitor the myeloperoxidase (MPO) activity of macrophages in rats with acute lung injury using in vivo endoscopic confocal fluorescence microscopy.48 The authors exploited the application of SNAPF – a red-emitting fluorophore upon reaction with the HOCl generated by MPO (see Section 2.1) – in intravital endoscopic microscopy to visualise alveolar macrophages in the inflamed lungs of rats. While further optimisation studies will be necessary to ensure the compatibility and biodistribution of smart fluorescent architectures in human tissue, these studies represent a first step towards non-invasive ‘virtual’ biopsies as point-of-care diagnostic tools. We anticipate that smart fluorescent probes for macrophages will also enhance the diagnostic resolution of other clinical imaging modalities, such as optical coherence tomography (OCT), to visualise atherosclerotic plaques in coronary arteries. Smart fluorescent probes able to discriminate between different states of macrophages will become excellent tools to improve the diagnosis and prognosis of cardiovascular diseases and cancer, wherein prevalence of NO-producing macrophages over other tumour-associated macrophages is predictive of survival of colorectal cancer.Medical imaging has also remarkably progressed in intraoperative modalities to assist surgeons in the operating room. These technologies reduce the risk of damaging critical organs or removing healthy tissue, and they can provide in situ confirmation that surgery was successful when tissue resection was involved. Some of the above described smart probes for imaging tumour-associated macrophages31 as well as lymph nodes47 have huge potential for intraoperative imaging to enhance the outcomes of cancer surgery. The recent report of Bogyo et al. employing cathepsin substrate-based probes with the FDA-approved da Vinci surgical system for fluorescence-guided tumour resection illustrates the compatibility and potential of smart fluorescent probes to help surgeons make rapid decisions in the operating room when dealing with macrophage-related lesions (e.g. cancer, inflammation).49
5.2 Not just a pretty image
Given the relevance of macrophage activity in many pathological disorders, smart fluorescent probes targeting macrophages will also play an important role in the emerging field of theranostics. The recent work of Blum et al. with cathepsin-triggered quenched photosensitizers is an excellent example of how these chemical architectures can be integrated for both imaging and therapeutic purposes.43 Such theranostic tools will have enormous impact in the field of cancer, where there is clinical and experimental evidence of the protumourigenic activity of tumour-associated macrophages, although many other inflammatory disorders may also benefit from therapies for selective macrophage ablation. Future generations of smart theranostic agents may not only rely on fluorophore/photosensitizers as imaging/therapeutic agents but also incorporate prodrugs to be specifically released by defined macrophage triggers (e.g. MMP or cathepsin cleavage, gradients of pH, ROS). These tools will be optimal for clinical translation, with enhanced cellular selectivity and potentially less side effects, as well as minimal dependence on photoactivation, which could become a limitation when accessing deep tissues.The emerging rise of nanomaterials (e.g. nanoparticles, polymers) will play an important role in the development of better diagnostic and therapeutic tools.2 For instance, novel biomaterials might be employed as nanotherapeutics to modulate macrophage activity in vivo. Bratlie et al. recently showed the potential of polymer chemistry to alter macrophage polarisation.50 With a library of poly-(N-isopropylacrylamide-co-acrylic acid) nanoparticles including different functional groups, the authors developed a quantitative structure–activity relationship method to determine the macrophage phenotypic response to specific biomaterials. While these studies will require further chemical collections to explore the vast array of macrophage functions, the incorporation of smart fluorophores into responsive biomaterials will create new exciting avenues to study macrophage polarisation in vivo.
5.3 Fine-tuning the fluorescent ‘bullet’
Addressing the phenotype complexity is one of the biggest challenges for those aiming at imaging macrophage activity in vivo. Recent studies using large transcriptomic analysis suggest that traditional classifications of macrophage cell states (e.g. M1/M2) may not correlate in vivo. Macrophages are very plastic cells, changing their functions in response to their microenvironment, with even marked differences between individual cells. From the chemical point of view, this means that future generations of smart fluorescent probes will have to exploit not only the differences between macrophages and other immune cell types (e.g. neutrophils, lymphocytes) but also between different subpopulations of macrophages. Potential strategies may involve the incorporation of ligands targeting subcellular organelles for additional selectivity. For instance, Chang et al. compared the behaviour of their HOCl-reactive fluorophores in the lysosomes and mitochondria of macrophages by simply conjugating morpholine (for lysosomes) and triphenylphosphine (for mitochondria) moieties to the fluorophore counterpart.20 Alternative strategies may involve the derivatization of reactive fluorophores with high affinity ligands (e.g. O(6)-benzylguanine derivatives for SNAP-tag proteins) to localise the smart probes in specific cells or subcellular regions.8 Peptides are a good source of targeting ligands, and they have been widely used to increase the cellular selectivity of small molecules and drugs. The integration of peptide sequences within smart fluorescent probes may become an interesting strategy for the development of highly selective imaging agents aiming at specific functions/states of macrophages. However, it is also possible that multiple ligands and/or activation triggers are necessary to target specific subpopulations of macrophages. This concept was nicely illustrated by Umezawa et al., who developed genetically-encoded reporters exploiting the synergistic activation by scavenger receptor class A type I and MMP-9 (i.e. key biomarkers for the progression of atherosclerosis) to discriminate between activated and resting macrophages.33 In the next few years, organic chemists and chemical biologists are facing the challenge of constructing translatable smart fluorescent probes that can achieve such high levels of specificity.Acknowledgements
A. F. acknowledges the funding from the Foundation Alfonso Martin Escudero (FAME, Spain). M. V. acknowledges funding from the Medical Research Council and the Marie Curie Career Integration Grant (333487). The authors also acknowledge Dr Steve Jenkins (University of Edinburgh) and Dr Martijn Verdoes (Radboud University Medical Center) for helpful discussions and advice.References
- W. Han, R. Zaynagetdinov, F. E. Yull, V. V. Polosukhin, L. A. Gleaves, H. Tanjore, L. R. Young, T. E. Peterson, H. C. Manning, L. S. Prince and T. S. Blackwell, Am. J. Respir. Cell Mol. Biol., 2015, 53, 50–59 CrossRef CAS PubMed.
- R. Weissleder, M. Nahrendorf and M. J. Pittet, Nat. Mater., 2014, 13, 125–138 CrossRef CAS PubMed.
- P. J. Murray and T. A. Wynn, Nat. Rev. Immunol., 2011, 11, 723–737 CrossRef CAS PubMed , and references therein.
- M. Vendrell, J. S. Lee and Y. T. Chang, Curr. Opin. Chem. Biol., 2010, 14, 383–389 CrossRef CAS PubMed.
- V. S. Lin, B. C. Dickinson and C. J. Chang, Methods Enzymol., 2013, 526, 19–43 CAS , and references therein.
- B. C. Dickinson, C. Huynh and C. J. Chang, J. Am. Chem. Soc., 2010, 132, 5906–5915 CrossRef CAS PubMed.
- K. Xu, B. Tang, H. Huang, G. Yang, Z. Chen, P. Li and L. An, Chem. Commun., 2005, 5974–5976 RSC.
- M. Abo, Y. Urano, K. Hanaoka, T. Terai, T. Komatsu and T. Nagano, J. Am. Chem. Soc., 2011, 133, 10629–10637 CrossRef CAS PubMed.
- M. Abo, R. Minakami, K. Miyano, M. Kamiya, T. Nagano, Y. Urano and H. Sumimoto, Anal. Chem., 2014, 86, 5983–5990 CrossRef CAS PubMed.
- D. Srikun, E. W. Miller, D. W. Domaille and C. J. Chang, J. Am. Chem. Soc., 2008, 130, 4596–4597 CrossRef CAS PubMed.
- L. Yuan, W. Lin, S. Zhao, W. Gao, B. Chen, L. He and S. Zhu, J. Am. Chem. Soc., 2012, 134, 13510–13523 CrossRef CAS PubMed.
- J. Kim, J. Park, H. Lee, Y. Choi and Y. Kim, Chem. Commun., 2014, 50, 9353–9356 RSC.
- T. Peng, N. K. Wong, X. Chen, Y. K. Chan, D. H. Ho, Z. Sun, J. J. Hu, J. Shen, H. El-Nezami and D. Yang, J. Am. Chem. Soc., 2014, 136, 11728–11734 CrossRef CAS PubMed.
- F. Yu, P. Li, G. Li, G. Zhao, T. Chu and K. Han, J. Am. Chem. Soc., 2011, 133, 11030–11033 CrossRef CAS PubMed.
- T. Nagano T, J. Clin. Biochem. Nutr., 2009, 45, 111–124 CrossRef PubMed , and references therein.
- L. Yuan, W. Lin, Y. Xie, B. Chen and J. Song, Chem. Commun., 2011, 47, 9372–9374 RSC.
- A. Beltran, M. I. Burguete, D. R. Abanades, D. Perez-Sala, S. V. Luis and F. Galindo, Chem. Commun., 2014, 50, 3579–3581 RSC.
- M. D. Pluth, M. R. Chan, L. E. McQuade and S. J. Lippard, Inorg. Chem., 2011, 50, 9385–9392 CrossRef CAS PubMed.
- J. Shepherd, S. A. Hilderbrand, P. Waterman, J. W. Heinecke, R. Weissleder and P. Libby, Chem. Biol., 2007, 14, 1221–1231 CrossRef CAS PubMed.
- L. Yuan, W. Lin, Y. Yang and H. Chen, J. Am. Chem. Soc., 2012, 134, 1200–1211 CrossRef CAS PubMed.
- J. J. Hu, N. K. Wong, Q. Gu, X. Bai, S. Ye and D. Yang, Org. Lett., 2014, 16, 3544–3547 CrossRef CAS PubMed.
- L. Yuan, L. Wang, B. K. Agrawalla, S. J. Park, H. Zhu, B. Sivaraman, J. Peng, Q. H. Xu and Y. T. Chang, J. Am. Chem. Soc., 2015, 137, 5930–5938 CrossRef CAS PubMed.
- H. Maeda, K. Yamamoto, Y. Nomura, I. Kohno, L. Hafsi, N. Ueda, S. Yoshida, M. Fukuda, Y. Fukuyasu, Y. Yamauchi and N. Itoh, J. Am. Chem. Soc., 2005, 127, 68–69 CrossRef CAS PubMed.
- K. Xu, X. Liu, B. Tang, G. Yang, Y. Yang and L. An, Chem. – Eur. J., 2007, 13, 1411–1416 CrossRef CAS PubMed.
- P. Li, T. Xie, X. Duan, F. Yu, X. Wang and B. Tang, Chem. – Eur. J., 2010, 16, 1834–1840 CrossRef CAS PubMed.
- X. Li, S. Zhang, J. Cao, N. Xie, T. Liu, B. Yang, Q. He and Y. Hu, Chem. Commun., 2013, 49, 8656–8658 RSC.
- L. Yuan and Q. P. Zuo, Chem. – Asian J., 2014, 9, 1544–1549 CrossRef CAS PubMed.
- A. Vazquez-Romero, N. Kielland, M. J. Arevalo, S. Preciado, R. J. Mellanby, Y. Feng, R. Lavilla and M. Vendrell, J. Am. Chem. Soc., 2013, 135, 16018–16021 CrossRef CAS PubMed.
- N. Kielland, M. Vendrell, R. Lavilla and Y. T. Chang, Chem. Commun., 2012, 48, 7401–7403 RSC.
- J. O. Deguchi, M. Aikawa, C. H. Tung, E. Aikawa, D. E. Kim, V. Ntziachristos, R. Weissleder and P. Libby, Circulation, 2006, 114, 55–62 CrossRef PubMed.
- M. Lohela, A. J. Casbon, A. Olow, L. Bonham, D. Branstetter, N. Weng, J. Smith and Z. Werb, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 5086–5095 CrossRef PubMed.
- T. Jiang, E. S. Olson, Q. T. Nguyen, M. Roy, P. A. Jennings and R. Y. Tsien, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 17867–17872 CrossRef CAS PubMed.
- H. Suzuki, M. Sato and Y. Umezawa, ACS Chem. Biol., 2008, 3, 471–479 CrossRef CAS PubMed.
- A. Cobos-Correa, J. B. Trojanek, S. Diemer, M. A. Mall and C. Schultz, Nat. Chem. Biol., 2009, 5, 628–630 CrossRef CAS PubMed.
- F. A. Jaffer, D. E. Kim, L. Quinti, C. H. Tung, E. Aikawa, A. N. Pande, R. H. Kohler, G. P. Shi, P. Libby and R. Weissleder, Circulation, 2007, 115, 2292–2298 CrossRef CAS PubMed.
- G. Blum, G. von Degenfeld, M. J. Merchant, H. M. Blau and M. Bogyo, Nat. Chem. Biol., 2007, 3, 668–677 CrossRef CAS PubMed.
- F. Cattaruzza, V. Lyo, E. Jones, D. Pham, J. Hawkins, K. Kirkwood, E. Valdez-Morales, C. Ibeakanma, S. J. Vanner, M. Bogyo and N. W. Bunnett, Gastroenterology, 2011, 141, 1864–1874 CrossRef CAS PubMed.
- M. Verdoes, L. E. Edgington, F. A. Scheeren, M. Leyva, G. Blum, K. Weiskopf, M. H. Bachmann, J. A. Ellman and M. Bogyo, Chem. Biol., 2012, 19, 619–628 CrossRef CAS PubMed.
- F. Fan, S. Nie, E. B. Dammer, D. M. Duong, D. Pan, L. Ping, L. Zhai, J. Wu, X. Hong, L. Qin, P. Xu and Y. H. Zhang, J. Proteome Res., 2012, 11, 5763–5772 CrossRef CAS PubMed.
- M. Verdoes, K. O. Bender, E. Segal, W. A. van der Linden, S. Syed, N. P. Withana, L. E. Sanman and M. Bogyo, J. Am. Chem. Soc., 2013, 135, 14726–14730 CrossRef CAS PubMed.
- K. O. Bender, L. Ofori, W. A. van der Linden, E. D. Mock, G. K. Datta, S. Chowdhury, H. Li, E. Segal, M. Sanchez-Lopez, J. A. Ellman, C. G. Figdor, M. Bogyo and M. Verdoes, J. Am. Chem. Soc., 2015, 137, 4771–4777 CrossRef PubMed.
- E. Segal, T. R. Prestwood, W. A. van der Linden, Y. Carmi, N. Bhattacharya, N. Withana, M. Verdoes, A. Habtezion, E. G. Engleman and M. Bogyo, Chem. Biol., 2015, 22, 148–158 CrossRef CAS PubMed.
- Y. Ben-Nun, E. Merquiol, A. Brandis, B. Turk, A. Scherz and G. Blum, Theranostics, 2015, 5, 847–862 CrossRef CAS PubMed.
- C. Leong, S. C. Lee, J. Ock, X. Li, P. See, S. J. Park, F. Ginhoux, S. W. Yun and Y. T. Chang, Chem. Commun., 2014, 50, 1089–1091 RSC.
- N. Y. Kang, S. J. Park, X. W. Ang, A. Samanta, W. H. Driessen, V. Ntziachristos, K. O. Vasquez, J. D. Peterson, S. W. Yun and Y. T. Chang, Chem. Commun., 2014, 50, 6589–6591 RSC.
- J. S. Yoo, R. K. Das, Z. Y. Jow and Y. T. Chang, PLoS One, 2014, 9, e103721 Search PubMed.
- J. S. Yoo, S. C. Lee, Z. Y. Jow, P. Y. Koh and Y. T. Chang, Cancer Res., 2014, 74, 44–55 CrossRef CAS PubMed.
- F. Chagnon, A. Bourgouin, R. Lebel, M. A. Bonin, E. Marsault, M. Lepage and O. Lesur, Am. J. Physiol.: Lung Cell. Mol. Physiol., 2015, 309, 543–551 CrossRef PubMed.
- L. O. Ofori, N. P. Withana, T. R. Prestwood, M. Verdoes, J. J. Brady, M. M. Winslow, J. Sorger and M. Bogyo, ACS Chem. Biol., 2015, 10, 1977–1988 CrossRef CAS PubMed.
- H. C. Bygd, K. D. Forsmark and K. M. Bratlie, Biomaterials, 2015, 56, 187–197 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cs00567a |
| This journal is © The Royal Society of Chemistry 2016 |