Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A redox-active radical as an effective nanoelectronic component: stability and electrochemical tunnelling spectroscopy in ionic liquids†
Alexander V.
Rudnev
*ab,
Carlos
Franco
c,
Núria
Crivillers
*c,
Gonca
Seber
c,
Andrea
Droghetti
d,
Ivan
Rungger
e,
Ilya V.
Pobelov
a,
Jaume
Veciana
 c,
Marta
Mas-Torrent
*c and
Concepció
Rovira
*c
c,
Marta
Mas-Torrent
*c and
Concepció
Rovira
*c
aUniversity of Bern, Department of Chemistry and Biochemistry, Freiestrasse 3, 3012 Bern, Switzerland. E-mail: rudnev@dcb.unibe.ch
bRussian Academy of Sciences A.N. Frumkin Institute of Physical Chemistry and Electrochemistry RAS, Leninskii pr. 31, Moscow, 119991, Russia
cDepartment of Molecular Nanoscience and Organic Materials, Institut de Ciència de Materials de Barcelona (ICMAB-CSIC) and CIBER-BBN, Campus la Universitat Autonoma Barcelona (UAB), 08193 Bellaterra, Spain. E-mail: ncrivillers@icmab.es; mmas@icmab.es; cun@icmab.es
dNano-Bio Spectroscopy Group and European Theoretical Spectroscopy Facility (ETSF), Universidad del Pais Vasco CFM, CSIC-UPV/EHU-MPC & DIPC, Avenida Tolosa 72, 20018 San Sebastian, Spain
eMaterials Division, National Physical Laboratory, Teddington, TW11 0LW, UK
First published on 26th September 2016
Abstract
A redox-active persistent perchlorotriphenylmethyl (PTM) radical chemically linked to gold exhibits stable electrochemical activity in ionic liquids. Electrochemical tunnelling spectroscopy in this medium demonstrates that the PTM radical shows a highly effective redox-mediated current enhancement, demonstrating its applicability as an active nanometer-scale electronic component.
Redox-active molecules have been shown to be promising active components for developing nanometer-scale electronic devices1–3 capable of acting as current rectifiers4 and switches,5–7 mechanical switches8,9 or active components in charge-storage devices.10 In order to progress in this field, the control of the molecular redox state as well as the understanding of its influence on the charge transport properties is crucial. Experiments on electrochemically-gated charge transport across redox-active molecular junctions have demonstrated an effective current modulation.11,12 In these systems, the Marcus-type reorganization energy strongly influences the efficiency of the current enhancement due to redox mediated electron tunnelling.
Organic radical perchlorotriphenylmethyl (PTM) is persistent and stable, both chemically and thermally, due to the steric hindrance at the central carbon atom caused by the ortho-chlorine atoms of the phenyl rings (Fig. 1a).13 Importantly, PTM radicals display a low reduction potential to the corresponding PTM anions,14 which makes them very interesting molecular redox switches.15–17 The charge transport through self-assembled monolayers18,19 and single-molecule junctions20 incorporating PTM radicals have been studied before, but the different redox states (radical and anion) were not addressed. Since the structure of both components of the redox pair is almost identical,21 the inner reorganization energy associated with the structural change upon reduction/oxidation is expected to be low. In addition, bulky redox systems are expected to show low outer-sphere reorganization energy associated with the surrounding solvent, and indeed their current enhancement efficiency in redox-active molecular junctions is typically high.22–24 In view of this, the bulky organic radical perchlorotriphenylmethyl (PTM) is expected to be a very good redox mediator.
 | ||
| Fig. 1 (a) Schematics of PTM-C8-SH adsorbed on Au(111) and (b) EPR spectrum of PTM-C8-SH SAM on Au(111) recorded at 300 K under ambient conditions. | ||
Here, the electrochemical gating of the charge transport through a surface-bound PTM radical has been explored using an electrochemical scanning tunnelling spectroscopy (EC-STS) technique. We demonstrated current enhancement due to the redox-mediated electron tunnelling (RMET) mechanism23–41 involving the PTM radical as the active redox-centre. Our results show that, in comparison with other redox-active moieties, the PTM radical is among the most efficient redox mediators in RMET, demonstrating its applicability as an active electronic component in electronic devices of nanoscale size.
A PTM radical bearing an alkenyl chain spacer with a thiol anchoring group (PTM-C8-SH) was synthesised as described previously18 and used to prepare self-assembled monolayers (SAMs) on Au(111) (Fig. 1a). Fig. 1a shows the schematics of PTM-C8-SH assembled on Au(111) (see the ESI† for the characterization and assembly details). The Electron Paramagnetic Resonance spectrum of the SAM on Au(111) recorded at 300 K exhibited a signal with a g value of 2.0032 and a line width of 2.6 Gauss (Fig. 1b), which shows that the PTM radical character is preserved once the molecule is immobilized on the gold surface. For the purpose of comparison, the electrochemically inactive αH-PTM-C8-SH (ESI,† Scheme S1) was isolated during the synthesis of the radical derivative and used to perform reference voltammetric and STS measurements.
Cyclic voltammograms (CVs) of PTM-C8-SH SAM on Au(111) in acetonitrile solution (ESI,† Fig. S1) showed the reduction and oxidation peaks assigned to the reversible reduction of the PTM radical moieties (PTM˙ ⇌ PTM−). The redox response was stable during a repetitive potential cycling and the linear dependence of the peak heights on the potential scan rate indicates the oxidation/reduction of a surface-confined species (ESI,† Fig. S1). However, organic solvents are not suitable for electrochemical STM/STS experiments due to their high volatility and/or insufficient stability of coatings employed to electrochemically insulate the STM tip. The PTM anion can be readily protonated in an acidic aqueous medium. Hence ionic liquids (ILs) appear to be the best media for EC-STM/STS measurements in our case.11,22
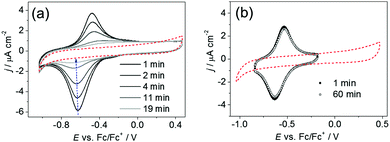
According to previous works, drying ILs by using molecular sieves (3 Å) may significantly decrease the water content.42,43 For this, hot molecular sieves activated at 200 °C were used to dry ILs before each experiment and they were also put into the electrochemical cell. The CVs of PTM-C8-SH on Au(111) were acquired using different ILs as electrolytes (ESI,† Table S1). A decrease of the redox signal with time (Fig. 2a) was observed and after 20 cycles (20 min) the CV response indicated that the radical SAM became redox-inactive (dashed curve). However, the capacitance current did not change and remained much smaller than for the bare Au(111) (ESI,† Fig. S2a), demonstrating that the desorption of the monolayer does not take place. Fig. S2b (ESI†) shows a representative EC-STM image of PTM-C8-SH on Au(111) in [Emim][EtSO4] recorded after the disappearance of the redox peaks. As illustrated by a cross-section (Fig. S2c, ESI†), the STM image shows a rather uniform corrugation height of ∼0.8 nm, which confirms unambiguously the presence of the adlayer. The above-mentioned results are in accordance with the well-known acidic character of the imidazolium cation44 (see ESI† for a more detailed explanation), which could promote the protonation of the electrochemically generated anion forming the non-electroactive αH-PTM-C8-SH SAM on Au(111) (ESI,† Scheme S1). In order to corroborate it, a solution of the non-functionalized PTM anion (PTM− K+[18-Crown-6])45 in [Emim][EtSO4] was monitored using UV-Vis spectroscopy. The characteristic band of the PTM− at 500 nm was observed to decrease gradually with time (ESI,† Fig. S3a), and completely disappeared after 24 h, confirming the protonation of the PTM anion in the IL, even after exhaustive drying. The same CV experiment was performed with the PTM SAM using [SEt3][Tf2N] in which, although a higher stability of the layer was observed, the redox peaks also diminished with time. We believe that small traces of water in the ionic medium could also induce the protonation of the PTM anion.
In order to overcome this problem, a soft base like triethylamine (Et3N) was added to the IL (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 v/v). According to the voltammetric and UV-Vis measurements, its presence greatly improved the stability of the PTM anion in the IL media. The CVs of PTM-C8-SH on Au(111) did not change significantly after 1 h of potential cycling (Fig. 2b) and the absorption band of PTM− remained unaltered after 24 hours (ESI,† Fig. S3b). We notice that the redox peaks of PTM-C8-SH on Au(111) in the ILs have a larger peak-to-peak separation as compared to those in acetonitrile (Fig. S1, ESI†). We attribute such a behaviour to the slow ion pairing of the PTM anion with the cations of the ILs.46 Moreover, peak-to-peak separation differs for different ionic liquids, also suggesting that the shape of the voltammograms is affected by the IL–cation and PTM anion interactions (Fig. 2 and Fig. S4, S5, ESI†).
15 v/v). According to the voltammetric and UV-Vis measurements, its presence greatly improved the stability of the PTM anion in the IL media. The CVs of PTM-C8-SH on Au(111) did not change significantly after 1 h of potential cycling (Fig. 2b) and the absorption band of PTM− remained unaltered after 24 hours (ESI,† Fig. S3b). We notice that the redox peaks of PTM-C8-SH on Au(111) in the ILs have a larger peak-to-peak separation as compared to those in acetonitrile (Fig. S1, ESI†). We attribute such a behaviour to the slow ion pairing of the PTM anion with the cations of the ILs.46 Moreover, peak-to-peak separation differs for different ionic liquids, also suggesting that the shape of the voltammograms is affected by the IL–cation and PTM anion interactions (Fig. 2 and Fig. S4, S5, ESI†).
Fig. 3 shows the CV of PTM-C8-SH SAM on Au(111) in [SEt3][Tf2N] + Et3N (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) recorded in the STM cell (Fig. 3a) and a representative EC-STM image (Fig. 3b). The redox response was stable and nearly identical to that obtained in the standard glass electrochemical cell. As expected, a highly ordered monolayer was not observed due to the bulkiness of the PTM moiety, which hinders the formation of a well-arranged molecular layer induced by the lateral interactions between the alkyl chains. However, the STM image demonstrates the formation of a compact adlayer (see also Fig. S6 in the ESI†). The coverage estimated from the integration of the CV peaks (with background current correction) was 1.2 × 10−10 mol cm−2, which is ≈70% of the theoretical maximum coverage calculated from the area of a single PTM-C8-S-Au (see the ESI† for details).
1 v/v) recorded in the STM cell (Fig. 3a) and a representative EC-STM image (Fig. 3b). The redox response was stable and nearly identical to that obtained in the standard glass electrochemical cell. As expected, a highly ordered monolayer was not observed due to the bulkiness of the PTM moiety, which hinders the formation of a well-arranged molecular layer induced by the lateral interactions between the alkyl chains. However, the STM image demonstrates the formation of a compact adlayer (see also Fig. S6 in the ESI†). The coverage estimated from the integration of the CV peaks (with background current correction) was 1.2 × 10−10 mol cm−2, which is ≈70% of the theoretical maximum coverage calculated from the area of a single PTM-C8-S-Au (see the ESI† for details).
After the radical monolayer was assembled on Au(111) and confirmed to be stable, electrochemical STS measurements in the constant bias mode were performed.23 In this mode, the potentials of both working electrodes, i.e. substrate ES and tip ET, were swept simultaneously while keeping the bias voltage Ebias = ET − ES constant. The STS curves (Itvs. ES) were recorded at a fixed geometry of the tunnelling junction with the feedback being temporarily switched off during the acquisition of each STS curve. The results obtained for PTM-C8-SH on Au(111) are shown in Fig. 4a, in which a significant enhancement of the tunnelling current flowing between the substrate and the tip is observed. As expected, the control STS experiment with the redox-inactive αH-PTM-C8-SH monolayer showed a constant current without tunnelling enhancement (Fig. 4b).
 | ||
| Fig. 4 2D occurrence histograms constructed from (a) 200 and (b) 100 constant bias current–voltage traces for SAM on Au(111): (a) PTM-C8-SH radical and (b) αH-PTM-C8-SH. Set-point current ISetPoint = 50 pA, Ebias = 0.1 V (constant), starting ES = −0.81 V and ET = −0.71 V. No data selection was applied. (c) Master current–voltage curves obtained from the data in panels (a) and (b): for the radical (squares) and αH-PTM (dashed line) forms. The solid green line represents fitting using the KU model (equations and fitting parameters are given in the ESI†). (d) Schematic energy level diagram of a two-step ET process mediated by a redox-active radical molecule. | ||
Fig. 4c shows the master curve obtained from the histogram where each data point corresponds to the value of tunnelling current measured with the highest probability at a given potential value. The curve has a bell shape with a maximum located close to the equilibrium potential of the PTM˙/PTM− redox pair (cf. the CV in Fig. 3a). Such behaviour resembles a transistor like functionality. With the initial value ISetPoint of 0.05 nA at ES = −0.80 V, the tunnelling current increases to ∼0.65 nA at the peak position, which corresponds to an on/off ratio of ∼13. The bell-shaped STS curves are typical for redox-mediated electron tunnelling (RMET) processes, which have been reported for a variety of redox-active molecules placed between the conductive substrate and the STM tip,22–24,26–32 as well as for single molecule junctions.11,47 However, it is the first time that a stable neutral free-radical is shown to mediate the tunnelling current enhancement under electrochemical conditions.
Similar to previous studies, the RMET phenomenon is rationalized by using the Kuznetsov–Ulstrup (KU) model,28i.e., a two-step electron transfer with a partial vibrational relaxation. When the sample and the tip potentials are located before or after the redox peak, the Fermi levels of the two electrodes are lower and higher than the single-unoccupied molecular orbital (SUMO) of the PTM radical (Fig. 4d). In the first electron transfer step, the electron transfers from the electrode with a higher Fermi level to the SUMO. Afterwards, the energy of the now occupied SUMO* decreases due to vibrational relaxation. However, the second electron transfer occurs before a complete vibrational relaxation and before the energy of the molecular level passes the Fermi level of the second electrode. These electron-transfer steps are repeated causing a multitude of electrons to be transferred between the tip and the sample through this pathway, which is additional to the direct (off-resonant) tip-substrate current. Thus, the tunnelling current through the junction can be modulated by tuning the potential of both electrodes around the equilibrium potential of the PTM˙/PTM− redox pair.
By fitting the experimental data (Fig. 4c) using the KU model the following model parameter values were obtained: total (inner- + outer-sphere) reorganization energy λT = 0.39 eV, fractions of overpotential and bias voltage at the redox site ξ = 0.57 and γ = 0.57, respectively (see the ESI†). These values allow us to evaluate the efficiency of the PTM radical as a mediator compared to those reported in the literature. It should be kept in mind that the direct comparison of the fitted KU parameters between different redox systems is not straightforward since not all mediators are measured at the same fixed junction geometry in the STS experiments. Practically, this is hardly possible in an STM configuration and it would require, for example, systematic measurements of a series of nanodevices (with different mediators) with fixed electrode separation and molecular assembly (see discussion in ref. 25). Instead, the STS experiments are typically carried out at fixed starting Ebias and ISetPoint (or tunnelling conductance ISetPoint/Ebias) which, via the STM feedback mechanism, is expected to result in a reproducible junction geometry for the same “gap composition”. Indeed, the established electrode separation will depend on the tunnelling efficiency through the gap in the absence of RMET (when the potentials of both electrodes are far from the equilibrium potential). Thus, diverse results attributed to a different gap geometry were obtained even for the same mediator in different starting redox states.23
Judging solely on the basis of the on/off ratio, the most effective mediators studied appear to be the Os bisterpyridine complex (Imax/ISetPoint ≈ 50).22 This result was obtained in an ionic liquid using Ebias = 0.7 V and ISetPoint = 0.05 nA, which is also the lowest tunnelling conductance used in EC-STS experiments. We cannot safely name this system as the most efficient mediator, as the on/off ratio tends to increase at lower tunnelling conductances.30 Formally, Imax/ISetPoint ≈ 50 was also obtained for viologens in one starting configuration (Ebias = 0.05 V, ISetPoint = 0.1 nA),23 but the off-resonance current was asymmetric indicating the contribution of non-RMET enhancement. Among the results obtained at spontaneously established standard settings for STS experiments, Ebias = 0.1 V and ISetPoint = 0.1 nA, the highest on/off ratios were obtained for perylene bisimide24 (Imax/ISetPoint ≈ 35) and Fc(CO)(CH2)7SH29 (Imax/ISetPoint ≈ 12). To the best of our knowledge, a ratio Imax/ISetPoint > 10 was not obtained for other mediators in either symmetric (covalently bonded to both contacts)11,47 or asymmetric (with a tunnelling gap)25,29,30,39 metal–molecule–metal junctions.
From the point of view of the KU model, an effective RMET mediator is characterized by a low total reorganization energy (λ), which depends on tunnelling resistance and therefore on electrode separation.30 For example, in systems where λ was estimated from the fitting of the STS results, the lowest values obtained were approximately 0.2 eV for mediators such as perylene bisimide19 and λ ≈ 0.35–0.4 eV for mediators such as the herein reported PTM and Fc(CO)(CH2)7SH.29 Interestingly, perylene bisimide and Os bisterpyridine complexes are bulky structures, which is expected to decrease the outer-sphere reorganization energy associated with the surrounding solvent, leading to a higher Imax/ISetPoint.
To get further information about the system presented in this work, the PTM inner-sphere reorganization energy (λi) has been calculated using Density Functional Theory by finite energy differences48 giving a λi of 0.08 eV (see the ESI† for further details). This value is comparable to λi calculated for perylene bisimide (0.10 eV, see the ESI†), but significantly higher than λi for ferrocene (∼0.005 eV, as estimated from the spectroscopic data49). Nevertheless, with the on/off ratio 13 and estimated λ = 0.39 eV, the PTM radical is one of the best reported redox mediators in the RMET mechanism. As expected, the outer-sphere reorganization energy (estimated from the difference between the calculated λi and the experimental λT) has a higher influence on the results.
In summary, in this work, we have prepared an organic radical based SAM on Au(111) exhibiting a reversible and stable redox signal in ionic liquids. Electrochemical scanning tunneling spectroscopy experiments demonstrated current enhancement due to the redox-mediated electron tunnelling mechanism. In comparison with other redox mediators studied to date, the PTM radical is among the most efficient ones, which indicates its potential applicability as an active electronic component in nanoelectronic devices.
We acknowledge the financial support from the EU projects ACMOL (FET Young Explorers, GA no. 618082), ERC StG 2012-306826 e-GAMES, ITN iSwitch (GA no. 642196), COST Action TD1002, the Swiss National Science Foundation (Grant No. 200020-144471), the Networking Research Center of Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN), the DGI (Spain) with project BE-WELL CTQ2013-40480-R, the Generalitat de Catalunya with project 2014-SGR-17, and the Severo Ochoa program. N. C acknowledges the RyC program. C. F. is enrolled in the Materials Science PhD program of UAB. We acknowledge support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).
Notes and references
- L. Sun, Y. A. Diaz-Fernandez, T. A. Gschneidtner, F. Westerlund, S. Lara-Avila and K. Moth-Poulsen, Chem. Soc. Rev., 2014, 43, 7378 RSC.
- S. Guo, J. M. Artés and I. Díez-Pérez, Electrochim. Acta, 2013, 110, 741 CrossRef CAS.
- Nat. Nanotechnol., 2013, 8, 377.
- C. A. Nijhuis, W. F. Reus and G. M. Whitesides, J. Am. Chem. Soc., 2010, 132, 18386 CrossRef CAS PubMed.
- M. Baghernejad, X. Zhao, K. Baruël Ørnsø, M. Füeg, P. Moreno-García, A. V. Rudnev, V. Kaliginedi, S. Vesztergom, C. Huang, W. Hong, P. Broekmann, T. Wandlowski, K. S. Thygesen and M. R. Bryce, J. Am. Chem. Soc., 2014, 136, 17922 CrossRef CAS PubMed.
- N. Darwish, I. Díez-Pérez, P. Da Silva, N. Tao, J. J. Gooding and M. N. Paddon-Row, Angew. Chem., Int. Ed., 2012, 51, 3203 CrossRef CAS PubMed.
- X. Xiao, L. A. Nagahara, A. M. Rawlett and N. Tao, J. Am. Chem. Soc., 2005, 127, 9235 CrossRef CAS PubMed.
- T. Avellini, H. Li, A. Coskun, G. Barin, A. Trabolsi, A. N. Basuray, S. K. Dey, A. Credi, S. Silvi, J. F. Stoddart and M. Venturi, Angew. Chem., Int. Ed., 2012, 51, 1611 CrossRef CAS PubMed.
- V. Kolivoska, M. Mohos, I. V. Pobelov, S. Rohrbach, K. Yoshida, W. J. Hong, Y. C. Fu, P. Moreno-Garcia, G. Meszaros, P. Broekmann, M. Hromadova, R. Sokolova, M. Valasek and T. Wandlowski, Chem. Commun., 2014, 50, 11757 RSC.
- V. Kaliginedi, H. Ozawa, A. Kuzume, S. Maharajan, I. V. Pobelov, N. H. Kwon, M. Mohos, P. Broekmann, K. M. Fromm, M.-A. Haga and T. Wandlowski, Nanoscale, 2015, 7, 17685 RSC.
- H. M. Osorio, S. Catarelli, P. Cea, J. B. G. Gluyas, F. Hartl, S. J. Higgins, E. Leary, P. J. Low, S. Martín, R. J. Nichols, J. Tory, J. Ulstrup, A. Vezzoli, D. C. Milan and Q. Zeng, J. Am. Chem. Soc., 2015, 137, 14319 CrossRef CAS PubMed.
- C. Huang, A. V. Rudnev, W. Hong and T. Wandlowski, Chem. Soc. Rev., 2015, 44, 889 RSC.
- M. Mas-Torrent, N. Crivillers, C. Rovira and J. Veciana, Chem. Rev., 2012, 112, 2506 CrossRef CAS PubMed.
- N. Crivillers, M. Mas-Torrent, J. Vidal-Gancedo, J. Veciana and C. Rovira, J. Am. Chem. Soc., 2008, 130, 5499 CrossRef CAS PubMed.
- C. Simão, M. Mas-Torrent, N. Crivillers, V. Lloveras, J. M. Artés, P. Gorostiza, J. Veciana and C. Rovira, Nat. Chem., 2011, 3, 359 CrossRef PubMed.
- C. Simão, M. Mas-Torrent, J. Veciana and C. Rovira, Nano Lett., 2011, 11, 4382 CrossRef PubMed.
- N. Crivillers, M. Paradinas, M. Mas-Torrent, S. T. Bromley, C. Rovira, C. Ocal and J. Veciana, Chem. Commun., 2011, 47, 4664 RSC.
- L. Yuan, C. Franco, N. Crivillers, M. Mas-Torrent, L. Cao, C. S. S. Sangeeth, C. Rovira, J. Veciana and C. A. Nijhuis, Nat. Commun., 2016, 7, 12066 CrossRef CAS PubMed.
- N. Crivillers, C. Munuera, M. Mas-Torrent, C. Simão, S. T. Bromley, C. Ocal, C. Rovira and J. Veciana, Adv. Mater., 2009, 21, 1177 CrossRef CAS.
- R. Frisenda, R. Gaudenzi, C. Franco, M. Mas-Torrent, C. Rovira, J. Veciana, I. Alcon, S. T. Bromley, E. Burzurí and H. S. J. van der Zant, Nano Lett., 2015, 15, 3109 CrossRef CAS PubMed.
- J. Guasch, X. Fontrodona, I. Ratera, C. Rovira and J. Veciana, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2013, 69, 255 CAS.
- T. Albrecht, K. Moth-Poulsen, J. B. Christensen, J. Hjelm, T. Bjørnholm and J. Ulstrup, J. Am. Chem. Soc., 2006, 128, 6574 CrossRef CAS PubMed.
- I. V. Pobelov, Z. H. Li and T. Wandlowski, J. Am. Chem. Soc., 2008, 130, 16045 CrossRef CAS PubMed.
- C. Li, A. Mishchenko, Z. Li, I. Pobelov, T. Wandlowski, X. Q. Li, F. Würthner, A. Bagrets and F. Evers, J. Phys.: Condens. Matter, 2008, 20, 374122 CrossRef CAS PubMed.
- A. Alessandrini and P. Facci, Eur. Polym. J., 2016, 83, 450 CrossRef CAS.
- I. Díez-Pérez, Z. Li, S. Guo, C. Madden, H. Huang, Y. Che, X. Yang, L. Zang and N. Tao, ACS Nano, 2012, 6, 7044 CrossRef PubMed.
- A. Mishchenko, M. Abdualla, A. Rudnev, Y. Fu, A. R. Pike and T. Wandlowski, Chem. Commun., 2011, 47, 9807 RSC.
- J. Zhang, A. M. Kuznetsov, I. G. Medvedev, Q. Chi, T. Albrecht, P. S. Jensen and J. Ulstrup, Chem. Rev., 2008, 108, 2737 CrossRef CAS PubMed.
- A. V. Rudnev, I. V. Pobelov and T. Wandlowski, J. Electroanal. Chem., 2011, 660, 302 CrossRef CAS.
- Z. Li, Y. Liu, S. F. L. Mertens, I. V. Pobelov and T. Wandlowski, J. Am. Chem. Soc., 2010, 132, 8187 CrossRef CAS PubMed.
- P. Salvatore, A. Glargaard Hansen, K. Moth-Poulsen, T. Bjornholm, R. John Nichols and J. Ulstrup, Phys. Chem. Chem. Phys., 2011, 13, 14394 RSC.
- J. M. Artés, I. Díez-Pérez and P. Gorostiza, Nano Lett., 2012, 12, 2679 CrossRef PubMed.
- P. Petrangolini, A. Alessandrini and P. Facci, J. Phys. Chem. C, 2013, 117, 17451 CAS.
- A. Alessandrini, S. Corni and P. Facci, Phys. Chem. Chem. Phys., 2006, 8, 4383 RSC.
- N. J. Tao, Phys. Rev. Lett., 1996, 76, 4066 CrossRef CAS PubMed.
- X. Li, J. Hihath, F. Chen, T. Masuda, L. Zang and N. Tao, J. Am. Chem. Soc., 2007, 129, 11535 CrossRef CAS PubMed.
- T. Albrecht, K. Moth-Poulsen, J. B. Christensen, A. Guckian, T. Bjornholm, J. G. Vos and J. Ulstrup, Faraday Discuss., 2006, 131, 265 RSC.
- T. Albrecht, A. Guckian, J. Ulstrup and J. G. Vos, IEEE Trans. Nanotechnol., 2005, 4, 430 CrossRef.
- A. Alessandrini, M. Salerno, S. Frabboni and P. Facci, Appl. Phys. Lett., 2005, 86, 133902 CrossRef.
- R. J. Nichols and S. J. Higgins, Electrocatalysis, Wiley-VCH Verlag GmbH & Co. KGaA, 2013, p. 99 Search PubMed.
- J. M. Artés, M. López-Martínez, A. Giraudet, I. Díez-Pérez, F. Sanz and P. Gorostiza, J. Am. Chem. Soc., 2012, 134, 20218 CrossRef PubMed.
- M. Gnahm and D. M. Kolb, J. Electroanal. Chem., 2011, 651, 250 CrossRef CAS.
- M. F. Suárez-Herrera, M. Costa-Figueiredo and J. M. Feliu, Langmuir, 2012, 28, 5286 CrossRef PubMed.
- D. R. MacFarlane, J. M. Pringle, K. M. Johansson, S. A. Forsyth and M. Forsyth, Chem. Commun., 2006, 1905 RSC.
- J. Veciana, J. Riera, J. Castañer and N. Ferrer, J. Organomet. Chem., 1985, 297, 131 CrossRef CAS.
- S. F. L. Mertens, G. Meszaros and T. Wandlowski, Phys. Chem. Chem. Phys., 2010, 12, 5417 RSC.
- N. J. Kay, S. J. Higgins, J. O. Jeppesen, E. Leary, J. Lycoops, J. Ulstrup and R. J. Nichols, J. Am. Chem. Soc., 2012, 134, 16817 CrossRef CAS PubMed.
- S. F. Nelsen, S. C. Blackstock and Y. Kim, J. Am. Chem. Soc., 1987, 109, 677 CrossRef CAS.
- A. S. Baranski, K. Winkler and W. R. Fawcett, J. Electroanal. Chem. Interfacial Electrochem., 1991, 313, 367 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, additional results and figures. See DOI: 10.1039/c6cp05658j |
| This journal is © the Owner Societies 2016 |