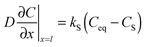Open Access Article
Open Access ArticleUntangling surface oxygen exchange effects in YBa2Cu3O6+x thin films by electrical conductivity relaxation†
P.
Cayado
 a,
C. F.
Sánchez-Valdés
a,
A.
Stangl
a,
M.
Coll
a,
C. F.
Sánchez-Valdés
a,
A.
Stangl
a,
M.
Coll
 a,
P.
Roura
b,
A.
Palau
a,
T.
Puig
a and
X.
Obradors
*a
a,
P.
Roura
b,
A.
Palau
a,
T.
Puig
a and
X.
Obradors
*a
aInstitut de Ciència de Materials de Barcelona (CSIC), Campus UAB, 08193, Bellaterra, Catalonia, Spain. E-mail: Xavier.obradors@icmab.es
bUniversity of Girona, Montilivi Campus, Edif. PII, E17o71, Girona, Catalonia, Spain
First published on 28th April 2017
Abstract
The kinetics of oxygen incorporation (in-diffusion process) and excorporation (out-diffusion process), in YBa2Cu3O6+x (YBCO) epitaxial thin films prepared using the chemical solution deposition (CSD) methodology by the trifluoroacetate route, was investigated by electrical conductivity relaxation measurements. We show that the oxygenation kinetics of YBCO films is limited by the surface exchange process of oxygen molecules prior to bulk diffusion into the films. The analysis of the temperature and oxygen partial pressure influence on the oxygenation kinetics has drawn a consistent picture of the oxygen surface exchange process enabling us to define the most likely rate determining step. We have also established a strategy to accelerate the oxygenation kinetics at low temperatures based on the catalytic influence of Ag coatings thus allowing us to decrease the oxygenation temperature in the YBCO thin films.
Introduction
The use of low-cost techniques such as chemical solution deposition (CSD) for the growth of high performance REBa2Cu3O6+x (REBCO) coated conductors (CCs) is one of the major requirements for a widespread use of superconductivity in large scale applications.1–6 CSD has become a very competitive, cost-effective and scalable methodology to produce REBCO, especially YBCO, epitaxial thin films, and so an extensive analysis of all the relevant processing steps is worthwhile.The last step in any CC manufacturing process is oxygenation. Achieving an optimal oxygen content in YBCO films is actually a critical issue in the development of high critical current superconductors. This process depends on the kinetics of the surface oxygen exchange and bulk oxygen diffusion processes. Therefore, an improved understanding of oxygen incorporation (in-diffusion process) and excorporation (out-diffusion process) in YBCO films is needed to design an optimal oxygenation.
The oxygen exchange processes have been investigated extensively in the past using different techniques such as secondary ion mass spectrometry,7–10 thermogravimetry,11,12 spectroscopic ellipsometry13 or electrical conductivity relaxation.14–20 However, in most of the cases the kinetics of oxygen exchange have been analyzed in terms of a volume diffusion process governed by Fick's laws21,22 and the surface reactions that the oxygen molecules experience on the YBCO films have been scarcely considered.18
However, in recent years, the mechanisms of oxygen exchange and bulk diffusion in functional oxides have become a topic generating high interest. The relevance of surface reactions, which may become the slowest step in the whole chain of oxygen exchange, has been stressed. This is particularly important in thin films, because the short diffusion distance makes bulk diffusion very fast leading to homogeneously oxygenated films. The research about this issue in the field of solid oxide fuel cells (SOFCs) where mixed ionic and electronic conductors (MIECs) are used is especially relevant.23–25 In these materials, the oxygen molecules have to complete four different steps before diffusing through the volume: (i) adsorption/ionization, (ii) surface diffusion to find a vacancy, (iii) dissociation and (iv) migration of the second ion to find another vacancy.26–28
Among these steps, the slowest one limits the overall rate of the surface reaction and, for this reason, it is known as the rate determining step (rds). The rds can vary depending on the particular case that is being considered. Similar proposals have been made for many MIECs, such as SrTiO3 (STO), Fe-doped STO or Ba1−xSrxCoyFe1−yO3−z.29–33 Also, extensive analyses of the oxygen surface reactions have been carried out in CeO2−z (ceria) and gadolinium doped ceria (GDC) as recognized oxygen-deficient compounds which may serve as model systems. The complexity of the surface oxygen-ion transfer was demonstrated on these systems and it was shown that surface phenomena can limit in some cases the rate of the overall oxygen exchange processes.34–40 However, the possibility that the surface reactions limited the oxygen exchange processes in YBCO films or single crystals was mostly discarded, and only recently the role of surface reactions was pointed out.10,13,18,41 Therefore, further analysis of the oxygen exchange mechanisms in YBCO films is needed to determine when this process is surface limited and under which conditions the volume bulk diffusion in films is indeed fast enough.
For this reason, in this work, we analyze, by electrical conductance relaxation measurements, the effect that several parameters, such as temperature (T) and oxygen partial pressure (PO2), have on the oxygenation kinetics of CSD YBCO films. After concluding that surface exchange effects play the key role in oxygenating our YBCO films, we also investigate the influence of including Ag as a catalyst to enhance the low-temperature oxygenation rate. The results are the base for designing optimal oxygenation processes in minimal time for YBCO films having different oxygen contents.
Theoretical aspects
The problem of oxygen diffusion in YBCO thin films is addressed, in a simple way, as a one-dimensional diffusion process in a homogeneous medium bound by two parallel infinite planes, e.g. the planes at x = 0 and x = l (actually, we only have one limiting surface because the other one is in contact with the substrate). These conditions apply in practice to diffusion into a plane sheet of material so thin that effectively all the diffusing substance enters through the plane faces and a negligible amount through the edges. In this case, the problem is reduced to solve Fick's second law in one dimension (eqn (1)):21,22,42 | (1) |
and a linear reaction rate at the free surface,
where D is the mean value of the bulk diffusion coefficient in the material, kS is the surface reaction rate constant, l is the film thickness and C, CS and Ceq are the oxygen concentration at any point, at the surface and that in equilibrium with the surrounding gas, respectively.
For an initial homogeneous oxygen concentration, C0, this problem has an exact analytical solution.42 This solution integrated over the film thickness delivers the time-dependence of the average oxygen concentration, ![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) (eqn (2)):
(eqn (2)):
 | (2) |
The values βn are the n'th positive roots of (eqn (3)):
βn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tan tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βn = δ βn = δ | (3) |
![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) with time can be described by a single time constant (eqn (4)):
with time can be described by a single time constant (eqn (4)): | (4) |
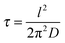 or τ = l/kS is the relaxation time if the process is volume-diffusion or surface exchange-controlled, respectively.
or τ = l/kS is the relaxation time if the process is volume-diffusion or surface exchange-controlled, respectively.
In the case when the process is volume-diffusion controlled, the exponential function should be multiplied by a factor very close to one (8/π2).
Finally, since the oxygen content controls the electronic carrier concentration in YBCO films, its evolution will result in a parallel evolution of the film conductance, G = 1/R. We will assume that ![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) and G are proportional (i.e., the mobility of the carriers is assumed to be constant during the course of relaxation) and, consequently, the electrical conductance transients will be fitted to the time dependence of eqn (5), where the concentrations must be substituted by the conductances (eqn (5)):
and G are proportional (i.e., the mobility of the carriers is assumed to be constant during the course of relaxation) and, consequently, the electrical conductance transients will be fitted to the time dependence of eqn (5), where the concentrations must be substituted by the conductances (eqn (5)):
 | (5) |
Results
Structural and superconducting characterization of the films
All the 250 nm CSD YBCO films employed in this study present excellent biaxial texture as observed in the 2D XRD θ–2θ frame (Fig. 1a) and confirmed by ϕ-scan and ω-scan measurements (typically Δω ∼ 0.6°). The YBCO grains exhibit a perfect epitaxial (00l) orientation (Fig. 1b). The presence of other grain orientations or secondary phases is not detected. X-ray diffraction was used to determine the film lattice parameters which are strongly sensitive to the YBCO oxygen content (see Fig. S2, ESI†). | ||
| Fig. 1 2D XRD θ–2θ (a) frame and (b) integrated patterns of a 250 nm CSD YBCO film studied in this work. The film displays a perfect 2D texture. | ||
On the other hand, the SEM image shown in Fig. 2 demonstrates that the obtained films have a dense and homogenous surface without the presence of precipitates or grains with the {100} planes oriented perpendicular to the substrate (a–b grains). The presence of pores (marked with white arrows) is also noticeable. These pores have actually a great importance in accelerating the oxygen diffusion processes, as will be discussed later, even if their density is not high enough to influence adversely the superconducting properties such as the critical current density.43–46 Actually, previous investigations of the origin of residual pores in YBCO CSD films showed that pores are formed at the stage of metallorganic pyrolysis and they are healed during the growth stage, therefore it is very important to perform a fine tuning of the processing conditions to reach this stage.47 The TEM images of the cross section of the CSD films after optimized growth confirm their high quality epitaxy and the limited density of pores (see Fig. S3, ESI†). The critical temperature of the superconducting films corresponds to Tonsetc and it was measured by inductive measurements using a superconducting quantum interference device (SQUID) magnetometer at a low magnetic field (H ∼ 1–5 Oe). This SQUID magnetometer was also used to determine the critical current density Jc of the YBCO films using the Bean critical state model of thin discs.48,49
 | ||
| Fig. 2 SEM image of a typical 250 nm CSD YBCO film showing a dense and homogeneous surface with the presence of some pores (white arrows). | ||
Temperature dependence of oxygenation kinetics
The electrical conductivity relaxation after consecutive steps of PO2, either going towards a lower value (PL = 2 × 10−4 atm) or towards a higher value (PH = 1 atm), can be observed in Fig. 3a. The consecutive conductance steps are measured at different temperatures. | ||
| Fig. 3 (a) Evolution of the conductance with time at different temperatures with alternating changes of PO2 (A: PH = 1 atm and B: PL = 2 × 10−4 atm). (b) Example of a non-saturated conductance relaxation measurement made at very long times (15 h) for an out-diffusion process at 400 °C. (c) Typical fitting (red curve) of an experimental conductivity relaxation measurement (black curve) using expression (5) to determine the τ values (in-diffusion process at 450 °C). | ||
When PO2 decreases, oxygen ions leave the YBCO structure (we will refer to this process as “out-diffusion”), thus decreasing the carrier concentration and so causing a decrease of the film conductance (Fig. 3b). This process leads to a final stoichiometry close to YBa2Cu3O6 and, therefore, a change from the orthorhombic phase to the tetragonal phase yielding a non-superconducting film. The oxygen level can be estimated from the T–PO2 phase diagram.50–52 On the other hand, when PO2 increases, oxygen ions enter into the YBCO structure (process referred as “in-diffusion”) and an increase of the conductance is observed (Fig. 3c). During the in-diffusion process, we return to the orthorhombic phase and the films become superconducting again. The different values of oxygen stoichiometry can be again estimated from the T and PO2 and this will determine the Tc of the films.53 In the ESI† (Fig. S2), we report typical examples of how the different oxygen contents of YBCO films influence the Tc, critical current density Jc(T) and c-axis parameter of the thin films. Notice that the values of saturation conductance at PL and at PH increase at lower temperatures. This evolution is mainly due to the increase of the equilibrium oxygen content at low temperatures and higher PO2 values.50,54 The saturation conductance is shown to decrease at high temperatures, independent of the metallic to semiconducting transition of the YBCO films at an oxygen content of δ ∼ 0.6. This would indicate that the influence of the carrier concentration change on conductance overcomes the intrinsic temperature dependence of the electric conductivity of the YBCO phase in the semiconductor or metallic regimes. Fig. 3a and b also show that at low temperatures (below 550 °C for out-diffusion and below 400 °C for in-diffusion), the saturation of the conductance is not reached. This is because, at such low temperatures, the oxygen exchange process is so slow that the equilibrium is not reached at lab scale times (maximum 2 h in the present experiments). This conclusion is also supported by the low Tc and Jc values measured on such partially oxygenated YBCO films (see Fig. S2, ESI†). Fig. 3b shows one example of the non-saturated out-diffusion conductance relaxation process measured at 400 °C, even if much longer annealing times are used (>15 h).
The conductance transients corresponding to oxygen out- and in-diffusion were fitted using eqn (6) to obtain the τ values (Fig. 3c). It is observed that good fits are achieved which correspond either to a process limited by surface reactions or by bulk diffusion, as discussed in Section 3. It has to be considered that only the transients that reach an equilibrium process can be properly fitted. The curves that are not saturated after the corresponding oxygen exchange process are not valid for the fitting and, therefore, all the graphs in this work only contain τ values obtained from equilibrium states. Fig. 4 displays the temperature dependence of τ values for the out- and in-diffusion processes. The τ values are longer for the out-diffusion process (∼7 min at 500 °C to ∼0.4 min at 800 °C) than for in-diffusion (∼4 min at 400 °C to ∼0.5 min at 500 °C). In the case of in-diffusion, above 500 °C, the process is so fast that it is not possible to obtain an accurate value of τ.
 | ||
| Fig. 4 Evolution of τ values with temperature and kSvs. 1000/T curves for the out-diffusion (black) and in-diffusion (red) processes of a 250 nm pristine YBCO film following the PO2 changes shown in Fig. 3. | ||
On the right Y axis of Fig. 4, the values of the surface reaction rates ks obtained by application of eqn (5) are also indicated. The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ksvs. 1/T series of points have been fitted to a linear dependence to extract the activation energy, Ea and k0 (ks = k0
ksvs. 1/T series of points have been fitted to a linear dependence to extract the activation energy, Ea and k0 (ks = k0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) expEa/kT). For out-diffusion, it is found that Ea = 0.25 ± 0.05 eV in the 550–800 °C range, whereas a much higher value of 0.8 ± 0.2 eV is obtained for in-diffusion Ea in the 400–500 °C range.
expEa/kT). For out-diffusion, it is found that Ea = 0.25 ± 0.05 eV in the 550–800 °C range, whereas a much higher value of 0.8 ± 0.2 eV is obtained for in-diffusion Ea in the 400–500 °C range.
The in-diffusion Ea value is similar to those previously reported for sputtered YBCO thin films and bulk and liquid phase epitaxy (LPE) samples,16,20,55,56 while the Ea value of the out-diffusion process is considerably smaller (no similar analyses have been previously reported). The k0 values determined from the intercepts of the straight lines in Fig. 4 also strongly differ between out- and in-diffusion processes: k0 = 1.5 × 10−6 ± 8 × 10−7 cm s−1 and 0.29 ± 0.09 cm s−1 for the out- and in-diffusion processes, respectively.
At this stage, we wonder if the asymmetric behavior between out- and in-diffusion processes is real or if it arises from an experimental artifact associated with the conductivity relaxation experiments which might be influenced by different electrical current percolation paths between both experiments.56,57 To ensure that this is not the case, we performed thermogravimetric analysis (TGA) experiments on YBCO powders with small particle size (10 μm of diameter) to ascertain if asymmetry is also observed in this case. We indeed found that under similar conditions of temperature and PH and PL values, similar τ values are obtained and the asymmetry is maintained (see Fig. 5). Hence, we confirm that the asymmetric behavior of oxygen exchange is an intrinsic characteristic of YBCO under the experimental conditions used in our experiments.
 | ||
| Fig. 5 TGA measurements of the mass change due to the oxygen out-diffusion (solid) and in-diffusion (dashed) processes in a YBCO polycrystalline powder sample. | ||
Oxygen pressure dependence of the conductivity relaxation
The oxygen partial pressure PO2 is a key parameter to analyze the mechanisms limiting the kinetics of oxygen exchange processes on any oxide. Hence, we have carried out a series of conductance relaxation measurements where PL was changed within the range PL = 5 × 10−5 to PH = 1 atm, while PH was kept constant to 1 atm. A first set of experiments was performed to analyze the temperature dependence of τ values at different PO2 values for the out-diffusion process. The experimental results show that PO2 has a strong influence on the corresponding τ values (Fig. 6).At higher PL, τ values are smaller at all temperatures (values shown in Fig. 6). Consequently, kS increases with PL at all temperatures and it follows a thermally activated behavior whose activation energy depends on PO2. Ea has been found to decrease with PL (Fig. 6).
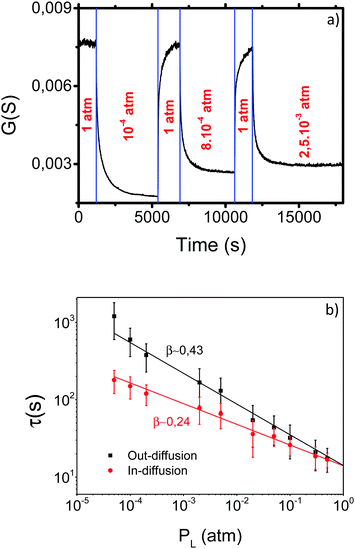
More insight into the influence of PO2 on the oxygenation kinetics was gained from a second series of experiments where the dependence of τ on PL was determined by measuring the conductance relaxation at 500 °C for both in-diffusion and out-diffusion processes. The procedure is illustrated in Fig. 7a where the conductance transients are observed when PO2 is switched from PL to PH and vice versaFig. 7b displays the corresponding τ values as a function of PL. The figure shows that τ follows a power law, i.e., τ ∝ PO2β with β values differing between in- and out-diffusion processes. Consequently, the corresponding τ values are progressively different when PL moves away from PH.
Actually, the reaction rates of surface reactions usually follow a power law dependence PO2β, where β depends on each particular reaction. Hence, the conductance relaxation times should also follow such a dependence (τ ∝ PO2β) if oxygen exchange is dominated by surface reactions.30,31,58 The power law dependence of the reaction rates has a thermodynamic origin derived from the equilibrium constant of the chemical reaction and also from the influence of oxygen adsorbate coverage on PO2.56,58 The analysis of the τ power dependence on PO2 can therefore provide more information on the rds of the surface reactions. It can also further clarify the origin of some controversial observations concerning the possible existence of an asymmetric behavior between in and out-diffusion oxygenation kinetics in YBCO.59–62 The results reported in Fig. 7b draw a plausible scenario for this controversy: in any experimental analysis of the asymmetry issue it is required that PL and PH differ by a significant amount to detect the corresponding effects.
At this stage, we should stress that bulk diffusion might also introduce some dependence of oxygenation kinetics on PO2 because the equilibrium oxygen content δ of YBCO depends on PO2,50 and this brings some dependence on PO2 on the chemical diffusion constant D, an usual feature in most MIEC oxides.63–66 However, in YBCO the dependence of D on δ seems to be rather weak, even if a thorough investigation of this effect in single crystals or epitaxial films would be worthwhile to handle properly the YBCO diffusion constant anisotropy effects.9,12,16,56,57,67–72
The results shown in Fig. 3–7 provide, therefore, an adequate framework to disentangle the corundum of the surface oxygen exchange mechanisms in CSD YBCO films. For instance, the strong divergence between in-diffusion and out-diffusion kinetics (i.e., modified τ values and a strong decrease of Ea for the out-diffusion process) can only be attributed to the asymmetry of the surface reactions.
An additional parameter which may influence the observed oxygenation kinetic asymmetries is the temperature, so we investigated the τ dependence on PL at different temperatures. Fig. 8 shows that the ratio of τ values for in- and out-diffusion follows a PO2 power law dependence with β independent of temperature within the investigated temperature range.
 | ||
| Fig. 8 Plot of the ratio of τ values of in- and out-diffusion at different temperatures vs. PL, when PH is fixed at 1 atm, as shown in Fig. 6. | ||
This behavior provides more understanding of the surface reaction processes limiting the oxygen exchange. Since, as commented above, one does not expect an asymmetry in the kinetics of in- and out-diffusion for a process controlled by bulk diffusion, the continuous lines of Fig. 8 are extrapolated to the different PL values where τin/τout ∼ 1, i.e., when the experimental behavior is indistinguishable from that of bulk diffusion. Under these conditions, the difference in PO2 between both processes become very small and the equilibrium states are reached very fast. Our analysis clarifies, therefore, why previous reports in single crystals showed that the τ values of in- and out-diffusion were indistinguishable: the departures from the equilibrium were too small.16,68 The asymmetric behavior associated with oxygen surface reactions, therefore, can only be detected when large enough PO2 steps are used.
Finally, we can conclude this section by analyzing in more detail the rds associated with surface reactions from the PO2 dependence of τ values.
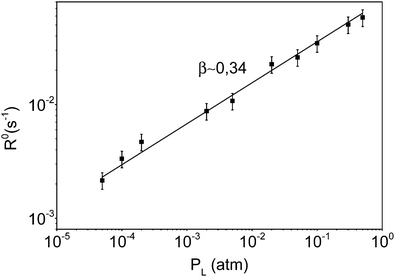
This analysis is mainly grounded on the determination of β exponents which always depend on the particular rds to be considered. In the case of STO films, Merkle and Maier described in detail the values of the exponent corresponding to each particular surface reaction.30 For the case of YBCO films, on the other hand, Chen et al. proposed that the β parameter associated with the surface reactions should be 1/2.18
In the most complete analysis of these issues, reported by Merkle and Maier for STO films,30 a useful approach was to determine the equilibrium reaction rate (R0) from the τ values of the in- and out-diffusion processes. The following equation was proposed (eqn (6)):
 | (6) |
The use of silver to accelerate the kinetics of the YBCO oxygen exchange
The analysis of the oxygenation kinetics in CSD YBCO films presented up to now has demonstrated that the surface reactions limit the rate of oxygen exchange. It was also found that at low temperatures (below 550 °C and 400 °C for out- and in-diffusion, respectively) the processes are too slow to reach the equilibrium state within the experimental time window. Consequently, it is not possible to carry out oxygenation processes below 400 °C in reasonable times because the oxygen incorporation will be incomplete (Fig. 3b). However, oxygenation at low temperatures would be interesting because one can further modify the oxygen content of the YBCO films.73–76 To overcome the low temperature limitation in the oxygenation of functional oxides, it has been shown that silver can be used as a catalyst to enhance the rate of oxygen incorporation. The use of silver as a catalyst for selective oxidation processes is well-known in different fields, especially in the hydrocarbon industry.77,78 Additionally, it has excellent oxygen solubility and permeability, two critical properties required to achieve a fast oxygen exchange.79 The role of silver is to facilitate the O2 molecule dissociation and reduction. When O2 molecules are adsorbed on a silver surface, there is an electron transfer from silver to the π* orbitals of the O2 molecules. This causes a decrease in the binding energy of the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond facilitating its rupture.80 This strategy was successfully tested in STO films.29 A layer of silver (100 nm) was deposited on top of STO by sputtering. After a 7-day annealing process at 1023 K, the silver layer transforms into micro/nanoparticles on the STO surface. The STO films with these silver nanoparticles on the top showed lower Ea and also much higher k0 values than the pristine samples.29
O bond facilitating its rupture.80 This strategy was successfully tested in STO films.29 A layer of silver (100 nm) was deposited on top of STO by sputtering. After a 7-day annealing process at 1023 K, the silver layer transforms into micro/nanoparticles on the STO surface. The STO films with these silver nanoparticles on the top showed lower Ea and also much higher k0 values than the pristine samples.29
In our case, we have introduced silver either directly in the YBCO solution or as evaporated metallic layers, on the YBCO surface. In the first case, a YBCO + 5% M Ag solution is prepared by adding Ag–TFA salt to the YBCO solution and the films were synthesized using similar growth processes as pristine YBCO films. The structure and superconducting properties are similar to those of the previously investigated films.81 Silver shows a tendency to diffuse towards the film surface before its sublimation and, at 810 °C, some traces of silver remain on the surface (at 720 °C, some silver NPs are still detected at the surface after growth).81
In the case of Ag coating of the YBCO films, its typical initial thickness is ∼100 nm, however, after thermal annealing at 300 °C for about 5 h, the typical surface microstructure corresponds to that of nanometric or micrometric Ag particles (Fig. S2 in ESI†).
Conductivity relaxation experiments, both for the out- and in-diffusion processes, have been performed on these films at temperatures below 350 °C where the pristine YBCO films displayed very long oxygenation times. A comparison of the typical conductance relaxation of pristine and YBCO–Ag coated films at 350 °C and 300 °C is shown in Fig. 10a and b. It can be observed that the equilibrium oxygenation in YBCO–Ag coated films is reached within reasonable oxygenation times, while in pristine YBCO films saturation is not reached, even after times as long as 12–15 h (Fig. 3b).
These results demonstrate that the oxygenation kinetics is much faster when Ag is deposited on the film surface.
It is clear that oxygen in-diffusion is favored at low temperatures by silver. When silver is deposited on the surface of YBCO, the number of O2 molecules that are adsorbed and dissociated is larger due to the reduction of the bond energy and, probably, also due to an enhanced sticking probability of the molecules. It has been suggested that oxygen atoms diffuse into bulk silver and reach the film surface. This oxygen path was proposed for a silver membrane kept at temperatures above 310 °C. Below this temperature, the atomic oxygen is found to be locked at the silver surface without diffusion into the silver layer.82–84 The overall result of this phenomenon is the acceleration of the surface reactions. Probably silver is able to accelerate the rds, which has been suggested to be the dissociation of O22− molecules (or the formation of O22−). A thorough analysis of the temperature and PO2 dependence of these films will be required to properly analyze the microscopic mechanisms responsible for such a strong modification of the oxygen exchange processes.
Discussion
The results presented in the previous sections have provided compelling evidence of the oxygen surface exchange reactions as the limiting step in the oxygenation processes of CSD YBCO films. In other words, the surface reactions are slower than the bulk diffusion process in these thin films. It is a useful exercise, however, to compare the surface reaction rates to the expected bulk diffusion rates in YBCO crystals. For this purpose, we need to take into account that along the a–b planes the bulk oxygen diffusion coefficient is much larger than the diffusion constant along the c-axis (Dab ≈ 106Dc).9,10 Since volume diffusion is faster than the surface oxygen surface exchange, we can estimate from our data an upper bound of the oxygen diffusion length parallel to the ab planes, based on previously determined chemical diffusion constants.A comparative analysis of oxygenation rates in bulk melt textured ceramics and thin films has been performed previously, where it is assumed that bulk oxygen diffusion is the limiting step. The upper bound of the grain size (or bulk diffusion length) can be determined using the following formula (eqn (7)):16
 | (7) |
Fig. 11 compares typical Ln(1/τ) vs. 1000/T curves for the oxygen in-diffusion process of the above mentioned YBCO samples. It is observed that the CSD film curve is shown in the top left part, thus indicating that the τ values are the smallest in this range of temperatures. The Ea values for the in-diffusion process of the CSD films are similar to those of LPE, sputtering or bulk ceramics. In the case of CSD YBCO films, the values vary in the range 0.8–1 eV, which are similar to the previously reported values (1.1–1.25 eV) in other types of samples. For the out-diffusion process, however, as we mentioned before, the Ea values are reduced to Ea = 0.2–0.3 eV, thus pointing towards a modified rds mechanism,23,85 or an asymmetry of the surface reactions during oxygen exchange.56
 | ||
| Fig. 11 In-diffusion Ln(1/τ) vs. 1000/T curves for the YBCO LPE films,55 sputtering films,16,68 MTG bulk samples20 and CSD films. | ||
With regard to the observed τ0 values in Fig. 11, the obtained results for each technique are quite different. The τ0 values for CSD and sputtering films are in the range of 10−5–10−7 s, while in the case of the LPE and MTG samples the τ0 values are in the range of 10−2–10−3 s, a reflection of the larger L values in these materials. We suggest therefore that in YBCO films with a thickness in the range of hundreds of nanometers and with small grain sizes (below ∼1 μm), the surface reactions are the rate limiting factor for oxygen exchange. When the sample thickness increases above several micrometers and the grain sizes also increases, as in the case of the LPE samples (10 μm thickness with large grains of 101–102 μm), the rate limiting factor is bulk diffusion.
The microscopic mechanisms underlying the obtained Ea values arise actually from different sources, depending on whether bulk diffusion or surface exchange is the rds. In the case of bulk controlled mechanisms, the Ea correspond to the energy barrier that the oxygen ions have to overcome to diffuse inside the YBCO structure. On the other hand, the Ea values in a surface controlled mechanism arise from one of the energy barriers that the oxygen molecules have to overcome: chemisorption, diffusion or dissociation.
After reaching the conclusion that oxygen diffuses along lengths shorter than 0.3–0.7 μm, we may wonder if these are reasonable values taking into account the microstructure of the CSD films. Actually, the scenario suggested for the present CSD YBCO films is quite similar to that previously suggested by other authors for epitaxial functional oxide films.18,19,86 These works proposed that the oxygen exchange in epitaxial films takes place through two different exchange channels: the native surface and dislocations usually present in the films. The claim is that the oxygen exchange rate through these two channels strongly differs. Oxygen diffusion through dislocations is much faster than through the native surface because in defective structures the oxygen mobility is higher87,88 and the number of oxygen vacancies is larger than in the bulk,87,89 thus enhancing the bulk diffusion.
In the case of the CSD YBCO films, we propose a slightly modified scenario. First, the surface reactions are completed at the native surface. In the case of YBCO films grown by CSD, no threading dislocations exist even at very low thicknesses,90 so it is considered that oxygen ion exchange towards the bulk of the film happens mainly through pores or low angle grain boundaries. Actually, as observed in Fig. 3, the density of pores in the 250 nm thick CSD YBCO films is high enough to facilitate oxygen diffusion through the small angle grain boundaries existing in CSD YBCO films. These low angle grain boundaries have been shown to play some role in the superconducting transport properties and so they are probably also active as fast oxygen diffusion paths.56,60,91 Due to the fact that bulk oxygen diffusion is faster than oxygen surface exchange rates, we should not expect that electronic percolation effects influence the measured bulk conductance. We propose, therefore, that oxygen ions penetrate into the films along the vertical direction through these pores, where the surface reactions may also occur, as well as along the low angle grain boundaries remaining in the CSD epitaxial films.62,67 These oxygen exchange paths should be much faster than bulk diffusion along the c-axis within the bulk of the film due to the low values of Dc mentioned before. Actually, we estimate that oxygen ions can diffuse over a distance between 300 and 700 times longer along the a–b planes than along the c-axis. We should note here that the mean separation distance between pores is in the range of the diffusion length (or probably grain size), L, calculated using eqn (7). Therefore, oxygen ions can diffuse fast enough along the a–b planes to achieve a homogeneous distribution.
Summarizing, the in-diffusion of oxygen proceeds through the five steps indicated in Fig. 12, four of them linked to the surface reactions and the last one linked to bulk diffusion. First, the O2 molecules are chemisorbed onto the free surface. Then, the ionized molecules diffuse into existing defects and dissociate leading to O2− ions which then require further surface diffusion to find available oxygen vacancies.92 Once the oxygen molecules complete the surface reactions, if the resulting ions are near a pore, they diffuse through it in the vertical direction and then along the a–b plane (Fig. 12). Molecular oxygen may probably also diffuse within the pores where the surface reactions can be also completed. Anyway, considering vertical diffusion through the pores or grain boundaries is the only way to justify the observed small τ values.
This scenario is compatible with the reported experimental results and it also clarifies the role of the silver nanoparticles at the surface. At low temperatures, when the oxygen exchange through pores and grain boundaries is still effective, the exchange rate is too small because the rds has been slowed down. When some silver nanoparticles are deposited on the film surface they accelerate the oxygen surface reactions, and probably the oxygen ion surface chemisorption as well. The huge τ values observed in pristine films at low temperatures are then strongly reduced and the conductivity transients become measurable. Essentially, the catalytic effect of silver nanoparticles accelerates the molecular oxygen dissociation reactions on the native surface thus increasing the formation rate of O2− ions at low temperatures and hence enhancing the efficiency of the oxygen exchange channels to diffuse towards the bulk of the films.
Conclusions
This work was devoted to the study of oxygen incorporation and excorporation processes in CSD YBCO films in order to clarify the limiting factors of the oxygen exchange in these kinds of films and we have unequivocally proved that the surface reactions are the limiting factor in the kinetics of the oxygen exchange processes in CSD YBCO films. The PO2 dependence of conductance relaxation times and the asymmetric behavior of the in- and out-diffusion processes cannot be explained without consideration of oxygen exchange as a surface controlled process. The study of the PO2 influence in the relaxation times is also essential to establish the rate determining step (rds) of the surface reactions. We suggest that the chemisorption process leading to the formation of ionized O22− molecules, or the dissociation of an O22− molecule to form two O− ions at the surface, is the rds in the present case. Finally, we have also shown that silver is an effective catalytic agent to accelerate the rate of the oxygen exchange rates at lower temperatures than those we can achieve in pristine YBCO films. The reduction of relaxation times in Ag coated YBCO films was quite significant at low temperatures, thus allowing the shortening of the oxygenation times of the films at low temperatures where the pristine films cannot be fully oxygenated. It is suggested that silver reduces the strength of the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and decreases drastically the overall surface reaction times. After completing the surface reactions, the oxygen ions diffuse parallel to the ab planes into the volume of the films benefiting from the existing pores and grain boundaries.
O bond and decreases drastically the overall surface reaction times. After completing the surface reactions, the oxygen ions diffuse parallel to the ab planes into the volume of the films benefiting from the existing pores and grain boundaries.
Experimental
The films studied in this work were prepared by CSD following the trifluoroacetate (TFA) route. YBCO metallorganic precursor solutions based on anhydrous TFA salts were prepared and characterized as described in detail previously.81,93 Basically, YBCO solid ceramic powder was dissolved in trifluoroacetic acid, trifluoroacetic anhydride and acetone. Further, the mixture was purified to finally obtain a 0.25 M (with respect to the Y content) YBCO–TFA solution. Alternative YBCO + 5% mol Ag solutions were prepared by adding the corresponding stoichiometric amount of Ag–TFA precursor salts into the original 0.25 M YBCO–TFA solution. These solutions were deposited by spin-coating on 5 × 5 mm2 LaAlO3(00l) (LAO) substrates. The subsequent pyrolysis and growth processes are also described elsewhere.93–95 The phase analysis of the YBCO films was performed by X-ray diffraction using a Bruker AXS GADDS diffractometer equipped with a 2D detector. The lattice parameters were determined using a Siemens model D-5000 X-ray diffractometer with a step size of 0.01°. The morphology of the fully grown films was studied to observe the presence of precipitates, porosity and the degree of grain disorientation. The scanning electron microscopy (SEM) images were obtained using a SEM Quanta 200 ESEM FEG equipment. TEM images were obtained on a Philips CM30 operated at 300 kV and a Jeol 2010 FEG operated at 200 kV.The films conductance was determined by electrical measurements carried out using a standard system reported elsewhere.18,19,95,96 It consists of a 4-probe contact in a Van der Pauw configuration. The sample is held in the center of a 22 mm furnace tube that allows controlling the temperature and gas atmosphere. All experiments have been performed at atmospheric pressure. The PO2 was set by mixing N2 and O2 using appropriate mass-flow meters. The in-diffusion conductance transients were measured after PO2 was switched from a low-value, PL, to a high value, PH; and the reverse was done for the out-diffusion experiments.
The influence of gas flow on the oxygen exchange kinetics was analyzed for out-diffusion experiments in order to evaluate the proper flow to be used in all the kinetic experiments. Out-diffusion experiments were performed at gas flows in the range from 0.15 to 0.6 l min−1 at different temperatures (300–800 °C) (Fig. S1 in the ESI†). It was found that some dependence on gas flow exists which is enhanced at low temperatures and which tends to stabilize at 0.6 l min−1. This gas flow dependence at low gas flow rates arises probably from insufficient oxygen diffusion across the boundary layer formed on top of the films. For this reason, all the reported experiments were performed under the highest gas flow allowed in our experimental set up, i.e., 0.6 l min−1.
Acknowledgements
The authors acknowledge the MICIN (NANOSELECT, CSD2007-00041 and MAT2014-51778-C2-1-R and C2-2-R), Generalitat de Catalunya (2014SGR 753 and Xarmae), the EU (EU-FP7 NMP-LA-2012-280432 EUROTAPES project, Cost Action MP1201) and ERC ADG-2014-669504. M. C. is grateful to the MINECO for the “Ramon y Cajal” contract RYC-2013-12448. We also acknowledge the MINECO for the Center of Excellence award Severo Ochoa (SEV-2015-0496). C. F. Sánchez-Valdés acknowledges the support received from CSIC under JAE-Predoctorals program. We also acknowledge useful discussions with Dr S. Ricart.References
- X. Obradors and T. Puig, Supercond. Sci. Technol., 2014, 27, 44003 CrossRef.
- X. Obradors, T. Puig, A. Pomar, F. Sandiumenge, N. Mestres, M. Coll, A. Cavallaro, N. Romà, J. Gázquez, J. C. González, O. Castaño, J. Gutiérrez, A. Palau, K. Zalamova, S. Morlens, A. Hassini, M. Gibert, S. Ricart, J. M. Moretó, S. Piñol, D. Isfort and J. Bock, Supercond. Sci. Technol., 2006, 19, S13–S26 CrossRef CAS.
- X. Obradors, T. Puig, A. Pomar, F. Sandiumenge, S. Piñol, N. Mestres, O. Castaño, M. Coll, A. Cavallaro, A. Palau, J. Gázquez, J. C. González, J. Gutiérrez, N. Romà, S. Ricart, J. M. Moretó, M. D. Rossell and G. van Tendeloo, Supercond. Sci. Technol., 2004, 17, 1055–1064 CrossRef CAS.
- T. Izumi, M. Yoshizumi, J. Matsuda, K. Nakaoka, Y. Kitoh, Y. Sutoh, T. Nakanishi, A. Nakai, K. Suzuki, Y. Yamada, A. Yajima, T. Saitoh and Y. Shiohara, Phys. C, 2007, 463, 510–514 CrossRef.
- Y. Shiohara, T. Taneda and M. Yoshizumi, Jpn. J. Appl. Phys., 2012, 51, 10007 CrossRef.
- Y. Shiohara, M. Yoshizumi, Y. Takagi and T. Izumi, Phys. C, 2013, 484, 1–5 CrossRef CAS.
- S. Tsukui, R. E. Koritala, M. Li, K. C. Goretta, M. Adachi, J. E. Baker and J. L. Routbort, Phys. C, 2003, 392, 841–846 CrossRef.
- R. Mogilevsky, R. Levi-Setti, B. Pashmakov, L. Liu, K. Zhang, H. M. Jaeger, D. B. Buchholz, R. P. H. Chang and B. W. Veal, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 6420–6423 CrossRef CAS.
- S. J. Rothman, J. L. Routbort, U. Welp and J. E. Baker, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 44, 2326–2333 CrossRef CAS.
- S. I. Bredikhin, G. A. Emelchenko, V. S. Shechtman, A. A. Zhokhov, S. Carter, R. J. Chater, J. A. Kilner and B. C. H. Steele, Phys. C, 1991, 179(4–6), 286–290 CrossRef CAS.
- T. B. Tang and W. Lo, Phys. C, 1991, 174, 463–466 CrossRef.
- K. Kishio, K. Suzuki, T. Hasegawa, T. Yamamoto, K. Kitazawa and K. Fueki, J. Solid State Chem., 1989, 82, 192–202 CrossRef CAS.
- A. Michaelis, E. A. Irene, O. Auciello and A. R. Krauss, J. Appl. Phys., 1998, 83, 7736 CrossRef CAS.
- G. S. Grader, P. K. Gallagher, J. Thomson and M. Gurvitch, Appl. Phys. A: Solids Surf., 1988, 45, 179–183 CrossRef.
- S. H. Lee, S. C. Bae, J. K. Ku and H. J. Shin, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 9142–9146 CrossRef CAS.
- S. Kittelberger, U. Bolz, R. P. Huebener, B. Holzapfel and L. Mex, Phys. C, 1998, 302, 93–101 CrossRef CAS.
- C. Krauns and H.-U. Krebs, Z. Phys. B: Condens. Matter, 1993, 92, 43–46 CrossRef CAS.
- L. Chen, C. L. Chen and A. J. Jacobson, IEEE Trans. Appl. Supercond., 2003, 13, 2882–2885 CrossRef CAS.
- T. Qu, Y. Xue, F. Feng, R. Huang, W. Wu, K. Shi and Z. Ha, Phys. C, 2013, 494, 148–152 CrossRef CAS.
- H. Zhang, H. Ye, K. Du, X. Y. Huang and Z. H. Wang, Supercond. Sci. Technol., 2002, 15, 317 Search PubMed.
- A. Fick, Ann. Phys. Chem., 1855, 170, 59–86 CrossRef.
- A. Fick, London, Edinburgh Dublin Philos. Mag. J. Sci., 1855, 10, 30–39 Search PubMed.
- S. B. Adler, Chem. Rev., 2004, 104, 4791–4844 CrossRef CAS PubMed.
- S. B. Adler, X. Y. Chen and J. R. Wilson, J. Catal., 2007, 245, 91–109 CrossRef CAS.
- A. J. Jacobson, Chem. Mater., 2010, 22, 660–674 CrossRef CAS.
- M. M. Kuklja, E. A. Kotomin, R. Merkle, Yu. A. Mastrikov and J. Maier, Phys. Chem. Chem. Phys., 2013, 15, 5443 RSC.
- M. V. Ananyev, E. S. Tropin, V. A. Eremin, A. S. Farlenkov, A. S. Smirnov, A. A. Kolchugin, N. M. Porotnikova, A. V. Khodimchuk, A. V. Berenov and E. Kh. Kurumchin, Phys. Chem. Chem. Phys., 2016, 18, 9102–9111 RSC.
- C. Körber, A. Wachau, P. Ágoston, K. Albe and A. Klein, Phys. Chem. Chem. Phys., 2011, 13, 3223 RSC.
- M. Leonhardt, R. A. De Souza, J. Claus and J. Maier, J. Electrochem. Soc., 2002, 149, J19 CrossRef CAS.
- R. Merkle and J. Maier, Phys. Chem. Chem. Phys., 2002, 4, 4140–4148 RSC.
- R. Merkle and J. Maier, Angew. Chem., Int. Ed., 2008, 47, 3874–3894 CrossRef CAS PubMed.
- L. Wang, R. Merkle and J. Maier, J. Electrochem. Soc., 2010, 157, B1802 CrossRef CAS.
- V. Metlenko, W. Jung, S. R. Bishop, H. L. Tuller and R. A. De Souza, Phys. Chem. Chem. Phys., 2016, 18, 29495–29505 RSC.
- Z. A. Feng, F. El Gabaly, X. Ye, Z.-X. Shen and W. C. Chueh, Nat. Commun., 2014, 5, 966–978 Search PubMed.
- A. Karthikeyan and S. Ramanathan, Appl. Phys. Lett., 2008, 92, 243109 CrossRef.
- Y. Shi, A. H. Bork, S. Schweiger and J. L. M. Rupp, Nat. Mater., 2015, 14, 721–727 CrossRef CAS PubMed.
- S. V. Kalinin, A. Borisevich and D. Fong, ACS Nano, 2012, 6, 10423–10437 CrossRef CAS PubMed.
- A. Sawa, Mater. Today, 2008, 11, 28–36 CrossRef CAS.
- C. Moreno, C. Munuera, S. Valencia, F. Kronast, X. Obradors and C. Ocal, Nano Lett., 2010, 10, 3828–3835 CrossRef CAS PubMed.
- N. Knoblauch, L. Dörrer, P. Fielitz, M. Schmücker and G. Borchardt, Phys. Chem. Chem. Phys., 2015, 17, 5849–5860 RSC.
- J. L. Routbort and S. J. Rothman, J. Appl. Phys., 1994, 76, 5615 CrossRef CAS.
- J. Crank, The mathematics of diffusion, Clarendon Press, 1975 Search PubMed.
- A. Pomar, J. Gutiérrez, A. Palau, T. Puig and X. Obradors, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 214–522 CrossRef.
- E. Bartolomé, A. Palau, J. Gutiérrez, X. Granados, A. Pomar, T. Puig, X. Obradors, V. Cambel, J. Soltys, D. Gregusova, D. X. Chen and A. Sánchez, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 895–899 CrossRef.
- A. Palau, T. Puig, X. Obradors, E. Pardo, C. Navau, A. Sánchez, A. Usoskin, H. C. Freyhardt, L. Fernández, B. Holzapfel and R. Feenstra, Appl. Phys. Lett., 2004, 84, 230 CrossRef CAS.
- O. Castaño, A. Cavallaro, A. Palau, J. C. González, M. Rosell, T. Puig, S. Piñol, N. Mestres, F. Sandiumenge, A. Pomar and X. Obradors, IEEE Trans. Appl. Supercond., 2003, 13, 2504–2507 CrossRef.
- K. Zalamova, A. Pomar, A. Palau, T. Puig and X. Obradors, Supercond. Sci. Technol., 2010, 23, 14012 CrossRef.
- C. P. Bean, Phys. Rev. Lett., 1962, 8, 250–253 CrossRef.
- C. P. Bean, Rev. Mod. Phys., 1964, 36(1), 31–39 CrossRef.
- J. Shimoyama, S. Horii, K. Otzschi and K. Kishio, MRS Proc., 2001, 689, E8.18 Search PubMed.
- E. D. Specht, C. J. Sparks, A. G. Dhere, J. Brynestad, O. B. Cavin, D. M. Kroege and H. A. Oye, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 7426–7434 CrossRef CAS.
- J. Ye and K. Nakamura, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 48, 7554–7564 CrossRef CAS.
- R. Liang, D. A. Bonn and W. N. Hardy, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 180505 CrossRef.
- T. B. Lindemer, J. F. Hunley, J. E. Gates, A. L. Sutton, J. Brynestad, C. R. Hubbard and P. K. Gallagher, J. Am. Ceram. Soc., 1989, 72, 1775–1788 CrossRef CAS.
- H. Zhang, X. Yao and X. Zeng, Phys. Status Solidi, 2004, 201, 2305–2311 CrossRef CAS.
- K. Conder, Mater. Sci. Eng., R, 2001, 32, 41–102 CrossRef.
- N. K. Tu, N. C. Yeh, S. I. Park and C. C. Tsuei, Phys. Rev. B: Condens. Matter Mater. Phys., 1989, 39, 304–314 CrossRef.
- Y. A. Mastrikov, R. Merkle, E. Heifets, E. A. Kotomin and J. Maier, J. Phys. Chem. C, 2010, 114, 3017–3027 CAS.
- J. R. LaGraff and D. A. Payne, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 3380–3390 CrossRef CAS.
- J. R. LaGraff and D. A. Payne, Phys. C, 1993, 212, 470–477 CrossRef CAS.
- J. R. LaGraff and D. A. Payne, Phys. C, 1993, 212, 478–486 CrossRef CAS.
- J. R. LaGraff and D. A. Payne, Phys. C, 1993, 212, 487–496 CrossRef CAS.
- M. Søgaard, P. Vang Hendriksen and M. Mogensen, J. Solid State Chem., 2007, 180, 1489–1503 CrossRef.
- I. Yasuda and M. Hishinuma, J. Solid State Chem., 1996, 123, 382–390 CrossRef CAS.
- J. A. Lane and J. A. Kilner, Solid State Ionics, 2000, 136, 927–932 CrossRef.
- D. Rupasov, T. Makarenko and A. J. Jacobson, Solid State Ionics, 2014, 265, 68–72 CrossRef CAS.
- M. D. Vázquez-Navarro, A. Kursumovic and J. E. Evetts, Supercond. Sci. Technol., 1999, 12, 1117–1122 CrossRef.
- S. Kittelberger, O. M. Stoll and R. P. Huebener, Supercond. Sci. Technol., 1998, 11, 744–750 CrossRef CAS.
- S. Elschner, W. Becker, H. Bestgen and M. Brand, Phys. C, 1992, 202, 401–407 CrossRef CAS.
- A. Erb, B. Greb and G. Müller-Vogt, Phys. C, 1996, 259, 83–91 CrossRef CAS.
- S. Kittelberger, U. Bolz, R. P. Huebener, B. Holzapfel, L. Mex and R. A. Schwarzer, Phys. C, 1999, 312, 7–20 CrossRef CAS.
- M. Burriel, H. Téllez, R. J. Chater, R. Castaing, P. Veber, M. Zaghrioui, T. Ishihara, J. A. Kilner and J.-M. Bassat, J. Phys. Chem. C, 2016, 120, 17927–17938 CAS.
- J. L. Tallon, IEEE Trans. Appl. Supercond., 2015, 25, 1–6 CrossRef CAS.
- E. F. Talantsev, S. C. Wimbush, N. M. Strickland, J. A. Xia, P. D’Souza, J. G. Storey, J. L. Tallon, B. Ingham, R. Knibbe and N. J. Long, IEEE Trans. Appl. Supercond., 2013, 23 Search PubMed.
- G. Deutscher, APL Mater., 2014, 2, 96108 CrossRef.
- G. Deutscher, J. Appl. Phys., 2012, 111, 112603 CrossRef.
- G. Ertl, Handbook of heterogeneous catalysis, Wiley-VCH, 2008 Search PubMed.
- R. A. Van Santen and H. P. C. E. Kuipers, Adv. Catal., 1987, 35, 265–321 CAS.
- C. C. Yu, J. D. Baek, C. H. Su, L. Fan, J. Wei, Y. C. Liao and P. C. Su, ACS Appl. Mater. Interfaces, 2016, 8, 10343–10349 CAS.
- S. T. Oyama, Mechanisms in homogeneous and heterogeneous epoxidation catalysis, Elsevier, 2008 Search PubMed.
- X. Obradors, F. Martínez-Julián, K. Zalamova, V. R. Vlad, A. Pomar, A. Palau, A. Llordés, H. Chen, M. Coll, S. Ricart, N. Mestres, X. Granados, T. Puig and M. Rikel, Phys. C, 2012, 482, 58–67 CrossRef CAS.
- C. Backx, C. P. M. De Groot and P. Biloen, Surf. Sci., 1981, 104, 300–317 CrossRef CAS.
- V. I. Bukhtiyarov, V. V. Kaichev and I. P. Prosvirin, J. Chem. Phys., 1999, 111, 2169 CrossRef CAS.
- B. A. Sexton and R. J. Madix, Chem. Phys. Lett., 1980, 76, 294–297 CrossRef CAS.
- J. Liu, G. Collins, M. Liu and C. Chen, APL Mater., 2013, 1, 31101 CrossRef.
- L. Yan and P. A. Salvador, ACS Appl. Mater. Interfaces, 2012, 4, 2541–2550 CAS.
- K. Szot, W. Speier, G. Bihlmayer and R. Waser, Nat. Mater., 2006, 5, 312–320 CrossRef CAS PubMed.
- K. Otsuka, A. Kuwabara, A. Nakamura, T. Yamamoto, K. Matsunaga and Y. Ikuhara, Appl. Phys. Lett., 2003, 82, 877 CrossRef CAS.
- R. F. Klie, W. Walkosz, G. Yang and Y. Zhao, J. Electron Microsc., 2009, 58, 185–191 CrossRef CAS PubMed.
- J. Gázquez, M. Coll, N. Romà, F. Sandiumenge, T. Puig and X. Obradors, Supercond. Sci. Technol., 2012, 25, 65009 CrossRef.
- V. Solovyov, I. K. Dimitrov and Q. Li, Supercond. Sci. Technol., 2013, 26, 13001 CrossRef.
- S. K. R. S. Sankaranarayanan and S. Ramanathan, J. Phys. Chem. C, 2010, 114, 6631–6639 CAS.
- N. Romà, S. Morlens, S. Ricart, K. Zalamova, J. M. Moretó, A. Pomar, T. Puig and X. Obradors, Supercond. Sci. Technol., 2006, 19, 521–527 CrossRef.
- X. Obradors, T. Puig, S. Ricart, M. Coll, J. Gázquez, A. Palau and X. Granados, Supercond. Sci. Technol., 2012, 25, 123001 CrossRef.
- C. F. Sánchez-Valdés, T. Puig and X. Obradors, Supercond. Sci. Technol., 2015, 28, 24006 CrossRef.
- H. Chen, K. Zalamova, A. Pomar, X. Granados, T. Puig and X. Obradors, J. Mater. Res., 2010, 25, 2371–2379 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cp01855j |
| This journal is © the Owner Societies 2017 |