Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Impact of the molecular structure and adsorption mode of D–π–A dye sensitizers with a pyridyl group in dye-sensitized solar cells on the adsorption equilibrium constant for dye-adsorption on TiO2 surface
Yousuke
Ooyama
*,
Naoya
Yamaguchi
,
Joji
Ohshita
and
Yutaka
Harima
*
Department of Applied Chemistry, Graduate School of Engineering, Hiroshima University, Higashi-Hiroshima 739-8527, Japan. E-mail: yooyama@hiroshima-u.ac.jp; Fax: +81 82-424-5494
First published on 15th November 2016
Abstract
D–π–A dyes NI-4 bearing a pyridyl group, YNI-1 bearing two pyridyl groups and YNI-2 bearing two thienylpyridyl groups as the anchoring group on the TiO2 surface have been developed as dye sensitizers for dye-sensitized solar cells (DSSCs), where NI-4 and YNI-2 can adsorb onto the TiO2 electrode through the formation of the coordinate bond between the pyridyl group of the dye and the Lewis acid site (exposed Tin+ cations) on the TiO2 surface, but YNI-1 is predominantly adsorbed on the TiO2 electrode through the formation of the hydrogen bond between the pyridyl group of the dye and the Brønsted acid sites (surface-bound hydroxyl groups, Ti–OH) on the TiO2 surface. The difference in the dye-adsorption mode among the three dyes on the TiO2 surface has been investigated from the adsorption equilibrium constant (Kad) based on the Langmuir adsorption isotherms. It was found that the Kad values of YNI-1 and YNI-2 are higher than that of NI-4, and more interestingly, the Kad value of YNI-2 is higher than that of YNI-1. This work demonstrates that that for the D–π–A dye sensitizers with the pyridyl group as the anchoring group to the TiO2 surface the number of pyridyl groups and the dye-adsorption mode on the TiO2 electrode as well as the molecular structure of the dye sensitizer affect the Kad value for the adsorption of the dye to the TiO2 electrode, that is, resulting in a difference in the Kad value among the D–π–A dye sensitizers NI-4, YNI-1 and YNI-2.
Introduction
Twenty-five years have passed since Grätzel and co-workers developed high-performance dye-sensitized solar cells (DSSCs) employing a Ru-complex dye-adsorbed TiO2 electrode in 1991,1 but DSSCs are still receiving considerable attention as one of the most promising new renewable photovoltaic cells from chemists, physicists, and engineers.2–9 To further improve the photovoltaic performances of DSSCs, much effort has been made toward the development of various types of organic dye sensitizers bearing a carboxyl group,10–16 aldehyde,17,18 nitro group,19 2-(1,1-dicyanomethylene)rhodanine,20 pyridine,21–24 or 8-hydroxylquinoline25 as the electron-withdrawing anchoring group that possesses high dye loading and high surface coverage of the TiO2 electrode, leading to good light-harvesting features over the wide spectral region of sunlight and high electron-injection efficiencies from the photoexcited dyes to the conduction band (CB) of the TiO2 electrode. In particular, many kinds of donor–acceptor π-conjugated (D–π–A) dyes bearing a carboxyl group have been designed and developed as one of the most promising classes of organic dye sensitizers because of their strong photoabsorption properties originating from the intramolecular charge transfer (ICT) excitation from the donor to the acceptor (carboxyl group) moiety in the D–π–A structures.3,4,6 The D–π–A dye sensitizers bearing a carboxyl group are adsorbed on the TiO2 electrode through the bidentate bridging linkage between the carboxyl group of the dye and Brønsted acid sites (surface-bound hydroxyl groups, Ti–OH) on the TiO2 surface (Fig. 1a and 2a; NI-221b as a typical D–π–A dye sensitizer bearing a carboxyl group). Thus, the photoabsorption properties associated with the ICT excitation of the D–π–A dye can lead to efficient electron transfer from the photoexcited dye through the carboxyl group to the CB of the TiO2 electrode. As the result, the DSSCs based on the D–π–A porphyrin dye sensitizers bearing a carboxyl group have achieved a solar energy-to-electricity conversion yield (η) of up to 13%.11c,dOn the other hand, we have designed and developed D–π–A dye sensitizers bearing a pyridyl group as the anchoring group to the TiO2 surface so far.21 It was found that the D–π–A dye sensitizer NI-4 bearing a pyridyl group is predominantly adsorbed on the TiO2 electrode through the coordination bond between the pyridyl group of the dye and the Lewis acid site (exposed Tin+ cations) on the TiO2 surface (Fig. 1b and 2b).21b,c The difference in the dye-adsorption mode between NI-2 and NI-4 on the TiO2 surface has been investigated from the Langmuir adsorption isotherms, and analysis of the Langmuir plots showed that the saturated dye-adsorption amounts (C0) of NI-2 and NI-4 on the TiO2 electrode are similar to each other. On the other hand, the adsorption equilibrium constant (Kad) of NI-4 is smaller than that of NI-2, that is, the fact indicates that the pyridyl group is a relatively weak anchoring ability to the TiO2 surface compared with the carboxyl group.22a Interestingly, the photovoltaic performance of DSSCs based on NI-4 is higher than that based on NI-2. Thus, it was revealed that the D–π–A dye sensitizers bearing a pyridyl group can inject electrons efficiently from the pyridyl group to the CB of the TiO2 electrode through the coordination bond, compared to the bidentate bridging linkages of the D–π–A dye sensitizers bearing a carboxyl group. Consequently, we demonstrated that the pyridyl group is a promising candidate as not only an electron-withdrawing anchoring group but also an electron-injecting group for D–π–A dye sensitizers. More interestingly, we found that the D–π–A dye sensitizer YNI-2 with two thienylpyridyl groups can adsorb onto the TiO2 electrode through the formation of the coordinate bond between the pyridyl group of the dye and the Lewis acid site on the TiO2 surface (Fig. 1d and 2b), but the D–π–A dye sensitizer YNI-1 with two pyridyl groups is predominantly adsorbed on the TiO2 electrode through the formation of the hydrogen bond between the pyridyl group of the dye and the Brønsted acid sites on the TiO2 surface (Fig. 1c and 2c).21d The photovoltaic performance of DSSCs based on YNI-2 is higher than that based on YNI-1. Consequently, our previous work demonstrated that the higher photovoltaic performance of YNI-2 is attributed to not only the red-shift and broadening of the photoabsorption band originating from the ICT and the stable oxidized state of the dye, but also to efficient electron injection by the formation of the coordinate bond at Lewis acid sites on the TiO2 surface. Recently, some researchers have reported high-performance DSSCs based on porphyrin dyes or D–π–A dyes bearing pyridyl groups;22–24 as a result, the η value reached up to 8.2%.24e However, a comprehensive understanding of the difference in the dye-adsorption modes among the dye sensitizers bearing the pyridyl group is still lacking.
Thus, in this work, to gain insight into the molecular structure and the adsorption mode of D–π–A dye sensitizers with a pyridyl group on the TiO2 surface, the difference in the dye-adsorption mode among NI-4, YNI-1 and YNI-2 on the TiO2 surface has been investigated from the Kad value based on the Langmuir adsorption isotherms. The Langmuir plots showed that the C0 value of NI-4 on the TiO2 electrode is slightly larger than those of YNI-1 and YNI-2, but the C0 values of YNI-1 and YNI-2 are similar to each other. Moreover, it was found that the Kad values of YNI-1 and YNI-2 are higher than that of NI-4, and more interestingly, the Kad value of YNI-2 is higher than that of YNI-1. Here, we report that the impacts of the molecular structure and adsorption mode of D–π–A dye sensitizers with a pyridyl group for DSSCs on the adsorption equilibrium constant for dye-adsorption to the TiO2 surface.
Results and discussion
A 9 μm (1.5 cm2) thick TiO2 electrode was immersed into THF solutions containing various concentrations (0.02, 0.04, 0.06, 0.08, 0.1, 0.2, 0.5, 1.0 or 1.0 mM) of NI-4, YNI-1 or YNI-2 for dye-adsorption on the TiO2 surface. In all cases, dye-adsorption was performed for 24 h in an incubator kept at 25 °C. The dye-adsorbed TiO2 electrode was immersed in a 1 M THF–DMSO–NaOH aq. mixed solvent (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
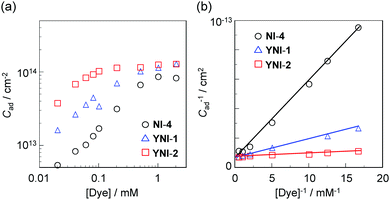
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to desorb the dye molecules, that is, the adsorption amount of NI-4, YNI-1 and YNI-2 was determined by recording the photoabsorption spectra of the solutions on a spectrophotometer. Consequently, the amount (Cad) of dyes adsorbed on the TiO2 electrode is expressed by the number of dye molecules per unit area of the TiO2 surface calculated using a specific surface area of 830 cm2 mg−1. The Cad−1versus [Dye]−1 (the concentration of the dye solution) plots in the double-logarithmic representation depicts the adsorption isotherms for NI-4, YNI-1 and YNI-2 adsorbed on the TiO2 surface (Fig. 3a). The adsorption isotherms showed that for the three dyes the adsorption amount of dye molecules adsorbed on the TiO2 electrode increased with the increasing concentration of the dye solution. However, it is seen clearly from the adsorption isotherms that the adsorption trends of the three dyes differ greatly from each other, reflecting the differences in the molecular structure and the adsorption mode among NI-4, YNI-1 and YNI-2. Thus, to investigate the difference in the adsorption trends among the three dyes from the Langmuir plots, the three adsorption isotherms were analyzed by using the following equation:
1) to desorb the dye molecules, that is, the adsorption amount of NI-4, YNI-1 and YNI-2 was determined by recording the photoabsorption spectra of the solutions on a spectrophotometer. Consequently, the amount (Cad) of dyes adsorbed on the TiO2 electrode is expressed by the number of dye molecules per unit area of the TiO2 surface calculated using a specific surface area of 830 cm2 mg−1. The Cad−1versus [Dye]−1 (the concentration of the dye solution) plots in the double-logarithmic representation depicts the adsorption isotherms for NI-4, YNI-1 and YNI-2 adsorbed on the TiO2 surface (Fig. 3a). The adsorption isotherms showed that for the three dyes the adsorption amount of dye molecules adsorbed on the TiO2 electrode increased with the increasing concentration of the dye solution. However, it is seen clearly from the adsorption isotherms that the adsorption trends of the three dyes differ greatly from each other, reflecting the differences in the molecular structure and the adsorption mode among NI-4, YNI-1 and YNI-2. Thus, to investigate the difference in the adsorption trends among the three dyes from the Langmuir plots, the three adsorption isotherms were analyzed by using the following equation:| Cad−1 = (KadC0)−1[Dye]−1 + C0−1 | (1) |
| Cad/C0 = Kad[Dye]/(1 + Kad[Dye]) | (2) |
| Dye | C 0/cm−2 | K ad/M−1 | R |
|---|---|---|---|
| a R denotes a correlation coefficient. | |||
| NI-4 | 1.7 × 1014 | 1.1 × 103 | 0.9969 |
| YNI-1 | 1.4 × 1014 | 6.1 × 103 | 0.9980 |
| YNI-2 | 1.3 × 1014 | 33.1 × 103 | 0.9653 |
Therefore, to gain insight into the impact of the molecular structure and the adsorption mode of D–π–A dye sensitizers with a pyridyl group on the adsorption equilibrium constant for dye-adsorption to the TiO2 surface, we evaluated the photovoltaic performance of DSSCs based on the co-adsorbed TiO2 electrode with the dye YNI-1 or YNI-2 and chenodeoxycholic acid (CDCA) capable of adsorbing at the Brønsted acid sites on the TiO2 electrode. For co-adsorption the concentration of CDCA was changed, with that of YNI-1 or YNI-2 kept constant at 0.1 mM, where 0.1 mM dye and 1.0 mM CDCA solution, and 0.1 mM dye and 5.0 mM CDCA solution were used. The photocurrent–voltage (I–V) characteristics of DSSCs based on the co-adsorbed TiO2 electrode with the dye YNI-1 or YNI-2 and CDCA were measured under simulated solar light (AM 1.5, 100 mW cm−2) and the I–V curves are shown in Fig. 4. The photovoltaic performances of the DSSCs are summarized in Table 2. We expected that for the co-adsorption of YNI-1 and CDCA the adsorption of YNI-1 onto the TiO2 electrode may compete with that of CDCA because YNI-1 and CDCA adsorb on the same acid sites (Brønsted acid sites), leading to a decrease in the short-circuit photocurrent density (Jsc) and the solar energy-to-electricity conversion yield (η) with the increase in the concentration of CDCA. On the other hand, YNI-2 and CDCA adsorb independently on the TiO2 surface because YNI-2 and CDCA adsorb at different acid sites, the Lewis acid sites and Brønsted acid sites on the TiO2 surface, respectively, and thus regardless of the concentration of CDCA the co-adsorption of YNI-2 and CDCA has little influence on the Jsc value and the η value. As is shown in Table 2, for the co-adsorption of YNI-1 and CDCA, with the increase in the concentration of CDCA, the η value as well as the Jsc value of DSSCs based on the co-adsorbed TiO2 electrode with YNI-1 and CDCA decreases. Therefore, the adsorption of YNI-1 onto the TiO2 surface may compete with that of CDCA, that is, by increasing the concentration of CDCA the adsorption amount of YNI-1 may decrease, being accompanied by an increase in that of CDCA. This may be explained by the fact that YNI-1 and CDCA adsorb on the same acid sites (Brønsted acid sites), and thus the increase in the concentration of CDCA leads to a decrease in the adsorption amount of YNI-1 by the competitive adsorption of CDCA. On the other hand, for the co-adsorption of YNI-2 and CDCA there are no appreciable changes in the Jsc value and the η value with the increase in the concentration of CDCA, suggesting that YNI-2 and CDCA adsorb independently on the TiO2 surface because YNI-2 and CDCA adsorb on different acid sites (Lewis acid sites for YNI-2 and Brønsted acid sites for CDCA, respectively). Therefore, the increase in the concentration of CDCA does not cause a decrease in the adsorption amount of YNI-2 without the competitive adsorption of CDCA, consistent with our previous result for NI-4.22a Consequently, these results indicate that the co-adsorption of YNI-1 and CDCA causes the competitive adsorption between the dye and CDCA, leading to a decrease in the amount of the dye adsorbed on the TiO2 electrode, thus resulting in a reduction of the photovoltaic performance of the DSSCs, but for the co-adsorption of YNI-2 and CDCA the competitive adsorption was not observed and the thus the co-adsorption has little influence on the photovoltaic performance of the DSSCs.
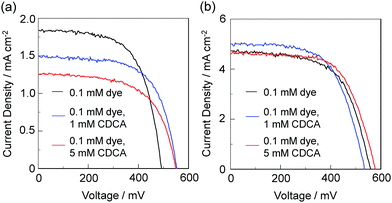 | ||
| Fig. 4 I–V curves of DSSCs based on (a) YNI-1 and (b) YNI-2 with and without CDCA as a co-adsorbent. | ||
| Dye | CDCA/mM | J sc /mA cm−2 | V oc /mV | ffd | η (%) |
|---|---|---|---|---|---|
| a A 9 μm thick TiO2 electrode was used. 0.1 mM dye solution in acetonitrile. b A 9 μm thick TiO2 electrode was used. 0.1 mM dye and 1 mM CDCA solution. c A 9 μm thick TiO2 electrode was used. 0.1 mM dye and 5 mM CDCA solution. d Under simulated solar light (AM 1.5, 100 mW cm−2). | |||||
| YNI-1 | 0a | 1.84 | 492 | 0.63 | 0.57 |
| 1b | 1.49 | 552 | 0.51 | 0.42 | |
| 5c | 1.25 | 548 | 0.61 | 0.42 | |
| YNI-2 | 0a | 4.72 | 556 | 0.61 | 1.60 |
| 1b | 5.00 | 532 | 0.60 | 1.60 | |
| 5c | 4.60 | 576 | 0.65 | 1.72 | |
On the basis of the Langmuir isotherms and the co-adsorption of the dye YNI-1 or YNI-2 and CDCA onto the TiO2 electrode, it is concluded that the high Kad value of YNI-2 relative to that of YNI-1 is attributed to the reasonable angle between two thienylpyridine moieties (based on MO calculations (MOPAC and AM1 method),21c,d it is defined as the angle between the two pyridines as the corner N2 for the nitrogen atom (N2) of carbazole and the nitrogen atoms (N1 and N3) of the two pyridyl groups, that is ca. 90° for YNI-1 and ca. 67° for YNI-2) as well as the accumulated electron density on the nitrogen atom of the pyridyl groups due to a large planar π-conjugated system extending from the electron donor moiety to the electron acceptor moiety by the introduction of a thiophene unit, leading to the construction of molecular structures capable of forming strong coordinate bonds between the two pyridyl groups of dyes and the Lewis acid sites on the TiO2 surface. On the other hand, YNI-1 is constructed with the rigid D–π–A structure due to the pyridyl groups directly bound to the carbazole skeleton. Therefore, it is reasonable to presume that the rigid molecular structure of YNI-1 makes it difficult to form the coordination bond between the pyridyl group of the dye and the Lewis acid site on the TiO2 surface, but instead prefers to form the hydrogen bond between the pyridyl group of the dye and the flexible surface-bound hydroxyl groups (Brønsted acid sites) on the TiO2 surface, resulting in a relatively low Kad value. Consequently, this work demonstrates that for the D–π–A dye sensitizers with the pyridyl group as the anchoring group to the TiO2 surface, the number of pyridyl groups and the dye-adsorption mode on the TiO2 electrode as well as the molecular structure of the dye sensitizer affect the Kad value for the adsorption of the dye to the TiO2 electrode, that is, resulting in a difference in the Kad value among the D–π–A dye sensitizers NI-4, YNI-1 and YNI-2.
Conclusions
The difference in the dye-adsorption modes among D–π–A dye sensitizers NI-4 with a pyridyl group, YNI-1 with two pyridyl groups and YNI-2 with two thienylpyridyl groups on the TiO2 electrode has been investigated from the adsorption equilibrium constant (Kad) based on the Langmuir adsorption isotherms. It was found that a rod-shaped D–π–A dye sensitizer with a pyridyl group such as NI-4 can effectively cover the TiO2 surface compared with relatively bulky bifurcation-shaped D–π–A dye sensitizer with two pyridyl groups such as YNI-1 and YNI-2; on the other hand, the bifurcation-shaped structure with the two pyridyl groups can strongly adsorb on the TiO2 surface compared with the rod-shaped D–π–A structure with a pyridyl group. More interestingly, the Kad value of YNI-2 is higher than that of YNI-1, which indicates that the adsorption ability of YNI-2 onto the TiO2 surface is superior to that of YNI-1. Thus, it was revealed that the number of pyridyl groups and the dye-adsorption mode on the TiO2 electrode as well as the molecular structure of the dye sensitizer affect the Kad value for the adsorption of the dye to the TiO2 electrode, resulting in a difference in the Kad value among the D–π–A dye sensitizers NI-4, YNI-1 and YNI-2. Consequently, this work provides useful knowledge of the molecular design of D–π–A dye sensitizers bearing a pyridyl group capable of controlling the dye-adsorption mode on the TiO2 electrode for DSSCs.Experimental
Preparation of the dye-adsorbed TiO2 electrode and DSSCs
The TiO2 paste (JGC Catalysts and Chemicals Ltd, PST-18NR) was deposited on a fluorine-doped-tin-oxide (FTO) substrate by doctor-blading, and sintered for 50 min at 450 °C. The 9 μm (1.5 cm2) thick TiO2 electrode was immersed into 0.02, 0.04, 0.06, 0.08, 0.1, 0.2, 0.5, 1.0 or 1.0 mM dye solution in THF for 24 h in an incubator kept at 25 °C. For the co-adsorption of the dye and chenodeoxycholic acid (CDCA), 0.1 mM dye and 1.0 mM CDCA solution, and 0.1 mM dye and 5.0 mM CDCA solution were used. BET surface areas of the TiO2 particles were 830 cm2 mg−1. Consequently, the total area for 1 cm2 geometric area of the electrode was ca. 1577 cm2 for 9 μm thick TiO2 electrode. The dye-coated electrode was immersed in a mixed solvent of THF–DMSO–NaOH aq. 1 M (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which was used to determine the amount of dye molecules adsorbed onto the electrode by measuring the absorbance. The quantification of dye was made based on the molar extinction coefficient for λabsmax of dye in the above solution. Photoabsorption spectra were observed with a Shimadzu UV-3150 spectrophotometer.
1), which was used to determine the amount of dye molecules adsorbed onto the electrode by measuring the absorbance. The quantification of dye was made based on the molar extinction coefficient for λabsmax of dye in the above solution. Photoabsorption spectra were observed with a Shimadzu UV-3150 spectrophotometer.
The DSSCs were fabricated by using the dye-adsorbed TiO2 electrode (0.5 × 0.5 cm2 in photoactive area), Pt-coated glass as a counter electrode, and a solution of 0.05 M iodine, 0.1 M lithium iodide, and 0.6 M 1,2-dimethyl-3-propylimidazolium iodide in acetonitrile as the electrolyte. The photocurrent–voltage characteristics were measured using a potentiostat under a simulated solar light (AM 1.5, 100 mW cm−2).
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (JSPS) KAKENHI, Grant Number 15H03859, by the Matching Planner Program (MP27115659061) from Japan Science and Technology Agency (JST) and by the TEPCO Memorial Foundation.Notes and references
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737 CrossRef.
- A. Mishra, M. K. R. Fischer and P. Bäuerle, Angew. Chem. Int. Ed., 2009, 48, 2474 CrossRef CAS PubMed.
- (a) Y. Ooyama and Y. Harima, Eur. J. Org. Chem., 2009, 2903 CrossRef CAS; (b) Y. Ooyama and Y. Harima, ChemPhysChem, 2012, 13, 4032 CrossRef CAS PubMed.
- (a) Z. Ning and H. Tian, Chem. Commun., 2009, 5483 RSC; (b) Z. Ning, Y. Fu and H. Tian, Energy Environ. Sci., 2010, 3, 1170 RSC.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595 CrossRef CAS PubMed.
- N. Manfredi, B. Cecconi and A. Abbotto, Eur. J. Org. Chem., 2014, 7069 CrossRef CAS.
- L. Zhang and J. M. Cole, ACS Appl. Mater. Interfaces, 2015, 7, 3427 CAS.
- C.-P. Lee, R. Y.-Y. Lin, L.-Y. Lin, C.-T. Li, T.-C. Chu, S.-S. Sun, J. T. Lin and K.-C. Ho, RSC Adv., 2015, 5, 23810 RSC.
- B. Pashaei, H. Shahroosvand, M. Graetzel and M. K. Nazeeruddin, Chem. Rev., 2016, 116, 9485 CrossRef CAS PubMed.
- (a) H. Imahori, T. Umeyama and S. Ito, Acc. Chem. Res., 2009, 42, 1809 CrossRef CAS PubMed; (b) L.-L. Li and E. W.-G. Diau, Chem. Soc. Rev., 2013, 42, 291 RSC; (c) K. Ladomenou, T. N. Kitsopoulos, G. D. Sharma and A. G. Coutsolelos, RSC Adv., 2014, 4, 21379 RSC; (d) T. Higashino and H. Imahori, Dalton Trans., 2015, 44, 448 RSC.
- (a) T. Bessho, S. M. Zakeeruddin, C.-Y. Yeh, E. W.-G. Diau and M. Grätzel, Angew. Chem. Int. Ed., 2010, 49, 6646 CrossRef CAS PubMed; (b) A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629 CrossRef CAS PubMed; (c) S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242 CrossRef CAS PubMed; (d) A. Yella, C.-L. Mai, S. M. Zakeeruddin, S.-N. Chang, C.-H. Hsieh, C.-Y. Yeh and M. Grätzel, Angew. Chem. Int. Ed., 2014, 53, 2973 CrossRef CAS PubMed; (e) T. Higashino, Y. Fujimori, K. Sugiura, Y. Tsuji, S. Ito and H. Imahori, Angew. Chem. Int. Ed., 2015, 54, 9052 CrossRef CAS PubMed; (f) J. P. Hill, Angew. Chem. Int. Ed., 2016, 55, 2976 CrossRef CAS PubMed; (g) F. Lodermeyer, R. D. Costa, J. Malig, N. Jux and D. M. Guldi, Chem. Eur. J., 2016, 22, 7851 CrossRef CAS PubMed; (h) G. Copley, D. Hwang, D. Kim and A. Osuka, Angew. Chem. Int. Ed., 2016, 55, 10287 CrossRef CAS PubMed.
- (a) T. Zhang, X. Qian, P. Zhang, Y.-Z. Zhu and J.-Y. Zheng, Chem. Commun., 2015, 51, 3782 RSC; (b) Y. Xie, Y. Tang, W. Wu, Y. Wang, J. Liu, X. Li, H. Tian and W.-H. Zhu, J. Am. Chem. Soc., 2015, 137, 14055 CrossRef CAS PubMed; (c) K. Sirithip, N. Prachumrak, R. Rattanawan, T. Keawin, T. Sudyoadsuk, S. Namuangruk, S. Jungsuttiwong and V. Promarak, Chem. Asian J., 2015, 10, 882 CrossRef CAS PubMed; (d) S. Mathew, N. A. Astani, B. F. E. Curchod, J. H. Delcamp, M. Marszalek, J. Frey, U. Rothlisberger, M. K. Nazeeruddina and M. Grätzel, J. Mater. Chem. A, 2016, 4, 2332 RSC; (e) L. Zeininger, F. Lodermeyer, R. D. Costa, D. M. Guldi and A. Hirsch, Chem. Commun., 2016, 52, 8842 RSC; (f) J. Luo, J. Zhang, K.-W. Huang, Q. Qi, S. Dong, J. Zhang, P. Wang and J. Wu, J. Mater. Chem. A, 2016, 4, 8428 RSC; (g) C. Li, L. Luo, D. Wu, R. Jiang, J. Lan, R. Wang, L. Huang, S. Yang and J. You, J. Mater. Chem. A, 2016, 4, 11829 RSC; (h) Y.-C. Liu, H.-H. Chou, F.-Y. Ho, H.-J. Wei, T.-C. Wei and C.-Y. Yeh, J. Mater. Chem. A, 2016, 4, 11878 RSC.
- (a) J.-J. Cid, J.-H. Yum, S.-R. Jang, M. K. Nazeeruddin, E. Martínez-Ferrero, E. Palomares, J. Ko, M. Grätzel and T. Torres, Angew. Chem. Int. Ed., 2007, 46, 8358 CrossRef CAS PubMed; (b) M. Kimura, H. Nomoto, N. Masaki and S. Mori, Angew. Chem. Int. Ed., 2012, 51, 4371 CrossRef CAS PubMed; (c) M.-E. Ragoussi, J.-J. Cid, J.-H. Yum, G. De La Torre, D. D. Censo, M. Grätzel, M. K. Nazeeruddin and T. Torres, Angew. Chem. Int. Ed., 2012, 51, 4375 CrossRef CAS PubMed; (d) L. Yu, K. Fan, T. Duan, X. Chen, R. Li and T. Peng, ACS Sustainable Chem. Eng., 2014, 2, 718 CrossRef CAS; (e) T. Ikeuchi, H. Nomoto, N. Masaki, M. J. Griffith, S. Mori and M. Kimura, Chem. Commun., 2014, 50, 1941 RSC; (f) T. Ikeuchi, S. Agrawal, M. Ezoe, S. Mori and M. Kimura, Chem. Asian J., 2015, 10, 2347 CrossRef CAS PubMed; (g) L. Tejerina, M. V. Martínez-Díaz, M. K. Nazeeruddin and T. Torres, Chem. Eur. J., 2016, 22, 4369 CrossRef CAS PubMed.
- (a) X. Wang, J. Yang, H. Yu, F. Li, L. Fan, W. Sun, Y. Liu, Z. Y. Koh, J. Pan, W.-L. Yim, L. Yan and Q. Wang, Chem. Commun., 2014, 50, 3965 RSC; (b) S.-G. Li, K.-J. Jiang, J.-H. Huang, L.-M. Yang and Y.-L. Song, Chem. Commun., 2014, 50, 4309 RSC; (c) D. K. Panda, F. S. Goodson, S. Ray and S. Saha, Chem. Commun., 2014, 50, 5358 RSC; (d) K. Kakiage, Y. Aoyama, T. Yano, T. Otsuka, T. Kyomen, M. Unno and M. Hanaya, Chem. Commun., 2014, 50, 6379 RSC; (e) A. Amacher, C. Yi, J. Yang, M. P. Bircher, Y. Fu, M. Cascella, M. Grätzel, S. Decurtins and S.-X. Liu, Chem. Commun., 2014, 50, 6540 RSC; (f) W.-I. Hung, Y.-Y. Liao, T.-H. Lee, Y.-C. Ting, J.-S. Ni, W.-S. Kao, J. T. Lin, T.-C. Wei and Y.-S. Yen, Chem. Commun., 2015, 51, 2152 RSC; (g) X. Li, Z. Zheng, W. Jiang, W. Wu, Z. Wang and H. Tian, Chem. Commun., 2015, 51, 3590 RSC; (h) K. Kakiage, Y. I. Aoyama, T. Yano, K. Oya, T. Kyomen and M. Hanaya, Chem. Commun., 2015, 51, 6315 RSC; (i) L. Yang, Z. Zheng, Y. Li, W. Wu, H. Tian and Z. Wang, Chem. Commun., 2015, 51, 4842 RSC; (j) N. Shibayama, Y. Inoue, M. Abe, S. Kajiyama, H. Ozawa, H. Miura and H. Arakawa, Chem. Commun., 2015, 51, 12795 RSC; (k) K. Kakiage, Y. Aoyama, T. Yano, K. Oya, J. Fujisawa and M. Hanaya, Chem. Commun., 2015, 51, 15894 RSC.
- (a) J.-S. Ni, Y.-C. Yen and J. T. Lin, Chem. Commun., 2015, 51, 17080 RSC; (b) Y.-D. Lin, B.-Y. Ke, Y. J. Chang, P.-T. Chou, K.-L. Liau, C.-Y. Liu and T. J. Chow, J. Mater. Chem. A, 2015, 3, 16831 RSC; (c) S. S. Soni, K. B. Fadadu, J. V. Vaghasiya, B. G. Solanki, K. K. Sonigara, A. Singh, D. Das and P. K. Iyer, J. Mater. Chem. A, 2015, 3, 21664 RSC; (d) X. Li, Y. Hu, I. Sanchez-Molina, Y. Zhou, F. Yu, S. A. Haque, W. Wu, J. Hua, H. Tian and N. Robertson, J. Mater. Chem. A, 2015, 3, 2173 Search PubMed; (e) Y. Hu, A. Ivaturi, M. Planells, C. L. Boldrini, A. O. Biroli and N. Robertson, J. Mater. Chem. A, 2016, 4, 2509 RSC; (f) H. Wu, L. Yang, Y. Li, M. Zhang, J. Zhang, Y. Guo and P. Wang, J. Mater. Chem. A, 2016, 4, 519 RSC; (g) J. Wu, G. Li, L. Zhang, G. Zhou and Z.-S. Wang, J. Mater. Chem. A, 2016, 4, 3342 RSC; (h) J.-S. Ni, Y.-C. Yen and J. T. Lin, J. Mater. Chem. A, 2016, 4, 6553 RSC; (i) A. J. Huckaba, A. Yella, P. Brogdon, J. S. Murphy, M. K. Nazeeruddin, M. Grätzel and J. H. Delcamp, Chem. Commun., 2016, 52, 8424 RSC; (j) T.-Y. Li, C. Su, S. B. Akula, W.-G. Sun, H.-M. Chien and W.-R. Li, Org. Lett., 2016, 18, 3386 CrossRef CAS PubMed; (k) Y. Gao, X. Li, Y. Hu, Y. Fan, J. Yuan, N. Robertson, J. Hua and S. R. Marder, J. Mater. Chem. A, 2016, 4, 12865 RSC.
- (a) Z. Yao, M. Zhang, H. Wu, L. Yang, R. Li and P. Wang, J. Am. Chem. Soc., 2015, 137, 3799 CrossRef CAS PubMed; (b) N. Zhou, K. Prabakaran, B. Lee, S. H. Chang, B. Harutyunyan, P. Guo, M. R. Butler, A. Timalsina, M. J. Bedzyk, M. A. Ratner, S. Vegiraju, S. Yau, C.-G. Wu, R. P. H. Chang, A. Facchetti, M.-C. Chen and T. J. Marks, J. Am. Chem. Soc., 2015, 137, 4414 CrossRef CAS PubMed; (c) Y.-S. Yen, J.-S. Ni, T.-Y. Lin, W.-I. Hung, J. T. Lin and M.-C. P. Yeh, Eur. J. Org. Chem., 2015, 7367 CrossRef CAS; (d) H. Jiang, G. Ferrara, X. Zhang, K. Oniwa, A. Islam, L. Han, Y.-J. Sun, M. Bao, N. Asao, Y. Yamamoto and T. Jin, Chem. Eur. J., 2015, 21, 4065 CrossRef CAS PubMed; (e) K. Matsumura, S. Yoshizaki, M. M. Maitani, Y. Wada, Y. Ogomi, S. Hayase, T. Kaiho, S. Fuse, H. Tanaka and T. Takahashi, Chem. Eur. J., 2015, 21, 9742 CrossRef CAS PubMed; (f) K. D. Seo, I. T. Choi and H. K. Kim, Chem. Eur. J., 2015, 21, 14804 CrossRef CAS PubMed; (g) B. Liu, F. Giordano, K. Pei, J.-D. Decoppet, W.-H. Zhu, S. M. Zakeeruddin and M. Grätzel, Chem. Eur. J., 2015, 21, 18654 CrossRef CAS PubMed; (h) Z. Yao, M. Zhang, R. Li, L. Yang, Y. Qiao and P. Wang, Angew. Chem. Int. Ed., 2015, 54, 5994 CrossRef CAS PubMed; (i) P. Brogdon, F. Giordano, G. A. Puneky, A. Dass, S. M. Zakeeruddin, M. K. Nazeeruddin, M. Grätzel, G. S. Tschumper and J. H. Delcamp, Chem. Eur. J., 2016, 22, 694 CrossRef CAS PubMed; (j) S. Irie, S. Fuse, M. M. Maitani, Y. Wada, Y. Ogomi, S. Hayase, T. Kaiho, H. Masui, H. Tanaka and T. Takahashi, Chem. Eur. J., 2016, 22, 2507 CrossRef CAS PubMed; (k) F.-L. Guo, Z.-Q. Li, X.-P. Liu, L. Zhou, F.-T. Kong, W.-C. Chen and S.-Y. Dai, Adv. Funct. Mater., 2016, 26, 5733 CrossRef CAS.
- J. Tang, S. Qu, J. Hu, W. Wu and J. Hua, Sol. Energy, 2012, 86, 2306 CrossRef CAS.
- Y. Ooyama, Y. Hagiwara, Y. Oda, T. Mizumo, Y. Harima and J. Ohshita, New J. Chem., 2013, 37, 2336 RSC.
- J. Cong, X. Yang, J. Liu, J. Zhao, Y. Hao, Y. Wang and L. Sun, Chem. Commun., 2012, 48, 6663 RSC.
- J. Mao, N. He, Z. Ning, Q. Zhang, F. Guo, L. Chen, W. Wu, J. Hua and H. Tian, Angew. Chem. Int. Ed., 2012, 51, 9873 CrossRef CAS PubMed.
- (a) Y. Ooyama, S. Inoue, R. Asada, G. Ito, K. Kushimoto, K. Komaguchi, I. Imae and Y. Harima, Eur. J. Org. Chem., 2010, 92 CrossRef CAS; (b) Y. Ooyama, S. Inoue, T. Nagano, K. Kushimoto, J. Ohshita, I. Imae, K. Komaguchi and Y. Harima, Angew. Chem. Int. Ed., 2011, 50, 7429 CrossRef CAS PubMed; (c) Y. Ooyama, T. Nagano, S. Inoue, I. Imae, K. Komaguchi, J. Ohshita and Y. Harima, Chem. – Eur. J., 2011, 17, 14837 CrossRef CAS PubMed; (d) Y. Ooyama, N. Yamaguchi, I. Imae, K. Komaguchi, J. Ohshita and Y. Harima, Chem. Commun., 2013, 49, 2548 RSC; (e) Y. Ooyama, Y. Hagiwara, T. Mizumo, Y. Harima and J. Ohshita, New J. Chem., 2013, 37, 2479 RSC; (f) Y. Ooyama, T. Sato, Y. Harima and J. Ohshita, J. Mater. Chem. A, 2014, 2, 3293 RSC; (g) Y. Ooyama, K. Uenaka and J. Ohshita, Eur. J. Org. Chem., 2015, 3713 CrossRef CAS; (h) Y. Ooyama, K. Uenaka, T. Kamimura, S. Ozako, M. Kanda, T. Koide and F. Tani, RSC Adv., 2016, 6, 16150 RSC.
- (a) Y. Harima, T. Fujita, Y. Kano, I. Imae, K. Komaguchi, Y. Ooyama and J. Ohshita, J. Phys. Chem. C, 2013, 117, 16364 CrossRef CAS; (b) N. Shibayama, H. Ozawa, M. Abe, Y. Ooyama and H. Arakawa, Chem. Commun., 2014, 50, 6398 RSC; (c) Y. Ooyama, K. Uenaka, T. Sato, N. Shibayama and J. Ohshita, RSC Adv., 2015, 5, 2531 RSC; (d) J. Ohshita, Y. Adachi, D. tanaka, M. Nakashima and Y. Ooyama, RSC Adv., 2015, 5, 36673 RSC; (e) Y. Harima, Y. Kano, T. Fujita, I. Imae, Y. Ooyama and J. Ohshita, RSC Adv., 2015, 5, 71387 RSC; (f) Y. Adachi, Y. Ooyama, N. Shibayama and J. Ohshita, Dalton Trans., 2016, 45, 13817 RSC.
- (a) D. Daphnomili, G. D. Sharma, S. Biswas, T. K. R. Justin and A. G. Goutsolelos, J. Photochem. Photobiol. A, 2013, 253, 88 CrossRef CAS; (b) J. Lu, X. Xu, Z. Li, k. Cao, J. Cui, Y. Zhang, Y. Shen, Y. Li, J. Zhu, S. Dai, W. Chjen, Y. Cheng and M. Wang, Chem.–Asian J., 2013, 8, 956 CrossRef CAS PubMed; (c) M.-D. Zhang, H.-X. Xie, X.-H. Ju, L. Qin, Q.-X. Yang, H.-G. Zheng and X.-F. Zhou, Phys. Chem. Chem. Phys., 2013, 15, 634–641 RSC; (d) D. Daphnomili, G. Landrou, P. Singh, A. Thomas, K. Yesudas, B. K. G. D. Sharma and A. G. Goutsolelos, RSC Adv., 2012, 2, 12899 RSC; (e) L. Wang, X. yang, S. Li, M. Cheng and L. Sun, RSC Adv., 2013, 3, 13677 RSC; (f) T. Sakurada, Y. Arai and H. Segawa, RSC Adv., 2014, 4, 13201 RSC; (g) J. Mao, D. Wang, S.-H. Liu, Y. Hang, Y. Xu, Q. Zhang, W. Wu, P.-T. Chou and J. Hua, Asian J. Org. Chem., 2014, 3, 153 CrossRef CAS; (h) T. Ikeuchi, S. Agrawal, M. Ezoe, S. Mori and M. Kimura, Chem. Asian J., 2015, 10, 2347 CrossRef CAS PubMed.
- (a) C. Stangel, A. Bagaki, P. A. Angaridis, G. Charalambidis, G. D. Sharma and A. G. Coutsolelos, Inorg. Chem., 2014, 53, 11871 CrossRef CAS PubMed; (b) M. N. K. P. Bolisetty, C.-T. Li, K. R. J. Thomas, G. B. Bodedla and K.-C. Ho, Tetrahedron, 2014, 70, 4203 Search PubMed; (c) D. Franchi, M. Calamante, G. Reginato, L. Zani, M. Peruzzini, M. Taddei, F. F. de Biani, R. Basosi, A. Sinicropi, D. Colonna, A. Di Carlo and A. Mordin, Tetrahedron, 2014, 70, 6285 CrossRef CAS; (d) E. V. Verbitskiy, E. M. Cheprakova, J. O. Subbotina, A. V. Schepochkin, P. A. Slepukhin, G. L. Rusinov, V. N. Charushin, O. N. Chupakhin, N. I. Makarova, A. V. Metelitsa and V. I. Minkin, Dyes Pigm., 2014, 100, 201 CrossRef CAS; (e) C.-L. Mai, T. Moehl, C.-H. Hsieh, J.-D. Décoppet, S. M. Zakeeruddin, M. Grätzel and C.-Y. Yeh, ACS Appl. Mater. Interfaces, 2015, 7, 14975 CrossRef CAS PubMed; (f) H. Jia, K. Shen, X. I. Ju, M. Zhanga and H. Zheng, New J. Chem., 2016, 40, 2799 RSC; (g) P. A. Angaridis, E. Ferentinos, G. Charalambidis, K. Ladomenou, V. Nikolaou, S. Biswas, G. D. Sharma and A. G. Coutsolelos, RSC Adv., 2016, 6, 22187 RSC; (h) U. Meinhardt, F. Lodermeyer, T. A. Schaub, A. Kunzmann, P. O. Dral, A. C. Sale, F. Hampel, D. M. Guldi, R. D. Costa and M. Kivala, RSC Adv., 2016, 6, 67372 RSC.
- H. He, A. Gurung and L. Si, Chem. Commun., 2012, 48, 5910 RSC.
- (a) A. Fillinger and B. A. Parkinson, J. Electrochem. Soc., 1999, 146, 4559 CrossRef CAS; (b) M. K. Nazeeruddin, P. Péchy, T. Renouard, S. M. Zakeeruddin, R. Humphry-Baker, P. Comte, P. Liska, L. Cevey, E. Costa, V. Shklover, L. Spiccia, G. B. Deacon, C. A. Bignozzi and M. Grätzel, J. Am. Chem. Soc., 2001, 123, 1613 CrossRef CAS PubMed; (c) S. Ushiroda, N. Ruzycki, Y. Lu, M. T. Spitler and B. A. Parkinson, J. Am. Chem. Soc., 2005, 127, 5158 CrossRef CAS PubMed; (d) K.-J. Hwang, W.-G. Shim, S.-H. Jung, S.-J. Yoo and J.-W. Lee, Appl. Surf. Sci., 2010, 256, 5428 CrossRef CAS; (e) C. Y. Lee, C. She, N. C. Jeong and J. T. Hupp, Chem. Commun., 2010, 46, 6090 RSC; (f) Y. Sakuragi, X.-F. Wang, H. Miura, M. Matsui and T. Yoshida, J. Photochem. Photobiol., A, 2010, 216, 1 CrossRef CAS; (g) Y. Hara, M. I. Tejedor-Tejedor, K. Lara, D. Lubin, L. J. Brzozowski, D. J. Severseike and M. A. Anderson, Electrochim. Acta, 2011, 56, 8873 CrossRef CAS.
| This journal is © the Owner Societies 2016 |