Neural network molecular dynamics simulations of solid–liquid interfaces: water at low-index copper surfaces†
Abstract
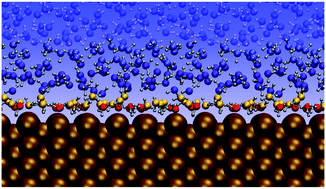
Solid–liquid interfaces have received considerable attention in recent years due to their central role in many technologically relevant fields like electrochemistry, heterogeneous catalysis and corrosion. As the chemical processes in these examples take place primarily at the interface, understanding the structural and dynamical properties of the interfacial water molecules is of vital importance. Here, we use a first-principles quality high-dimensional neural network potential built from dispersion-corrected density functional theory data in molecular dynamics simulations to investigate water–copper interfaces as a prototypical case. After performing convergence tests concerning the required supercell size and water film diameter, we investigate numerous properties of the interfacial water molecules at the low-index copper (111), (100) and (110) surfaces. These include density profiles, hydrogen bond properties, lateral mean squared displacements and residence times of the water molecules at the surface. We find that in general the copper–water interaction is rather weak with the strongest interactions observed at the Cu(110) surface, followed by the Cu(100) and Cu(111) surfaces. The distribution of the water molecules in the first hydration layer exhibits a double peak structure. In all cases, the molecules closest to the surface are predominantly allocated on top of the metal sites and are aligned nearly parallel with the oxygen pointing slightly to the surface. The more distant molecules in the first hydration layer at the Cu(111) and Cu(100) surfaces are mainly found in between the top sites, whereas at the Cu(110) surface most of these water molecules are found above the trenches of the close packed atom rows at the surface.


 Please wait while we load your content...
Please wait while we load your content...