Open Access Article
Open Access ArticleChiral recognition by fullerenes: CHFClBr enantiomers in the C82 cage†
Helena
Dodziuk
*a,
Kenneth
Ruud
b,
Tatiana
Korona
c and
Taye B.
Demissie
b
aInstitute of Physical Chemistry, Polish Academy of Sciences, ul. Kasprzaka 44/52, 01-264 Warsaw, Poland. E-mail: hdodziuk@gmail.com
bCentre for Theoretical and Computational Chemistry, Department of Chemistry, UiT The Arctic University of Norway, 9037 Tromsø, Norway
cFaculty of Chemistry, University of Warsaw, ul. Pasteura 1, 02-093 Warsaw, Poland
First published on 18th August 2016
Abstract
Density-functional theory and symmetry-adapted perturbation theory calculations on complexes of the enantiomers of CHFClBr with the most stable isomer of C82-3 fullerene show that despite the guests being too large for the host cage, they are nevertheless stabilized by electrostatic interactions. The complexation leads to considerable strain on the cage and the guests accompanied by compression of the bonds of the guest molecule, resulting in considerable complexation-induced changes in the infrared (IR), vibrational circular dichroism (VCD), nuclear magnetic resonance (NMR), and UV-vis spectra. The effect of chiral recognition is pronounced only for the 19F signal in the NMR spectra and in a sign reversal of the rotational strength of the νCH stretching vibration of S-CHFClBr@C82-3 in the VCD spectrum as compared to that of the free guest, making the sign of this band for the C82 complexes with the S- and R-guest enantiomers the same. This is a surprising result since vibrational circular dichroism is considered a reliable method for determining the absolute chirality of small molecules and for establishing dominant conformations in biopolymers.
1. Introduction
Fullerenes display fascinating electrochemical,1 magnetic,2 optical,3,4 and other properties.5–8 In the early fullerene days in the 1990s, the proposals of their applications abounded.9 They encompassed, among others, the use of doped C60 as a superconductor, perfluorinated C60F60 as the ideal lubricant and even C60 with a door and a guest drug inside (released at a suitable place and time into the organism through the opened door) as a drug carrier.10 Some fullerene applications in medicine have also been proposed.11,12 Nevertheless, with the exception of using C60 as an AFM tip,13 to our best knowledge no applications of pure endohedral fullerene complexes have been reported. The most exciting prospects for using fullerene derivatives (among which phenyl-C61-butyric acid methyl ester – PCBM – is the most popular) is in quantum computing14,15 and solar cells,16 whereas the applications proposed for use in hydrogen storage appear to be mostly misleading.17Most studies of endohedral fullerene complexes focus on metallic guest(s) inside the cage because of their availability and their interesting properties. These complexes are untypical salts since, upon inclusion, the guest becomes a cation, transferring its electron(s) to the host cage. Extending this notion to small polar guest molecules, their encapsulation offers a unique possibility of studying subtle effects on e.g. translational–rotational–vibrational motions caused by the reduced mobility of the small guest molecule, the lowering of the high cage symmetry of the host due to perturbations from the guest, or the interconversion of the ortho and para spin isomers of the guests. Such studies, performed mostly using IR, far-IR, NMR or inelastic neutron scattering spectroscopy, have been carried out for complexes such as H2@C6018–20 or H2O@C60,21,22 and were predicted theoretically for CO@C60.23 Although such studies do not lead to immediate applications, they are nonetheless enlightening from the theoretical point of view, allowing one to analyze the predictive power of the calculations for complexes where subtle interactions are dominant.
It should be emphasized that encapsulation of a guest inside a host molecule can lead to spectacular changes in the properties. Exciting examples are the creation of a stable cyclobutadiene inside a hemicarcerand cage synthesized by Cram and coworkers,24 the production of negative alkaline ions in alkalides,25 the stability of white phosphorus in air within a self-assembled capsule26 and an isolated nitrogen atom incarcerated inside the C60 fullerene cage.27 In all these cases, the properties of the guest molecule or ion dramatically change upon complexation.
Other molecular properties that can be affected by encapsulation arise from the chirality of the host and the guest. Chirality is an important property of molecules that are not superimposable on their mirror image28 and chiral recognition refers to the differentiation of these enantiomers by a third chiral agent. It is an important property since the majority of organic molecules, from which living creatures are built, are chiral. Moreover, drug chirality is an important issue for the pharmaceutical industry in view of different, sometimes dangerous, biological activity of drug enantiomers. Due to their high symmetry, C60 and C70 are achiral but higher fullerenes are usually a mixture of chiral and achiral isomers. The lowest stable fullerene having a pair of chiral isomers, C76, was resolved into enantiomers more than 20 years ago.29
The present study of chiral recognition by a fullerene cage was carried out as a continuation of our calculations on endohedral fullerene complexes.30–33 We have shown that C60 can host only one hydrogen molecule whereas for C70 we demonstrated the possible existence of both complexes with one or two H2 guests,34 the calculated energy difference between them, partly accidentally, was equal to a later determined experimental result.35 One of the present authors (HD) postulated as early as in 2001 that for applications of endohedral fullerene complexes, efficient synthetic and purification procedures for higher fullerenes are needed.30
Except the methyl halides involving hydrogen isotopes (CHDTX and CHDXY, etc., X, Y = halides), CHFClBr (1a/1b) is the smallest chiral molecule with a chiral center. Therefore, it was chosen as the guest for studying chiral recognition by fullerene cages. The molecule was separated into enantiomers36,37 and their absolute configuration was determined.38 Interestingly, 1a/1b has been considered to be of importance for studies of parity violation.39,40
Except for some chiral stationary phases involving cyclodextrins, 1a/1b has been found to be complexed only by a few cryptophanes capable of differentiating the two enantiomers.41,42 The 1H and 19F NMR signals in this molecule have been reported together with the spectra of the cryptophane complexes.
The smallest fullerene capable of forming complexes with 1a/1b is C82. This higher fullerene seems to be of importance in view of the ease of formation of its complexes with metallic guests,43 anticancer activity of its polyol derivative with a gadolinium guest44,45 and other applications of the endohedral complexes in medicine.46 Of interest are also a C82 complex with an unusual guest structure,47 a non-IPR (non-Isolated Pentagon Rule) isomer of the endohedral complex involving C82,48 and the so-called peapods – that is, nanotubes with Ga@C82 inside.49–53
Of the 9 IPR isomers of C82, only three (isomers 1, 3, and 5 of C2 symmetry; see The Atlas of Fullerenes54 for the nomenclatures) are chiral (Fig. 1).54 Kikuchi et al. showed that three isomers of C2, C2v and C3v symmetry are formed in the C82 manufacturing, with the first one prevailing with more than 80% abundance.55 The most stable isomer 3 of C2 symmetry was isolated and its structure determined mainly on the basis of the comparison between the measured and calculated NMR spectra.56,57 Marcelli et al.58 synthesized C2 and C2v isomers of C82 and characterized them. Some endohedral metallofullerenes in which there is a strong interaction between the host cage and the guest were shown to have C2v symmetry, in which the cage corresponded to the chiral isomer 9.58,59 Several calculations for C82 were carried out, all showing that isomer 3 has the lowest energy.57,60,61 Khamatgalimov and Kovalenko carried out calculations for all 9 isomers by DFT using the B3LYP functional and the 6-31G, 6-31G*, and 6-31+G* basis sets60 and found isomer 3 to be the most stable for all basis sets used. However, contrary to the experimental finding,55 the energy of all isomers of C2 symmetry was calculated to be lower than those of other symmetries. Gao and coworkers62 calculated UPS, NEXAFS, XPS, and RIXS spectra for isomers 3, 6 and 9 of C2, Cs and C2v symmetry, respectively. They found a strong dependence of the spectra on the isomers and claimed agreement with experimental data. Sun and Kertesz carried out calculations for all IPR isomers with a nonvanishing HOMO–LUMO gap at the B3LYP/6-31G* level (isomers 1–6). By comparing their calculated NMR spectra with experiment, they concluded, in agreement with experimental finding by Kikuchi et al.,55 that isomer 3 prevails.
 | ||
| Fig. 1 The guest (CHFClBr) enantiomers 1a and 1b, one enantiomer of the fullerene C82-3 2, and the 3a and 3b complexes R- and S-CHFClBr@C82-3. | ||
As mentioned above, preliminary calculations have shown that C82 is the smallest fullerene cage capable of hosting the CHFClBr guest. We have therefore carried out calculations of the energy, infrared (IR), vibrational circular dichroism (VCD), UV-vis and nuclear magnetic resonance (NMR) spectra of one enantiomer of the C82 fullerene, the guests and complexes of one host enantiomer with the S and R guest enantiomers, with the goal of studying the chiral recognition of the fullerene cage and its manifestations on the spectra.
2. Computational methods
The optimized geometries for isomers of C82, for an isolated CHFClBr molecule and the endohedral complexes CHFClBr@C82 were obtained by density-functional theory (DFT), using the B97D functional63 and the 6-31G(d) basis set.64 It should be emphasized that the size of the species studied prevented us from using larger basis sets. However, several studies have confirmed that the geometries provided by this combination of method and the basis set are sufficient.65 Noteworthily, the B97D functional includes dispersion corrections, so it should give reliable results for the dispersion-bound intermolecular complexes. However, in contrast to the previous guests we have studied,31–33 in this case not only dispersion but also electrostatic interactions with the labile π-electron systems are of importance. The stationary points resulting from the B97D/6-31G(d) optimization were verified to correspond to minima on the potential energy surface by performing a harmonic frequency analysis at the same level of theory. These frequencies were later used to calculate the zero-point vibrational energy (ZPVE) for the molecules studied here.Because we in this work are focused on chiral recognition, only chiral IPR (Isolated Pentagon Rule) isomers, i.e. isomers 1, 3, and 5 according to The Atlas of Fullerenes,54 were taken into account and structurally optimized. The insertion of the R or S enantiomers of the guest into one of the host enantiomers thus resulted in six distinct complexes, denoted in the following by the guest chirality as 1R, 1S, 3R, 3S, 5R and 5S, irrespective of the cage chirality. Such a procedure enabled us to define all diastereomeric complexes. The optimized geometric structures were then utilized in all subsequent calculations.
In agreement with the supermolecular approach which we applied previously,31–33 the interaction energy is defined as
| Eint(A@B) = E(A@B) − (E(A) + E(B)), | (1) |
As an alternative perturbational approach, the symmetry-adapted perturbation theory (SAPT) with a DFT description of the constituent molecules was applied71–73 using the PBE functional (with the asymptotic correction of Grüning et al.74) to account for the intramolecular electron correlation. The energies of the highest occupied molecular orbitals of the host and the guest were calculated using the PBE functional and the same basis set as in the main SAPT calculations. Ionization potentials (IPs) were either calculated at the PBE/def2-QZVP level (for C82) or taken from experiment (for CHFClBr).75 The calculated energies were 6.52, 6.67, and 6.76 eV for the 1, 3, and 5 isomers of C82, respectively, while the experimental IP value for the guest amounts to 11.15 eV. The latter value is close to 10.67 eV obtained using the same computational method as that used for C82. The asymptotic correction for the guest molecule was set to 0.142 hartree. Since the HOMO energies of the three isomers of C82 were shifted according to the IP values, it turned out that the asymptotic corrections for all three cage types were the same and equal to 0.05 hartree.
The SAPT interaction energy was obtained in the form of a sum of several components, such as the electrostatic, induction, dispersion terms and their exchange counterparts, resulting from imposing an antisymmetry condition on the approximate wave functions. Higher-order terms were estimated through the so-called delta Hartree–Fock term (δEHF).76 Summarizing, the SAPT interaction energy is obtained using the equation:
| Eint(SAPT) = E(1)elst + E(2)ind + E(2)disp + E(1)exch + E(2)exch-ind + E(2)exch-disp + δEHF. | (2) |
The stability of the complex is best described through the stabilization energy, which accounts for the deformation of the constituent molecules forming a complex, Edef(X), and for the zero-point vibration energy differences, ΔZPVE, of the complex A@B and molecules A and B,
| Estab = Eint(A@B) + Edef(A) + Edef(B) + ΔZPVE | (3) |
Most SAPT(DFT) calculations have been performed using the def2-TZVP basis set. However, since the dispersion energy is known to be difficult to saturate with respect to the basis set size, we additionally calculated this energy in a larger def2-QZVP basis set. From the values of E(2)disp in both basis sets, one can further estimate the complete-basis set (CBS) limit for this energy component. Since the dispersion energy is a pure electron-correlation contribution, a suitable formula for the CBS limit can be found e.g. in ref. 77:
| E(2)disp(CBS) = E(2)disp[X] + C/X3, | (4) |
Geometry optimizations, single-point calculations of the interaction energies with DFT, MP2, and SCS-MP2, as well as the TD-DFT calculations with the CAM-B3LYP functional were performed using the Gaussian (G09) program package.79 The SAPT(DFT) calculations and the calculations of the C82 ionization potential with unrestricted DFT were performed using the Molpro package.80
Another energy parameter describing the stability of the complex is the binding energy. Binding energy is defined as the difference between the energies at the optimized geometries for A@B and the sum of the energies of the isolated A and B molecules. In order to avoid estimating the BSSE, the binding energy is calculated from the following formula:
| Ebind = Eint(A@B) + Edef(A) + Edef(B), | (5) |
| Estab = Ebind + ΔZPVE | (6) |
The infrared (IR) and vibrational circular dichroism (VCD) spectra of the complexes were obtained within the harmonic approximation using the B97D/6-31G(d) approach. Although the absolute values of the frequencies obtained in this way differ from experiment, they should allow us to evaluate trends of frequency shifts upon complexation and eventually reveal manifestations of chiral recognition of the CHFClBr enantiomers by the chiral C82 cage. Since the resulting identical sign of the rotatory strength of the stretching C–H vibration mode of both the S and R guest enantiomers in the C82-3 host is unusual, we performed additional calculations of this property using the B97D functional and the SVP and 6-311G(d,p) basis sets, as well as using one more functional (PBE) and the same basis set (6-31G(d)). The results show that the rotatory strength of the C–H stretching mode is negative for all combinations of functionals and basis sets, and that the S enantiomer always has a higher rotatory strength than the R one.
The nuclear magnetic resonance (NMR) spectra are particularly sensitive to the environment. NMR chemical shifts were calculated using the spin–orbit zero-order regular approximation (SO-ZORA)85,86 as implemented in the Amsterdam Density Functional (ADF) program.87 We used the PBE functional including Grimme's dispersion correction (PBE-D3)66,67,88 and the relativistically optimized all-electron TZ2P basis set.89 Solvent effects were accounted for by using chloroform in a conductor-like screening model (COSMO)90 as implemented in ADF. The chemical shifts of the carbon atoms of the cage relative to tetramethylsilane (TMS) (in ppm) were obtained by using benzene as the secondary reference as given in the following equation:
| δ(Ci, C82) = [σ(C, C6H6) − σ(Ci, C82) + 128.5] | (7) |
3. Results and discussion
3.1. Energies and geometries of the complexes
As already mentioned, there are 9 IPR isomers of C82, and among these, isomer 3 was determined to prevail both experimentally55 and theoretically (see ref. 57, 60, 62 and references cited therein). Kikuchi et al.55 observed three isomers of the fullerene: 80% of the prevailing isomer 3, and the rest arising from achiral isomers of C2v and C3v symmetry.As in all previous calculations,57,60,62 the analysis of the free energies under normal conditions and total electronic energies confirms that the most stable isomer of the isolated C82 is the chiral isomer 3. Only isomer 3 and the less stable isomers 1 and 5 (all of C2 symmetry) of all possible IPR isomers of C82 are chiral. Therefore, we will in the following focus on these isomers and their complexes. The calculated differences between the free energy ΔG under standard (298.15 K, 1 atm) conditions and the total electronic energy ΔE of the isomers 1, 3 and 5 of C82 with respect to the most stable isomer 3 calculated at the B97D/6-31G(d), B97D/6-311G(d,p) (in parentheses) and PBE-D3(BJ)/6-31G(d) [in brackets] levels are collected in Table S1 in the ESI.† The ΔG energy differences between the isomers relative to that of isomer 3 are larger than 4 kcal mol−1 for isomers 1 and 5, whereas the corresponding ΔE values are larger than 6 kcal mol−1 for all functionals and basis sets used. Thus, in agreement with the experimental finding of Kikuchi et al.,55 the population of the other chiral isomers (1 and 5) should be negligible at room temperature.
The calculated energies of the diastereomeric complexes of the chiral isomers with both enantiomers of the CHFClBr guest is collected in Table 1 and indicate that stable complexes should be formed only with the most stable isomer 3, and that the complex with the S enantiomer should be more stable than that with the R enantiomer by ca. 0.5 kcal mol−1. To allow future experimental investigations to verify this prediction, the IR, VCD, UV-vis and NMR spectra of both complexes, i.e. R-CHFClBr@C82 and S-CHFClBr@C82, have been calculated.
| CHFClBr@C82 | ΔG | ΔE |
|---|---|---|
| 1R |
3.7
(3.7) |
3.4
(3.6) |
| 1S |
7.1
(6.8) |
7.0
(6.7) |
| 3R |
0.4
(0.7) |
0.9
(1.0) |
| 3S |
0.0
(0.0) |
0.0
(0.0) |
| 5R |
13.4
(12.8) |
15.0
(14.3) |
| 5S |
12.2
(11.7) |
13.5
(13.2) |
Interestingly, the energy ordering of the complexes involving isomers 1 and 5 changes upon complexation. The 1R complex gains the most with respect to isomer 3, i.e. the difference between 1R and 3S or 3R is about 4 kcal mol−1 less than for the isolated C82-1. On the other hand, isomer 5 increases its relative energy upon complexation (the difference between isolated isomer 5 and 3 amounts to 6 kcal mol−1, whereas for the 5S and 5R complexes it is more than two times larger). However, the 3R and 3S complexes are more stable than those with 1 and 5 cage isomers by more than 3 kcal mol−1, and the 3S complex is more stable than the 3R one by 0.4 kcal mol−1.
DFT calculations of the complexes reveal that the guests are too large for the cage, since the distances between their substituents and the closest carbon cage atom dXC are smaller than the sums of the van der Waals radii of the corresponding atoms by approximately 0.4–0.6 Å (Table 2). The Δ values are smaller for the more electronegative halogen substituent (as expressed by Pauling electronegativity).94,95 It should be stressed that such a short distance between the atoms is associated with strong repulsion, resulting in a considerable strain to which both the host and guests are exposed. Interestingly, only the smallest distances to the hydrogen atom show a large chiral recognition effect of ca. 0.1 Å, for all other distances the differences between the enantiomeric guests atoms and the cage are insignificant.
| Nonbonding distance | van der Waals radii | Distances to the nearest carbon dXC and the Δ values | ||||
|---|---|---|---|---|---|---|
| Of the substituent | Sum of the radii of the carbon cage atom (1.70 Å) and of the substituent | d XC(R) | Δ(R) | d XC(S) | Δ(S) | |
| H⋯C82 | 1.20 | 2.90 | 2.42 (2.40) | −0.48 (−0.50) | 2.33 (2.30) | −0.57 (−0.60) |
| F⋯C82 | 1.47 | 3.17 | 2.57 (2.56) | −0.60 (−0.61) | 2.59 (2.58) | −0.58 (−0.59) |
| Cl⋯C82 | 1.75 | 3.45 | 3.01 (2.98) | −0.56 (−0.53) | 3.02 (3.00) | −0.57 (−0.55) |
| Br⋯C82 | 1.85 | 3.65 | 3.10 (3.13) | −0.45 (−0.42) | 3.09 (3.12) | −0.44 (−0.43) |
Too large guests inserted inside the cage lead to its considerable distortion: a reduction of some bond lengths and considerable changes in bond angles (Table 3). They raise the question to the origin of the stability of the complexes (vide infra). The CBr bond of the guest displays the largest compression (by almost 0.1 Å) upon complex formation, the CCl bond a somewhat smaller compression and the smallest reduction in bond length is observed for the bond with the largest polarity, the CF bond. Interestingly, also in this case the Δ values of the reduced C–halogen bond lengths correlate with the decrease in the Pauling electronegativity parameter of the halogen substituent. All guest bond angles involving the hydrogen atom in the complexes are increased in comparison to the values for the free guest, whereas the angles involving only the heavier atoms are reduced. The maximum values of the change (of ca. 4°) are obtained for the HCBr angle. No significant changes in the bond lengths or bonds of the guests that could be related to chiral recognition are found, except, perhaps the ∠FCBr bond angle. For obvious reasons, the distortions of bond lengths and bond angles of the C82 cage are much smaller than those observed for the guests since they are spread over a much larger structure.
| Guest R/S | R@C82 | S@C82 | |||
|---|---|---|---|---|---|
| d CX/∠XCY | Δ | d CX/∠XCY | Δ | ||
| C–H | 1.095 (1.092) | 1.087 (1.085) | −0.008 (−0.007) | 1.087 (1.084) | −0.008 (−0.008) |
| C–F | 1.350 (1.351) | 1.347 (1.345) | −0.003 (−0.006) | 1.346 (1.344) | −0.004 (−0.007) |
| C–Cl | 1.797 (1.794) | 1.734 (1.728) | −0.063 (−0.066) | 1.732 (1.726) | −0.065 (−0.068) |
| C–Br | 1.990 (1.991) | 1.893 (1.884) | −0.097 (−0.107) | 1.896 (1.888) | −0.094 (−0.103) |
| ∠HCF | 110.7 (110.3) | 111.6 (111.4) | 0.9 (1.1) | 111.5 (111.2) | 0.8 (0.9) |
| ∠HCCl | 108.6 (108.4) | 111.2 (111.1) | 2.6 (2.7) | 111.7 (111.5) | 3.1 (3.3) |
| ∠HCBr | 106.6 (106.7) | 111.0 (111.3) | 4.4 (4.6) | 110.4 (110.8) | 3.8 (4.1) |
| ∠FCCl | 109.9 (109.7) | 107.7 (107.9) | −2.2 (−1.8) | 107.0 (107.3) | −2.9 (−2.4) |
| ∠FCBr | 109.3 (109.3) | 106.2 (106.2) | −3.1 (−3.1) | 107.4 (107.2) | −1.9 (−2.1) |
| ∠ClCBr | 111.8 (112.4) | 108.9 (108.7) | −2.9 (−3.7) | 108.7 (108.6) | −3.1 (−3.8) |
The sum of NBO97 charges on the guests and host in the complexes calculated using B97D/6-31G(d) and B97D/6-311G(d,p) (in parentheses) and those calculated using B97D/6-31G(d) applying the QTAIM98 method are collected in Table S2 (ESI†). They show very small net charges on the host and guest, with considerably larger values calculated by QTAIM.
As mentioned in the previous section, factors influencing the stability of the complexes with respect to the constituent molecules were determined by calculating the interaction energies using several methods. The binding energies were obtained by adding the deformation energies of the constituent molecules of the complex to the interaction energies (see Computational methods section for details), and the stabilization energies by additionally correcting the binding energy with the ZPVE of the nuclear motion. The results of these calculations are listed in Table 4.
| Complex | SAPT (DFT)a def2-TZVP/QZVP | B97D def2-TZVP | B97D def2-QZVP | PBE + D3 def2-TZVP | PBE + D3 def2-QZVP | MP2 def2-TZVP | SCS-MP2 def2-TZVP | ΔZPVE | E def | E stab |
|---|---|---|---|---|---|---|---|---|---|---|
| a The SAPT(DFT) results in parenthesis are obtained from the CBS extrapolation of the E(2)disp energy (def2-TZVP → def2-QZVP), see Computational details. The results without parenthesis are obtained by a simple replacement of the E(2)disp(def2-TZVP) value in the SAPT(DFT)/def2-TZVP energy by the E(2)disp(def2-QZVP) result. | ||||||||||
| 1R | −5.3 (−8.5) | −3.7 | −5.3 | 0.8 | −0.1 | −37.3 | −14.7 | 1.1 | 5.5 | 1.3 (−1.9) |
| 1S | −3.3 (−6.8) | −0.8 | −2.4 | 2.9 | 1.8 | −35.1 | −12.3 | 1.0 | 5.5 | 3.3 (−0.3) |
| 3R | −4.0 (−7.6) | −0.4 | −2.0 | 2.5 | 1.5 | −35.1 | −12.7 | 0.5 | 6.6 | 3.1 (−0.6) |
| 3S | −4.3 (−7.8) | −0.9 | −2.5 | 2.2 | 1.1 | −35.5 | −13.1 | 0.7 | 6.2 | 2.7 (−0.9) |
| 5R | −2.3 (−6.0) | 3.4 | 1.8 | 4.3 | 3.3 | −33.1 | −10.8 | 0.3 | 9.3 | 7.3 (+3.6) |
| 5S | −2.3 (−6.1) | 3.2 | 1.6 | 4.4 | 3.4 | −33.5 | −11.0 | 0.4 | 8.3 | 6.4 (+2.6) |
As can be seen from Table 4, the MP2 and SCS-MP2 interaction energies are unphysically large in absolute values. The MP2 overbinding is well known for stacked conjugated systems99 and apparently this effect is here amplified by the electron-rich guest. The SCS-MP2 approach, which is known to correct MP2 in many cases,68 also gives unsatisfactory results in the present case. Both sets of results are presented here only as a warning that in some cases the MP2 (and SCS-MP2) methods are unable to give reliable interaction energies in supermolecular calculations. A similar huge overestimation of the binding energy by the MP2 and SCS-MP2 methods has recently been observed in ref. 100 where large intermolecular complexes involving a fullerene with a buckycatcher molecule were studied.
The PBE + D3 method produces a slightly negative energy for the 1R case in the largest basis set. It should be emphasized that in this case, the pure PBE contribution to the energy is large and positive, and the dispersion “correction” is as large in absolute value as the PBE interaction energy, but of the negative sign, almost canceling each other. This casts some doubts on the reliability of the PBE + D3 interaction energies for systems of interacting nonpolar molecules, bound mostly by dispersion. On the other hand, the B97D functional as well as SAPT(DFT) gives reasonable values for the interaction energies. The CBS estimate of the dispersion energy (see the Computational methods section for details) enhances the attraction energy by about 3 to 4 kcal mol−1 compared to the non-CBS results. The stabilization energies computed with the CBS-extrapolated energy give slightly negative (attractive) results for four of the six complexes. It should be noted that the CBS procedure turns out to be indispensable. This is due to a well-known feature of the dispersion energy of intermolecular interactions (see, e.g.ref. 100 for an analysis of complexes of a similar size to the present case), being very sensitive to the quality of the orbital basis set. The dispersion energy calculated in a triple or even quadruple-zeta basis is thus often still far from saturated. Nevertheless, these values are of sufficient quality to estimate the CBS limit.
When comparing the energy ordering resulting from the ΔE values in Table 1 with that of the binding energies from eqn (5) in Table 4, one should keep in mind that their ordering will only be identical for the ideal case when both quantities are calculated using the same method in the complete basis set limit. Because the 6-31G(d) basis set is very far away from the CBS, the BSSE is quite large (in fact, it is of the order of the binding energy itself), and irregularities in the energy ordering can appear. However, some trends can be seen in both tables, i.e. for the same C82 isomer, the energetic ordering of the R and S guest isomers of the complex is the same.
According to all methods used, the largest stabilization upon complexation is obtained for the 1R complex, and the next is the 3S complex as far as the interaction energies are concerned. The third most stable complex according to the interaction energies is the 3R complex when using SAPT(DFT), and 1S using B97D, but the energy differences are in this case small. Finally, the 5S and 5R complexes are the least stabilized. The deformation energies are quite substantial in view of the smallness of the interaction energies, and the final values for the stabilization energies are thus the result of a delicate balance between the attractive interaction energy and the repulsive deformation energy (with a small additional contribution from the nuclear motion).
Although the 1R complex has the largest stabilization energy (Table 4), it is isomer 3 that is the most abundant according to both the calculations and experimental results. Thus, the complexes of this isomer with CHFClBr should be observable. We therefore present in the next section their IR, VCD, UV-vis, and NMR spectra to enable a future observation.
3.2 IR and VCD spectra
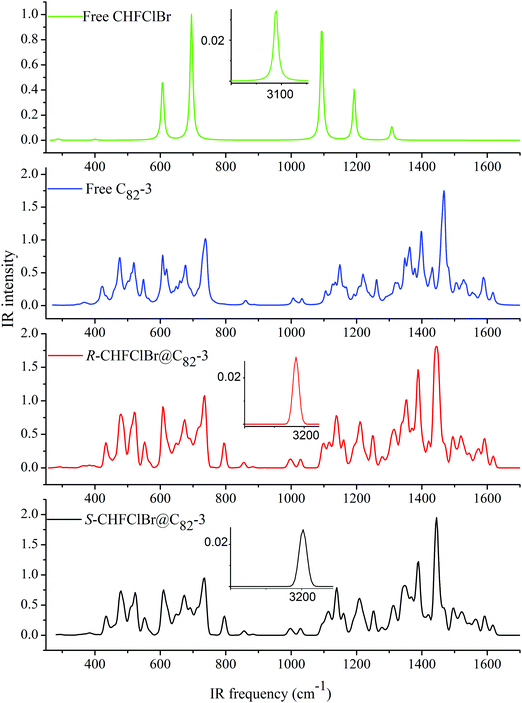
The calculated IR spectra of the free host and guest and their complexes are shown in Fig. 2 (a full list of the normal frequencies is presented in Table S3 of the ESI†) and reveal a strong influence of both the complexation and the guest chirality on the calculated spectra. Not unexpectedly, the most prominent effect is seen on the νCH stretching vibration band. However, in contrast to the usual IR manifestations of hydrogen bonding, the bands are shifted to higher frequencies by more than 100 cm−1. This is due to the considerable shortening of the CH bonds upon the formation of the complex (Table 3). The calculated effects of chiral recognition on the vibrational frequencies are quite sensitive to the method of calculation. Because CH stretching vibration is a well-localized characteristic vibration, the IR spectra could in this case be used as a marker for both molecular and chiral recognition. On the other hand, the region below 1650 cm−1 contains numerous nonlocalized vibrations of the C82 cage, which are likely to appear together with guest vibrations and vibrational overtones. For instance, the bands at 1093 cm−1, 1101 cm−1, and 1107 cm−1 contain a significant contribution from the νCF of the guest and of the cage vibrations whereas for the bands at 695 cm−1, 795 cm−1, and 796 cm−1, the contribution of νCCl of the guest as well as cage vibrations is considerable, and they are also likely to be influenced by Fermi resonances in the experiment. Thus, although one can see clear differences in the spectra of the host, guest, and the complexes in the region below 1650 cm−1, they are of a negligible diagnostic value.VCD is one of the most important methods for determining the absolute configuration of organic molecules and for establishing the dominant conformations in biopolymers.101–104 The rotational strengths of the vibrational bands of free guests 1 and host 2 and complexes 3a and 3b were therefore calculated.
The rotational strengths calculated for the stretching CH vibrations for 3a and 3b (Fig. 3 and Table 5 contain results obtained using several functionals and basis sets) show that both the νCH frequencies and the rotational strengths depend on the functionals and basis sets used. However, in all cases the rotational strengths for both complexes with the R- and the S-guest enantiomers have the same sign. Such a sign reversal for the νCH band for the complex with the S guest enantiomer relative to the free molecule, independently of the choice of the exchange–correlation functional and the basis set used, has not been previously reported for diastereomeric complexes.101–104
 | ||
| Fig. 3 VCD spectra of 1a and 1b (top), 2 (middle) and 3a and 3b (bottom), calculated using B97D/6-31G(d). The inset depicts the VCD band of the νCH stretching mode of 1. | ||
| Molecule | Vibrational frequency | Rotational strength |
|---|---|---|
| B97D/6-31G(d) | ||
| R-CHFClBr/S-CHFClBr | 3089.4 | −1.30/+1.30 |
| R-CHFClBr@C82-3 | 3185.9 | −2.03 |
| S-CHFClBr@C82-3 | 3202.6 | −1.00 |
| B97D/6-311G(d,p) | ||
| R-CHFClBr/S-CHFClBr | 3080.1 | −0.92/+0.92 |
| R-CHFClBr@C82-3 | 3178.3 | −2.35 |
| S-CHFClBr@C82-3 | 3174.7 | −0.81 |
| B97D/SVP | ||
| R-CHFClBr/S-CHFClBr | 3060.0 | −0.54/+0.54 |
| R-CHFClBr@C82-3 | 3161.2 | −4.15 |
| S-CHFClBr@C82-3 | 3170.3 | −1.00 |
| PBE/6-31G(d) | ||
| R-CHFClBr/S-CHFClBr | 3093.7 | −1.26/+1.26 |
| R-CHFClBr@C82-3 | 3143.2 | −1.46 |
| S-CHFClBr@C82-3 | 3146.5 | −0.96 |
3.3. NMR spectra
The complexes contain several nuclei that are sensitive to their environment. Therefore, predicting changes in e.g. chemical shifts can be useful for the future detection of S or R isomers of the endohedral complexes with C82. We have therefore calculated 1H, 13C, 19F, 35Cl, and 79Br chemical shifts for the complexes and for the free host and guest. In view of the numerous close-lying carbon signals of the host cage, only signals pertaining to the guest are collected in Table 6 and discussed in the following. First, it should be mentioned that the negative values for the fluorine chemical shifts as opposed to the positive values for all other nuclei are caused by the choice of reference for this nucleus in the experimental work.105 The proton, carbon, and fluorine guest signals will be discussed first since for these nuclei the experimental values have been reported. The calculated chemical shift of the carbon nucleus differs considerably from the reported experimental value, highlighting the challenge for DFT of calculating properties of even small molecules with electron-rich substituents. However, because the chemical shifts of CHFClBr for the different nuclei are determined in separate experiments and because the chemical shift of the carbon atom of the guest is well separated from the signals of the cage atoms, we can nevertheless draw some qualitative conclusions from the calculations.| Free CHFClBr | R-CHFClBr@C82-3 | S-CHFClBr@C82-3 | Δδ | |||||
|---|---|---|---|---|---|---|---|---|
| δ exp | δ calc | Δδexp-calc | δR | Δδfree-complex | δS | Δδfree-complex | ||
| a Chemical shifts of the guest atoms referenced to TMS.41 (Other 1H for the free guest reported by Doyle and Vogl37 is 7.62 ppm). b Chemical shifts of the guest atoms referenced to CFCl3. c Chemical shifts of the guest atoms referenced to NaCl. d Chemical shifts of the guest atoms referenced to NaBr. e Average values of 13C signals taken from ref. 37. f Ref. 41. | ||||||||
| 1Ha | 7.65f | 8.15 | −0.50 | 2.85 | 5.30 | 2.73 | 5.42 | 0.12 |
| 13Ca | 91.4e | 116.5 | −25.1 | 100.5 | 16.0 | 100.5 | 16.0 | 0.0 |
| 19Fb | −80.1f | −92.8 | 12.7 | −103.1 | 10.3 | −107.0 | 14.2 | 3.9 |
| 35Clc | — | 608 | — | 591 | 17 | 597 | 11 | −6 |
| 79Brd | — | 1572 | — | 1518 | 54 | 1506 | 66 | 12 |
An inspection of the data collected in Table 6 reveals that (a) all guest signals are considerably shifted upon complexation with a very large value for the proton and carbon shifts; (b) the fluorine, and to a lesser extent, the proton signals, are sensitive to the guest chirality. On the other hand, differences in chemical shifts of the guest carbon, chlorine and bromine nuclei in the spectra of the 3R and 3S complexes lie within the experimental error bars.
The signals of the cage carbon atoms are collected in Table S4 of the ESI.† Interestingly, the differences between the experimental and calculated values are reasonably small, not exceeding 2 ppm. The complexation shifts for the cage carbon atoms are also usually small; they only exceed 2 ppm for three signals of the 3R complex and for one signal of the 3S complex. The differences in ΔδCcage between the chemical shifts of the diastereomeric 3R and 3S complexes pertaining to chiral recognition are also too small to be significant.
To summarize, all guest signals (except the 35Cl signals) exhibit considerable complexation shifts. However, only the signals of the fluorine nucleus are suitable for the detection of chiral recognition of the CHFClBr enantiomers by the C82 cage by NMR.
3.4. Electronic spectra
The calculated vertical excitation energies (in eV) and oscillator strengths (f) for the 10 lowest singlet excited states of the empty C82-3 cage and the complexes with the 3R, 3S enantiomers are presented in Table S5 of the ESI.† Most of them depend only weakly on complexation and guest chirality. As the lowest excitation energy of CHFClBr is placed in the UV region outside normal experimental observation ranges, we do not discuss the excited states of the guest molecule.The data in the table reveal that the vertical excitation energy of the first excited state is significantly shifted upon complexation by about 0.05 eV, and for the second and third states the shift amounts to more than 0.02 eV. On the other hand, all intensities are largely independent of the complexation. There are also no noticeable differences in the vertical excitation energies and oscillator strengths for the diastereomeric complexes with the R and S guests. Thus, whereas UV-vis spectra could provide information on the formation of the complex of C82 with the CHFClBr guest, they cannot be used to detect and study chiral recognition of the guest enantiomers by the fullerene cage.
4. Conclusions
Quantum chemical calculations (using density-functional theory and symmetry-adapted perturbation theory) of the chiral recognition of complexes involving the CHFClBr enantiomers by the most stable C82-3 isomer reveal that the guests are too large for the fullerene cage and that they undergo considerable squeezing (shortening of bond lengths and distortions of bond angles) upon complex formation. However, despite the considerable steric strain, the guests are stabilized inside the host cavity by electrostatic interactions. The complexation results in considerable changes in the IR, VCD, NMR and UV-vis spectra, but the significant effect of chiral recognition between the guests was observed only by 19F signals in the NMR spectrum and by the sign reversal of the νCH vibrational band of S-CHFClBr in the VCD spectrum.101–104What are the prospects for the experimental verification of these predictions? Both C82-3 enantiomers, probably as a mixture, and the CHFClBr separated into enantiomers37 are known. It would be feasible to obtain the complexes studied here by using a ‘molecular surgery’ approach applied successfully in the case of H2@C60106 and a few other fullerene complexes. To avoid tedious separation of the fullerene C82-3 enantiomers, one could insert the S guest enantiomer into both enantiomers of the fullerene cage. By measuring the VCD spectra of R- and S-CHFClBr@C82-3, it should be possible to experimentally verify the results of our calculations. To check the structural predictions, the separation of the C82-3 enantiomers and crystallization of the complexes will be necessary.
Acknowledgements
This research was supported by The Research Council of Norway [grant no. 179568/V30] and a European Research Council starting grant [grant no. 279619]. Computer time was provided by the Norwegian Supercomputing Program NOTUR [grant no. NN4654K] and by PL-Grid Infrastructure.References
- L. Echegoyen and L. E. Echegoyen, Acc. Chem. Res., 1998, 31, 593–601 CrossRef CAS.
- J. E. Grose, E. S. Tam, C. Timm, M. Scheloske, B. Ulgut, J. J. Parks, H. D. Abruna, W. Harneit and D. C. Ralph, Nat. Mater., 2008, 7, 884–889 CrossRef CAS PubMed.
- E. Xenogiannopoulou, S. Couris, E. Koudoumas, N. Tagmatarchis, T. Inoue and H. Shinohara, Chem. Phys. Lett., 2004, 394, 14–18 CrossRef CAS.
- P. Norman, Y. Luo, D. Jonsson and H. Ĺgren, J. Chem. Phys., 1997, 106, 8788, DOI:8710.1063/8781.473961.
- M. S. Dresselhaus, G. Dresselhaus and P. C. Eklund, Science of Fullerenes and Carbon Nanotubes: Their Properties and Applications, Elsevier, Oxford, 1995 Search PubMed.
- Y. Yasutake, Z. J. Shi, T. Okazaki, H. Shinohara and Y. Majima, Nano Lett., 2005, 5, 1057–1060 CrossRef CAS PubMed.
- G. Gu, H. Huang, S. Yang, P. Yu, J. Fu, G. K. Wong, X. Wan, J. Dong and Y. Du, Chem. Phys. Lett., 1998, 289, 167–173 CrossRef CAS.
- M. S. Dresselhaus, G. Dresselhaus and P. C. Eklund, Science of Fullerenes and Carbon Nanotubes: Their Properties and Applications, Elsevier, Oxford, 1995, pp. 670–673 Search PubMed.
- Strained Hydrocarbons. Beyond the van't Hoff and Le Bel hypothesis, ed. H. Dodziuk, Wiley-VCH, Weinheim, 2009 Search PubMed.
- J. F. Stoddart, Angew. Chem., Int. Ed. Engl., 1991, 30, 70–71 CrossRef.
- R. D. Bolskar, Nanomedicine, 2008, 3, 201–213 CrossRef CAS PubMed.
- R. Bakry, R. M. Vallant, M. Najam-ul-Haq, M. Rainer, Z. Szabo, C. W. Huck and G. K. Bonn, Int. J. Nanomed., 2007, 2, 639–649 CAS.
- V. J. Morris, A. R. Kirby and A. P. Gunning, Atomic Force Microscopy for Biologists, World Scientific, 2009, p. 14 Search PubMed.
- C. Y. Yu, Phys. Rev. A: At., Mol., Opt. Phys., 2007, 75, art. 012318 Search PubMed.
- M. S. Garelli and F. V. Kusmartsev, Eur. Phys. J. B, 2005, 48, 199–206 CrossRef CAS.
- M. Gong, T. A. Shastry, Y. Xie, M. Bernardi, D. Jasion, K. A. Luck, T. J. Marks, S. Ren and M. C. Hersam, Nano Lett., 2014, 14, 5308–5314 CrossRef CAS PubMed.
- H. Dodziuk, J. Nanosci. Nanotechnol., 2007, 7, 1102–1110 CrossRef CAS PubMed.
- M. Ge, U. Nagel, D. Hüvonen, T. Rõõm, S. Mamone, M. H. Levitt, M. Carravetta, Y. Murata, K. K. Komatsu, J. Y.-C. Chen and N. J. Turro, J. Chem. Phys., 2011, 134, 054507 CrossRef PubMed.
- G. Min, U. Nagel, D. Hüvonen, T. Rőőm, S. Mamone, M. H. Levitt, M. Carravetta, Y. Murata, K. Komatsu, J. Y.-C. Chen and N. J. Turro, J. Chem. Phys., 2011, 134, art. 054507 Search PubMed.
- S. Mamone, J. Y.-C. Chen, R. Bhattacharyya, M. H. Levitt, R. G. Lawler, A. J. Horsewill, T. Rõõm, Z. Bačić and N. J. Turro, Coord. Chem. Rev., 2011, 255, 938–948 CrossRef CAS.
- S. Mamone, M. Concistre, E. Carignani, B. Meier, A. Krachmalnicoff, O. G. Johannesen, X. Lei, Y. Li, M. Denning, M. Carravetta, K. Goh, A. J. Horsewill, R. J. Whitby and M. H. Levitt, J. Chem. Phys., 2014, 140, 194306 CrossRef PubMed.
- K. Kurotobi and Y. Murata, Science, 2011, 333(6042), 613–616 CrossRef CAS PubMed.
- E. H. T. Olthof, A. van der Avoird and P. E. S. Wormer, J. Chem. Phys., 1996, 104, 832–847 CrossRef CAS.
- D. J. Cram, M. E. Tanner and R. Thomas, Angew. Chem., Int. Ed., 1991, 30, 1024–1027 CrossRef.
- J. L. Dye, J. Phys. IV, 1991, 1, C5-259–C5-282, DOI:10.1051/jp4:1991531.
- J. R. Nitschke, P. Mal, B. Breiner and K. Rissanen, Science, 2009, 324(5935), 1697–1699, DOI:1610.1126/science.1175313.
- P. Jakes, K.-P. Dinse, C. Meyer, W. Harneit and A. Weidinger, Phys. Chem. Chem. Phys., 2003, 5, 4080–4083 RSC.
- H. Dodziuk, Modern Conformational Analysis. Elucidating Novel Exciting Molecular Structures, VCH Publishers, New York, 1995, pp. 119–134 Search PubMed.
- R. Ettl, I. Chao, F. Diederich and R. L. Whetten, Nature, 1991, 353, 149–153 CrossRef CAS.
- H. Dodziuk, G. Dolgonos and O. Lukin, Carbon, 2001, 39, 1907–1911 CrossRef CAS.
- T. Korona, M. Hesselmann and H. Dodziuk, J. Chem. Theory Comput., 2009, 5, 1585–1596 CrossRef CAS PubMed.
- T. Korona and H. Dodziuk, J. Chem. Theory Comput., 2011, 7, 1476–1483, DOI:1410.1021/ct200111a.
- H. Dodziuk, T. Korona, E. Lomba and C. Bores, J. Chem. Theory Comput., 2012, 8, 4546–4555 CrossRef CAS PubMed.
- H. Dodziuk, Chem. Phys. Lett., 2005, 410, 39–41 CrossRef CAS.
- M. Murata, S. Maeda, Y. Morinaka, Y. Murata and K. Komatsu, J. Am. Chem. Soc., 2008, 130, 15800–15801 CrossRef CAS PubMed.
- Z. Jiang, J. Crassous and V. Schurig, Chirality, 2005, 17, 488–493 CrossRef CAS PubMed.
- T. R. Doyle and O. Vogl, J. Am. Chem. Soc., 1989, 111, 8510–8511 CrossRef CAS.
- J. Costante, L. Hecht, P. L. Polavarapu, A. Collet and L. D. Baron, Angew. Chem., Int. Ed., 1997, 36, 885–887 CrossRef CAS.
- B. Darquie, C. Stoeffler, A. Shelnikovnikov, C. Daussy, A. Amy-Klein, C. Chardonnet, S. Zrig, L. Guy, J. Crassous, P. Soulard, P. Asselin, T. R. Huet, P. Schwerdtfeger, R. Bast and T. Saue, Chirality, 2010, 22, 870–884 CrossRef CAS PubMed.
- J. Crassous, F. Monier, J. P. Dutasta, M. Ziskind, C. Daussy, C. Grain and C. Chardonnet, ChemPhysChem, 2003, 4, 541–548, DOI:510.1002/cphc.200200536.
- J. Crassous and S. Hediger, J. Phys. Chem. A, 2003, 107, 10233–10240, DOI:10.1021/jp0305685.
- J. Canceill, L. Lacombe and A. Collet, J. Am. Chem. Soc., 1985, 107, 6993–6996 CrossRef CAS.
- A. Hirsch and M. Brettreich, Fullerenes. Chemistry and reactions, Wiley-VCH, 2005 Search PubMed.
- Y. Liu, C. Chen, P. Qian, X. Lu, B. Sun, X. Zhang, L. Wang, X. Gao, H. Li, Z. Chen, J. Dong, R. Ru Bai, P. E. Lobie, Q. Wu, S. Liu, H. Zhang, F. Zhao, M. S. Wicha, T. Zhu and Y. Zhao, Nat. Commun., 2015, 1, 5988 CrossRef PubMed.
- J. Meng, X. Liang, X. Chen and Y. Zhao, Integr. Biol., 2013, 5, 43–47 RSC.
- D. W. Cagle, S. J. Kennel, S. Mirzadeh, J. M. Alford and L. J. Wilson, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 5182–5187 CrossRef CAS.
- S. Yang, C. Chen, F. Fupin Liu, Y. Xie, F. Li, M. Jiao, M. Suzuki, T. Wei, S. Wang, C. Chen, X. Lu and T. Akasaka, Sci. Rep., 2013, 3 DOI:10.1038/srep01487.
- W. Fu, L. S. Xu, H. Azurmendi, J. Ge, T. Fuhrer, T. Zuo, J. Reid, C. Shu, K. Harich and H. C. Dorn, J. Am. Chem. Soc., 2009, 131, 11762–11769, DOI:10.1021/ja902286v.
- T. Shimada, Y. Ohno, T. Okazaki, T. Sugai, K. Suenaga, S. Kishimoto, T. J. Mizutani, T. Inoue, R. Taniguchi, N. Fukui, H. Okubo and H. Shinohara, Physica E, 2004, 21, 1089–1092 CrossRef CAS.
- T. Shimada, Y. Ohno, K. Suenaga, T. Okazaki, S. Kishimoto, T. Mizutani, R. Taniguchi, H. Kato, B. P. Cao, T. Sugai and H. Shinohara, Jpn. J. Appl. Phys., Part 1, 2005, 44, 469–472 CrossRef CAS.
- T. Shimada, T. Okazaki, R. Taniguchi, T. Sugai, H. Shinohara, K. Suenaga, Y. Ohno, S. Mizuno, S. Kishimoto and T. J. Mizutani, Appl. Phys. Lett., 2002, 81, 4067–4069 CrossRef CAS.
- Y. Nakayama, S. Fujiki, Y. Hirado, H. Shiozawa, H. Ishii, T. Miyahara, Y. Maniwa, T. Kodama, Y. Achiba, H. Kataura, Y. Kubozono, M. Nakatake and T. Saitoh, Phys. Status Solidi B, 2008, 245, 2025–2028 CrossRef CAS.
- A. A. Taherpour, Chem. Phys. Lett., 2009, 483, 233–240 CrossRef CAS.
- P. W. Fowler and D. E. Manolopoulos, An Atlas of Fullerenes, Clarendon Press, Oxford, 1995 Search PubMed.
- K. Kikuchi, N. Nakahara, T. Wakabayashi, S. Suzuki, H. Shiromaru, Y. Miyake, K. Saito, I. Ikemoto, M. Kainosho and Y. Achiba, Nature, 1992, 357, 142 CrossRef CAS.
- M. Zalibera, A. A. Popov, M. Kalbac, P. Rapta and L. Dunsch, Chem. – Eur. J., 2008, 14, 9960–9967 CrossRef CAS PubMed.
- G. Sun and M. Kertesz, J. Phys. Chem. A, 2001, 105, 5468–5472 CrossRef CAS.
- A. Marcelli, W. Xu, L. Liu, C. Wang, W. Chu and Z. Wu, J. Nanophotonics, 2009, 3, 031975 CrossRef.
- L. Feng, T. Wakahara, T. Tsuchiya, Y. Maeda, Y. Lian, T. Akasaka, N. Mizorogi, K. Kobayashi, S. Nagase and K. M. Kadish, Chem. Phys. Lett., 2005, 405, 274–277 CrossRef CAS.
- A. R. Khamatgalimov and V. I. Kovalenko, J. Phys. Chem. A, 2011, 115, 12315–12320 CrossRef CAS PubMed.
- H. Gao, W. H. Zhu, C. M. Tang, F. F. Geng, C. D. Yao, Y. L. Xu and K. M. Deng, Acta Phys. Sin., 2010, 59, 1707–1711 Search PubMed.
- B. Gao, L. Liu, C. Wang, Z. Wu and Y. Luo, J. Chem. Phys., 2007, 127, 164314 CrossRef PubMed.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- P. C. Hariharan and J. A. Pople, Theor. Chim. Acta, 1973, 28, 213–222 CrossRef CAS.
- R. Yamakado, K. Mikami, K. Takagi, I. Azumaya, S. Sugimoto, S.-I. Matsuoka, M. Suzuki, K. Katagiri, M. Uchiyama and A. Muranaka, Chem. – Eur. J., 2013, 19, 11853–11857 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- S. Grimme, J. Comput. Chem., 2004, 25, 1463–1473 CrossRef CAS PubMed.
- S. Grimme, J. Chem. Phys., 2003, 118, 9095–9102 CrossRef CAS.
- S. F. Boys and F. Bernardi, Mol. Phys., 1970, 19, 553–566 CrossRef CAS.
- N. Godbout, D. R. Salahub, J. Andzelm and E. Wimmer, Can. J. Chem., 1992, 70, 560–571 CrossRef CAS.
- B. Jeziorski, R. Moszynski and K. Szalewicz, Chem. Rev., 1994, 94, 1887–1930 CrossRef CAS.
- A. Hesselmann, G. Jansen and M. Schütz, J. Chem. Phys., 2005, 122, art. 054306 Search PubMed.
- R. Podeszwa and K. Szalewicz, Chem. Phys. Lett., 2005, 412, 488–493 CrossRef CAS.
- M. Grüning, O. V. Gritsenko, S. J. A. van Gisergen and E. J. Baerends, J. Chem. Phys., 2001, 114, 652–660 CrossRef.
- D. M. P. Holland, A. W. Potts, L. Karlsson, I. L. Novak, I. L. Zaytseva, A. B. Trofimov, E. V. Gromov and J. Schirmer, J. Phys. B: At., Mol. Opt. Phys., 2010, 43, 135101 CrossRef.
- M. Jeziorska, B. Jeziorski and J. Cizek, Int. J. Quantum Chem., 1987, 32, 149–164 CrossRef CAS.
- T. Helgaker, W. Klopper, H. Koch and J. Noga, J. Phys. Chem., 1997, 106, 9639–9646 CrossRef CAS.
- A. Anoop, W. Thiel and F. Neese, J. Chem. Theory Comput., 2010, 6, 3137–3144 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- H.-J. Werner, P. J. Knowles, G. Knizia, F. R. Manby, M. Schuetz, P. Celani, T. Korona, R. Lindh, A. Mitrushenkov, G. Rauhut, K. R. Shamasundar, T. B. Adler, R. D. Amos, A. Bernhardsson, A. Berning, D. L. Cooper, M. J. O. Deegan, A. J. Dobbyn, F. Eckert, E. Goll, C. Hampel, A. Hesselmann, G. Hetzer, T. Hrenar, G. Jansen, C. Koeppl, Y. Liu, A. W. Lloyd, R. A. Mata, A. J. May, S. J. McNicholas, W. Meyer, M. E. Mura, A. Nicklass, D. P. O'Neill, P. Palmieri, D. Peng, K. Pflueger, R. Pitzer, M. Reiher, T. Shiozaki, H. Stoll, A. J. Stone, R. Tarroni, T. Thorsteinsson and M. Wang, Molpro, version 2012.1, a package of ab initio programs, Cardiff, UK, 2012 Search PubMed.
- R. Bauernschmitt and R. Ahlrichs, Chem. Phys. Lett., 1996, 256, 454–464 CrossRef CAS.
- M. E. Casida, C. Jamorski, K. C. Casida and D. R. Salahub, J. Chem. Phys., 1998, 108, 4439–4449 CrossRef CAS.
- R. E. Stratmann, G. E. Scuseria and M. J. Frisch, J. Chem. Phys., 1998, 109, 8218–8224 CrossRef CAS.
- T. Yanai, D. P. Tew and N. C. Handy, Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS.
- E. V. Lenthe, J. G. Snijders and E. J. Baerends, J. Chem. Phys., 1996, 105, 6505–6516 CrossRef.
- E. V. Lenthe, E. J. Baerends and J. G. Snijders, J. Chem. Phys., 1993, 99, 4597–4610 CrossRef.
- E. J. Baerends, J. Autschbach, A. Berces, F. M. Bickelhaupt, C. Bo, P. M. Boerrigter, L. Cavallo, D. P. Chong, L. Deng, R. M. Dickson, D. E. Ellis, M. van Faassen, L. Fischer, T. H. Fan, C. Fonseca Guerra, S. J. A. van Gisbergen, J. A. Groeneveld, O. V. Gritsenko, M. Gruning, F. E. Harris, P. van den Hoek, C. R. Jacob, H. Jacobsen, G. Jensen, G. van Kessel, F. Kootstra, E. van Lenthe, D. A. McCormack, A. Michalak, J. Neugebauer, V. P. Osinga, S. Patchkovskii, P. H. T. Philipsen, D. Post, C. C. Pye, W. Ravenek, P. Ros, P. R. T. Schipper, G. Schreckenbach, J. G. Snijders, M. Sola, M. Swart, D. Swerhone, G. teVelde, P. Vernooijs, L. Versluis, L. Visscher, O. Visser, F. Wang, T. A. Wesolowski, E. van Wezenbeek, G. Wiesenekker, S. Wolff, T. Woo, A. Yakovlev and T. Ziegler, http://www.scm.com/, Vrije Universiteit: Amsterdam, The Netherlands, 2014.
- S. Grimme, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2011, 1, 211–228 CrossRef CAS.
- E. V. Lenthe and E. J. Baerends, J. Comput. Chem., 2003, 24, 1142–1156 CrossRef PubMed.
- A. Klamt and G. Schuurmann, J. Chem. Soc., Perkin Trans. 2, 1993, 799–805 RSC.
- T. Kupka, M. Stachów, M. Nieradka and L. Stobiński, Magn. Reson. Chem., 2011, 49, 549–557 CrossRef CAS PubMed.
- T. Kupka, M. Stachów, E. Chełmecka, K. Pasterny and L. Stobiński, Synth. Met., 2012, 162, 573–583 CrossRef CAS.
- T. Kupka, M. Stachów, E. Chełmecka, K. Pasterny, M. Stobińska and L. Stobiński, J. Chem. Theory Comput., 2013, 9, 4275–4286 CrossRef CAS PubMed.
- R. T. Sanderson, J. Am. Chem. Soc., 1983, 105, 2259–2261 CrossRef CAS.
- L. Pauling, J. Am. Chem. Soc., 1932, 54, 3570–3582 CrossRef CAS.
- A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
- A. E. Reed, L. A. Curtiss and F. Weinhold, Chem. Rev., 1988, 88, 899–926 CrossRef CAS.
- R. F. W. Bader, Atoms in Molecules – A Quantum Theory, Oxford University Press, Oxford, 1990 Search PubMed.
- P. Hobza, H. L. Selzle and H. W. Schlag, J. Phys. Chem., 1996, 100, 18790–18794 CrossRef CAS.
- M. Hesselmann and T. Korona, J. Chem. Phys., 2014, 141, 094107 CrossRef PubMed.
- P. J. Stephens, F. J. Devlin and J. R. Cheeseman, VCD Spectroscopy for Organic Chemists, CRC Press, Boca Raton, 2012 Search PubMed.
- T. A. Keiderling, in Circular Dichroism: Principles and Applications, ed. N. Berova, K. Nakanishi and R. W. Woody, Wiley-VCH, New York, 2nd edn, 2000, pp. 621–666 Search PubMed.
- P. J. Stephens, F. J. Devlin and J.-J. PAN, Chirality, 2008, 20, 643–663 CrossRef CAS PubMed.
- B. Ranjbar and P. Gill, Chem. Biol. Drug Des., 2009, 74, 101–120, DOI:10.1111/j.1747-0285.2009.00847.x.
- J. Crassous and S. Hediger, J. Phys. Chem. A, 2003, 107, 10233–10240 CrossRef CAS.
- K. Komatsu, M. Murata and Y. Murata, in XIX International Winterschool on Electronic Properties of Novel Materials, ed. H. Kuzmany, Kirchberg, Tirol, 2005 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Excitation energies, changes in free energies, charges, IR and NMR results as well as the optimized geometries of the molecules studied. See DOI: 10.1039/c6cp05030a |
| This journal is © the Owner Societies 2016 |