 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Modelling the chemistry of Mn-doped MgO for bulk and (100) surfaces†
Andrew J.
Logsdail
*ab,
Christopher A.
Downing
ac,
Thomas W.
Keal
c,
Paul
Sherwood
c,
Alexey A.
Sokol
 a and
C. Richard A.
Catlow
abd
a and
C. Richard A.
Catlow
abd
aKathleen Lonsdale Materials Chemistry, Department of Chemistry, University College London, 20 Gordon Street, London, WC1H 0AJ, UK. E-mail: a.logsdail@ucl.ac.uk; Tel: +44 (0)20 7679 0312
bCardiff Catalysis Institute, School of Chemistry, Cardiff University, Cardiff, CF10 3AT, UK
cScientific Computing Department, STFC Daresbury Laboratory, Daresbury, Warrington, WA4 4AD, UK
dUK Catalysis Hub, Research Complex at Harwell, Science and Technology Facilities Council Rutherford Appleton Laboratory, Harwell Science and Innovation Campus, Oxon, OX11 0QX, UK
First published on 26th September 2016
Abstract
We have investigated the energetic properties of Mn-doped MgO bulk and (100) surfaces using a QM/MM embedding computational method, calculating the formation energy for doped systems, as well as for surface defects, and the subsequent effect on chemical reactivity. Low-concentration Mn doping is endothermic for isovalent species in the bulk but exothermic for higher oxidation states under p-type conditions, and compensated by electrons going to the Fermi level rather than cation vacancies. The highest occupied dopant Mn 3d states are positioned in the MgO band gap, about 4.2 eV below the vacuum level. Surface Mn-doping is more favourable than subsurface doping, and marginally exothermic on a (100) surface at high O2 pressures. For both types of isovalent Mn-doped (100) surfaces, the formation energy for catalytically important oxygen defects is less than for pristine MgO, with F0 and F2+-centres favoured in n- and p-type conditions, respectively. In addition, F+-centres are stabilised by favourable exchange coupling between the Mn 3d states and the vacancy-localised electrons, as verified through calculation of the vertical ionisation potential. The adsorption of CO2 on to the pristine and defective (100) surface is used as a probe of chemical reactivity, with isovalent subsurface Mn dopants mildly affecting reactivity, whereas isovalent surface-positioned Mn strongly alters the chemical interactions between the substrate and adsorbate. The differing chemical reactivity, when compared to pristine MgO, justifies further detailed investigations for more varied oxidation states and dopant species.
1 Introduction
Rocksalt alkali-earth oxides, such as CaO and MgO, are amongst the materials currently under investigation for adsorbing and catalysing the transformation of CO2.1,2 The bulk and surface structures of these oxides have been extensively studied,3–5 owing both to the simplicity of the rocksalt geometry and also the high degree of ionicity, which gives rise to a relatively simple electronic structure.6,7 Additionally, basic oxides such as MgO are popular as catalyst support materials but have displayed interesting reactivity when dopants or defects are included,8 which opens up the possibility for their use as catalysts in their own right.3,9–12 Whilst the surface chemistry and reactivity of MgO is well studied, only recently have investigations begun to show the potential of MnO in areas such as photocatalytic water splitting and CO2 reduction applications when alloyed with ZnO,13 and the (100) MnO surface has also been considered for CO2 adsorption as part of a systematic study of transition metal monoxides.14 The doping of rocksalt alkali-earth oxides with transition metals has proven to result in novel physical and catalytic properties15–17 and thus the catalytic reactivity of MnO, coupled with the stability of the MgO surface, makes Mn-doped MgO an appealing material when designing novel catalytic systems. Furthermore, Mn2+ can directly replace Mg2+ without significant structural distortion as the ions are roughly equivalent in size; Mn-doped MgO is therefore structurally very similar to pristine MgO but can be differing in chemical reactivity, due to the range of stable oxidation states for the Mn-dopant.18–21Previous studies on Mn-doped MgO, which we refer to henceforth as Mn–MgO, have focused on the enthalpies of mixing for MgO and MnO,22–24 which are generally positive (endothermic) and small (<0.1 eV), as well as formation energies and defect properties for low concentration isovalent Mn-dopants with the aim of finding a dilute magnetic semiconductor (DMS) for use in spintronic applications.25–31 In particular, recent work has emphasised understanding the consequences of increasing Mn concentration and the coupling between extrinsic and intrinsic defects for both bulk and discrete systems. Experimental and modelling work by Azzaza et al. has shown that increasing the Mn content in MgO decreases the band gap and Curie temperature for dopant concentrations of 5 to 40%, and magnetic interaction between isovalent Mn dopants and oxygen vacancies is postulated as influencing the stability.27 Panigrahi et al. showed via computation that isovalent Mn dopants are favourably positioned at (100) surfaces, rather than in the bulk, for an MgO slab model, and that short-range anti-parallel coupling between multiple Mn dopants is favoured in the absence of intrinsic defects;30 they then additionally highlighted that exchange coupling between isovalent Mn dopants and oxygen vacancies was also energetically favourable for bulk and surface models.30,31 MgO surfaces are commonly host to significant concentrations of oxygen defects8,32 and, in the presence of the Mn 3d dopants, strong hybridisation between the Mn 3d and O 2p bands30 means that novel chemistry of the material could be driven by the interchange of electrons between the redox active manganese atom and oxygen vacancy, e.g.:
| Mn+ + F2+ ⇌ Mn2+ + F+ ⇌ Mn3+ + F0 | (1) |
 ,
,  and
and  , respectively, and the equivalent equations are:
, respectively, and the equivalent equations are: | (2) |
2 Computational methodology
Calculations were performed using the hybrid quantum mechanical and molecular mechanical (QM/MM) embedded-cluster methodology that has previously proven to be accurate in modelling the bulk and surface chemistry of MgO.8,34–43 Our chosen QM/MM implementation is the software environment ChemShell,44–46 with QM and MM energy evaluations performed with the NWChem47 and GULP48–50 software packages, respectively, and geometry optimisation was performed using the DL-FIND software routines.45For the QM calculations, density functional theory (DFT) was used with the B3LYP51–53 and B97-354 exchange–correlation (XC) functionals. Results obtained using B3LYP provide a direct comparison with previous work for MgO,42 while B97-3 provides comparison with an XC functional that was fitted to a more extensive set of molecular thermochemical data. A Def2 triple-zeta valence plus polarisation (TZVP) basis set55 was used for all atoms except Mn, where a Stuttgart/Dresden relativistic small-core effective core potential (ECP) was used with a complementing modified basis set:56 the most diffuse functions were removed to prevent the artificial spreading of charge density outside the QM region. Large core ECP57 for Mg2+ ions were placed on cations at the border of the QM region, representing the QM/MM boundary. The redox-active Mn dopant can adopt different energetically-competitive spin configurations, particularly when neighbouring an unpaired electron on an oxygen vacancy (F+), and therefore the spin-states were explicitly checked. High-spin (HS) configurations were energetically favoured for defect-free systems, which preserves the unpaired Mn d5 configuration in Mn–MgO in agreement with previous calculations.26,28 For pure MgO, a closed-shell configuration was always preferable. Unless otherwise stated, all calculations use these configurations.
The MM energy is calculated using a polarisable interatomic forcefield with Coulomb (ECoulMM), Buckingham (EBuckMM) and spring (EspringMM) terms that are defined as:49
 | (3) |
 | (4) |
 | (5) |
| Coul. & spring | q core | q shell | k 2 | k 4 |
|---|---|---|---|---|
| Mg | 0.415 | 1.585 | 361.60 | 0.0 |
| Mn | −1.420 | 3.420 | 95.00 | 0.0 |
| O | 1.000 | −3.000 | 54.76 | 0.0 |
| Buck. | A | ρ | C | r c |
|---|---|---|---|---|
| Mgshell–Oshell | 1428.5 | 0.2945 | 0.0 | 12.0 |
| Mnshell–Oshell | 1007.4 | 0.3262 | 0.0 | 12.0 |
| Oshell–Oshell | 22764.3 | 0.1490 | 23.0 | 12.0 |
A spherical or hemispherical QM/MM cluster was used for bulk and surface models, respectively, with a total radius of 30 Å centred on either an oxygen atom or vacancy. The central QM region contained a total of 33 atoms for the bulk and from 22 to 26 atoms for the surface (e.g.Fig. 2); this setup previously produced accurate results for MgO.42 The radius of the active MM region was set to 15 Å as atoms beyond this distance did not move during previous geometry optimisations.42 This partitioning meant that ∼1500(∼850) atoms underwent ionic relaxation in our bulk (slab) models, with atoms beyond 15 Å fixed in their lattice positions. The lattice constant was obtained for MgO using GULP and the forcefield of choice. Once cut, the surface clusters were oriented so that the x- and y-axes are in the plane of the surface, and the z-axis is perpendicular to the surface.
The total energy of the system is defined additively as:
| ETotal = EQM + EMM + EJost | (6) |
 | (7) |
 | (8) |
3 Mn-doped MgO bulk
In order to test and validate our QM/MM model, we calculated the energy required to replace Mg with an Mn atom in MgO. The reaction scheme of this substitution is:| MgO(s) + Mn(ref) → Mn–MgO(s) + Mg(ref), | (9) |
| Ef(Mn–MgO) = E(Mn–MgO) − E(MgO) + E(Mg) − E(Mn) + qεf, | (10) |
 ) formed as:
) formed as: | (11) |
 can be replaced with Mg×at with the corresponding holes instead localised on the extrinsic defects, with substitution of the formation energy for the Mg vacancy in the place of εf in eqn (10) giving:
can be replaced with Mg×at with the corresponding holes instead localised on the extrinsic defects, with substitution of the formation energy for the Mg vacancy in the place of εf in eqn (10) giving: | (12) |
 appears due to the double charge on the Mg vacancy. In all cases, the energy of the Mg reference state is:
appears due to the double charge on the Mg vacancy. In all cases, the energy of the Mg reference state is:| E(Mg) = E(Mgat) + μ(Mg), | (13) |
Mg(s) and Mn(s) are the reference states at low oxygen partial pressure, p(O2), and thus their vaporisation enthalpies of 143.38 and 279.58 kJ mol−1 are used to derive μ(Mg) − μ(Mn) = 136.2 kJ mol−1 (1.41 eV). In a physical sense, a positive value means the stronger bonding in the Mn(s) reactant would inhibit the reaction. In contrast, for high p(O2) the reference states are MgO(s) and MnO(s), respectively. In this case, the formation enthalpy for MgO (−601.6 kJ mol−1) and MnO (−385.2 kJ mol−1) are combined with the values above to give μ(Mg) − μ(Mn) = −80.2 kJ mol−1 (−0.83 eV), with the negative value indicating favourability for the MgO product over the MnO reactant.
Our results for isovalent Ebulkf(Mn–MgO) are presented in Table 2. The formation of Mn-dopants at low and high p(O2) are endothermic, with energies of 2.73(2.55) and 0.49(0.31) eV, respectively, for the B3LYP(B97-3) XC functional. Our high p(O2) results match previous work by Konigstein et al. and Heath et al. for the enthalpies of mixing (ΔHmix) of MgO and MnO: 12.5% MnO in MgO was found to be endothermic by 0.03 and 0.05–0.06 eV at the GULP and Hartree–Fock levels of theory, respectively,22,24 in good agreement with experiment (0.02 eV).23 We consider that ΔHmix(MgO/MnO) is lower than for our results due to the relaxation of the bulk lattice constants in this previous work;22 an effect that is not included in our calculations. Interestingly, all of these results are contrary to the Mn-doping DFT calculations of Panigrahi et al.30 where results using the generalised-gradient approximation65 give Ebulkf(Mn–MgO) = −0.1 eV for a 64-atom unit cell, relative to atomic reference states, and adjustment for a high p(O2) environment gives Ef(Mn–MgO) = −0.93 eV, which is surprisingly exothermic.
| B3LYP | B97-3 | MM | |
|---|---|---|---|
| Mnbulk | 2.73(0.49) | 2.55(0.31) | −(0.23) |
| Mnsub | 2.67(0.43) | 2.53(0.28) | −(0.23) |
| Mnsurf | 2.16(−0.08) | 2.00(−0.24) | −(−0.35) |
| 5.354(516.566) |
Structural analysis of the B3LYP calculation shows that the Mn–O bond length in the doped system is 2.18 Å, which is an extension of the Mg–O bond length (2.12 Å); identical extension of the metal–oxygen bond occurs when the XC is B97-3. Our results are in broad agreement with experimental lattice parameters for MgO (2.11 Å) and MnO (2.22 Å),64 and with previous computational reports for isolated Mn dopants in MgO, which give an Mn–O bond distance of ∼2.1 Å.30
Fig. 1 illustrates the formation energy for Mn dopants of differing charge states in MgO, in the range 0 ≤ q ≤ 5, within the limits of εf. This graph can be considered as a guide to the favoured oxidation state of an Mn-dopant in either p- or n-type conditions. The lower bound for εf is the valence band maximum (VBM), which we position as the negative of the vertical ionisation potential (VIP) for MgO. QM/MM allows us to calculate the VIP for MgO as 6.27(6.51) eV for the B3LYP(B97-3) XC functional using the ΔSCF technique,66 which is a slight underestimate of experiment (7.16 eV)67 and lower than our previous estimates using XC functionals tuned for bulk materials (PBE0: 6.89 eV; PBESol0: 6.97 eV).6 The upper bound for εf would typically be the conduction band minimum (CBM) but the band gap of MgO (7.83 eV)68 is greater than the VIP, which means the conduction band is actually above the vacuum level. As electrons would not favour going into a CBM with a positive energy, the vacuum level (0 eV) becomes the upper bound for εf. Comparison of the Mulliken analysis for the bulk MgO and [MgO]+ models shows that the electrons are ionised from the 2p states of the central oxygen, though the remaining electron hole is delocalised over neighbouring atoms as also previously documented for MgO surfaces.35 In addition to these observations, we also calculated the 1st and 2nd VIPs for Mnbulk–MgO to be 4.27(4.38) and 5.62(5.86) eV, respectively, which means that the highest occupied Mn 3d states are positioned in the bandgap, in agreement with previous reports.25–27 For higher order VIPs, the Mn 3d states hybridise with the O 2p states in the energy range of −8 to −11 eV to form ill-defined resonance states.
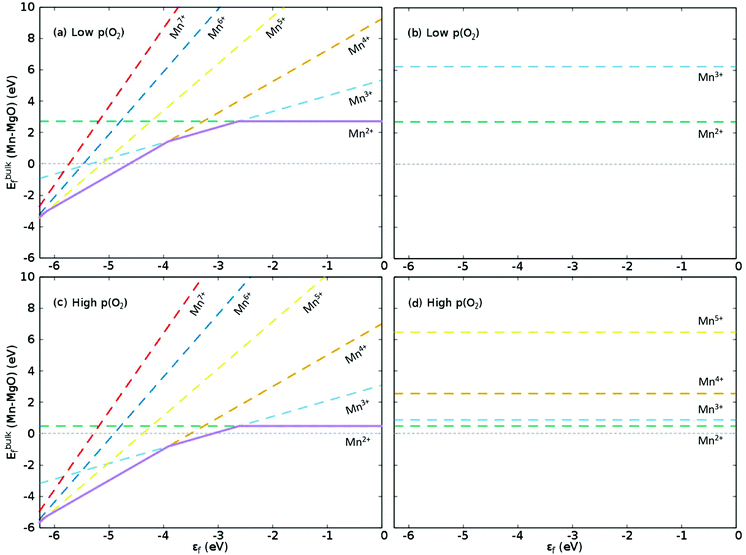 | ||
Fig. 1
E
f for bulk Mn dopants in MgO when using the B3LYP XC functional at (a and b) low and (c and d) high p(O2), with εf ranging from the VBM (−6.27 eV) through to vacuum level (0 eV). (a and c) Show Ef as compensated by electrons transferring to the Fermi level, εf, as calculated with eqn (10) whilst (b) and (d) show Ef as compensated by cation (Mg) vacancies (eqn (12)). Dashed green, aqua, gold, yellow, navy blue and red lines show Ef when the defect charge is respectively q = 0, 1, 2, 3, 4 and 5, i.e. ranging from Mn2+ to Mn7+ as Mn×Mg,  , ,  , ,  , ,  and and  , respectively. The overall preferred defect charge as a function of εf, which is found to be compensated by the Fermi level in all cases, is given as a solid purple line in (a) and (c). , respectively. The overall preferred defect charge as a function of εf, which is found to be compensated by the Fermi level in all cases, is given as a solid purple line in (a) and (c). | ||
The graphs for Fermi level-compensated Mn-dopants in (a) low and (c) high p(O2) in Fig. 1 are clear translations of each other, with both showing that a like-for-like replacement of Mg with Mn is preferred when εf is close to 0 eV. Reducing εf results in a shift towards higher oxidation states, which may be attributed to the stability of Mn in 3+, 4+ and 5+ oxidation states; in experiment the position of εf would be strongly dependent on factors such as the dopant concentration. For low p(O2), Ef for Mn4+ is exothermic below εf = −4.63 eV, and for high p(O2) the formation of Mn3+ and Mn4+ is exothermic below εf = −3.10 and −3.51 eV, respectively, with formation of higher oxidation states also exothermic at lower εf in both cases: Mn5+ is the most favourable dopant state in both environments in the range of εf from −6.13 to −6.27 eV. We note from graphs (b) and (d) in Fig. 1, and Table 3, that using Mg vacancies as compensating defects results in higher formation energies than using electron compensation, though the Mn3+ defect is competitive with the isovalent defect at high p(O2), differing by 0.38 eV. This observation is general to our calculations for B3LYP and B97-3, as shown in Table 3, with only the former plotted in Fig. 1 and discussed in detail here: the difference in Ef for isovalent (2+) and 3+ Mn-dopants when using the latter XC functional is also 0.38 eV.
| q | ε f = 0 eV | ε f = VBM | |||
|---|---|---|---|---|---|
| Mnbulk | 0 | 0.49 | (0.31) | 0.49 | (0.31) |
| 1 | 3.10 | (3.11) | −3.18 | (−3.40) | |
| 2 | 7.01 | (7.23) | −5.53 | (−5.79) | |
| 3 | 13.15 | (13.49) | −5.67 | (−6.03) | |
| 1a | 0.87 | (0.68) | 0.87 | (0.68) | |
| 2a | 2.55 | (2.37) | 2.55 | (2.37) | |
| 3a | 6.45 | (6.20) | 6.45 | (6.20) | |
| Mnsub | 0 | 0.43 | (0.28) | 0.43 | (0.28) |
| 1 | 3.00 | (3.00) | −3.15 | (−3.40) | |
| 2 | 7.13 | (7.37) | −5.17 | (−5.42) | |
| 3 | 13.67 | (13.95) | −4.78 | (−5.23) | |
| 1a | 0.76 | (0.58) | 0.76 | (0.58) | |
| 2a | 2.67 | (2.50) | 2.67 | (2.50) | |
| 3a | 6.98 | (6.65) | 6.98 | (6.65) | |
| Mnsurf | 0 | −0.08 | (−0.24) | −0.08 | (−0.24) |
| 1 | 2.54 | (2.56) | −3.60 | (−3.83) | |
| 2 | 7.54 | (7.72) | −4.76 | (−5.06) | |
| 3 | 14.16 | (14.32) | −4.29 | (−4.85) | |
| 1a | 0.31 | (0.13) | 0.31 | (0.13) | |
| 2a | 3.08 | (2.86) | 3.08 | (2.86) | |
| 3a | 7.46 | (7.03) | 7.46 | (7.03) | |
In general, it is noted that Mn-doping is exothermic in p-type conditions, both at low and high p(O2). As the dopant concentration increases the material will become more n-type due to the presence of Mn-defect states in the band gap, resulting in changes to the most stable Mn oxidation state: at εf = −4.27(−4.38) eV, Mn4+ is thermodynamically favoured. Lower oxidation states, such as isovalent Mn2+, are endothermic in formation and would require n-type conditions during synthesis. Typically, n-type conditions are achieved by using low p(O2), which results in oxygen vacancies with defect states high in the band gap, as discussed later; surface oxygen vacancies are also of interest for catalytic purposes, where the F-centres can play an important role in reactivity. Therefore, the material properties could be tuned with careful synthesis protocols.
Due to the similarity of our results thus far for the different XC functionals, only those acquired using the B3LYP XC functional will be discussed subsequently unless explicitly stated.
4 Mn-doped MgO(100) surface
4.1 Position of Mn-dopant
As illustrated in Fig. 2, two different QM/MM clusters were used for the Mn–MgO surfaces: (a) the Mn dopant positioned in the surface layer (Layer 1, L1), adjacent to the central oxygen; (b) the Mn dopant situated in the first subsurface layer (Layer 2, L2), directly beneath the central oxygen. Henceforth, these two configurations will be referred to as Mnsurf and Mnsub, respectively. The Cartesian axes of the QM/MM cluster have been defined so that the xy-plane aligns with the surface-plane of the model; thus Mnsub occupies the Mg lattice position (0,0,z) and Mnsurf occupies the Mg lattice position (x,0,0), with the primary oxygen lattice site (O5c or F-centre) at (0,0,0). | ||
| Fig. 2 QM cluster illustrating the Mn-dopant in (a) surface (Mnsurf) and (b) subsurface (Mnsub) positions. O atoms are shown in red, Mg in green and Mn in purple. Bond lengths are given in Å. | ||
Our results, which are presented in Tables 2 and 3, show that formation of isovalent Mn-doped surface layers is exothermic at high p(O2) when compensated using εf, whereas formation of subsurface dopants is very similar to bulk behaviour, being endothermic for all p(O2). With reduction of εf from 0 eV towards the VBM, higher oxidation states become preferable with Mn4+ the most stable at εf = VBM. In contrast, cation-vacancy compensated defects remain endothermic (Table 3). Overall, Mnsurf is energetically favoured over Mnsub (and Mnbulk) for q = 0 and 1 as the electronic and structural flexibility of the surface minimises any strain induced by the discrepancy in radius between Mn and Mg (0.83 vs. 0.72 Å for 2+ cations, respectively).69 In addition, at these charge states Ef(Mnsurf) < Ef(Mnbulk), whereas Ef(Mnsub) ≈ Ef(Mnbulk). We note that low-spin (LS) Mn2+ is in fact structurally smaller (0.67 Å) than HS Mn2+,69 in octahedral coordination, but a half-filled d-shell with no degenerate occupancy (HS d5) is energetically preferable, which also means no Jahn–Teller distortion is observed, in agreement with previous calculations.30 For q > 1, it is apparent that long-distance bulk electrostatics are more important, with Mn5+ unstable with respect to Mn4+ at the surface and sub-surface, yet preferred in the bulk.
On the un-doped MgO(100) surface, the Mg–O bond lengths are 2.12 Å in the xy-plane and the z-direction (L1 to L2). These bonds extend to 2.17 and 2.23 Å, respectively, for isovalent Mnsurf and the O–Mnsurf–O bond angles are 90.0° in the xy-plane but as low as 88.5° and 87.3° in the xz- and yz-planes, respectively. The same bond angles are 91.6° for O–Mg–O, showing that the Mn dopants protrude outwards from the (100) surface whereas Mg withdraws inwards. For isovalent Mnsub, Mn–O bond lengths are similarly extended to 2.17 Å in the xy-plane (L2–L2) and 2.22(2.19) Å in the z-direction between L1 and L2 (L2 and Layer 3, L3), which is partly due to the relaxation of the surface oxygen atom outwards from the surface.7 For comparison, the same bonds for Mg–O in the pure surface are 2.18 and 2.07 Å for L1–L2 and L2–L3 interactions, which shows that the subsurface bond lengths increase by >0.1 Å due to the Mnsub-dopant. The O–Mnsub–O bond angles within the xy-plane were maintained at 90.0°, similar to Mnsurf, whilst the elongation of the bonds in the z-direction, and upwards movement of Mnsub, creates bond angles of up to 90.6° in the xz- and yz-planes between the L1 and L2 oxygens.
In order to place the VBM for the surface, as well as to ascertain which atoms contribute to the band edges and dopant states, we calculated the VIP and the adiabatic ionisation potential (AIP) of the pure and Mn-doped MgO(100) surfaces (Fig. 3). For the MgO surface a VIP of 6.15(6.39) eV is calculated with the B3LYP(B97-3) XC functional, which is in fair agreement with experiment (6.7 ± 0.4 eV)70 though slightly lower than previous results from periodic DFT using XC functionals for bulk materials (6.52–6.57 eV)7 and QM/MM calculations with the B3LYP XC functional (6.46, ∼6.5 eV).35,42 We attribute this difference to the incomparable XC potentials used in the previous periodic DFT,7 the improvements in the MM forcefield and basis sets when compared to our own prior work,42 and an absence of long-range corrections (EJost) in the work of Sushko et al.;35 if we remove EJost from our calculations to match the latter work, the agreement between results improves with our VIP becoming 6.39(6.63) eV for the B3LYP(B97-3) XC functional. In all cases, Mulliken analysis shows that the electron removed during ionisation is taken from the surface oxygen atoms, which associates the VBM with O 2p states, in agreement with well-developed theories of metal oxide band structures.71,72 We also calculated the AIP of MgO(100) to be 5.21(5.23) eV, for the B3LYP(B97-3) XC functional, i.e. the relaxation of the nuclei around an electronic defect lowers the energy of the system by 0.94(1.16) eV. The smaller VIP of the MgO surface when compared to bulk MgO is due to surface band bending, as we have discussed previously elsewhere.7
The VIP for Mnsurf– and Mnsub–MgO is calculated as 6.16(6.41) and 6.14(6.32) eV, respectively, when using the B3LYP(B97-3) XC functional with a septuplet multiplicity i.e. an ionised electron is removed from the O 2p orbitals, with the HS Mn d5 configuration preserved. Unsurprisingly, these results match the VIP for the pure MgO surface, reported above, as the O 2p states contribute to the VBM. However, changing our setup to an intermediate quintet spin-configuration, with the ionised electron removed instead from the Mn 3d states to give an Mn d4 electronic configuration, the VIP is 4.40(4.63) and 4.20(4.32) eV for the same dopant systems and XC functionals, respectively (Fig. 3). The smaller VIP indicates that the highest occupied Mn 3d states are 1.76–1.94 eV above the O 2p states and thus in the band gap, matching the bulk systems (Section 3); the VIP of Mnsub–MgO is noted as being similar to the bulk, for which the VIP was calculated as 4.27(4.38) eV. Mulliken analysis further confirms that the electrons are removed from the Mn 3d and O 2p orbitals on ionisation calculated with multiplicities of 5 and 7, respectively. Full localisation of the remaining holes is not achieved with 0.55 and 0.2 e removed from the Mn and central O in each respective calculation; however the hole does fully localise on the Mn dopant after geometry relaxation of the HS configuration. The AIP for the intermediate spin-state (IS) was calculated for Mnsurf– and Mnsub–MgO as 2.62(2.81) and 2.56(2.71) eV, respectively, using the B3LYP(B97-3) XC functional; the energetic relaxation of the nuclei, and localisation of the hole, results in significant stabilisation by 1.61–1.83 eV. In addition, higher-order 2nd and 3rd VIPs were calculated for Mnsurf– and Mnsub–MgO: the results for Mnsub–MgO match those of Mnbulk with the 2nd Mn 3d state at −6.02 eV, just above the VBM, whilst the 3rd Mn 3d state is in the valence band i.e. a resonance state. For Mnsurf–MgO, the 2nd Mn 3d state is already in the valence band, showing that the surface strongly stabilises the 3d states and indicating a reason for the improved stability of the Mn-dopant at the surface.
4.2 Oxygen vacancy formation
Oxygen vacancies that contain trapped electrons on the surface of a material, i.e. surface F-centres,8 have previously been postulated as active sites for the conversion of CO2 to CH3OH on metal oxides, e.g. Cu/ZnO/Al2O3.46,73–77 As discussed in Section 3, the formation of such vacancies would require careful material preparation, however their presence would be beneficial for catalytic applications. Therefore, we have calculated the formation energies of oxygen vacancies at the Mn–MgO(100) surface, as presented in Table 4. The formation energy, Ef(Fq+), was calculated relative to the energy of the Mn-doped surface, E(Mn–MgO), and half an oxygen dimer, :
: | (14) |
| q | Spin | Mnsurf | Mnsub | MgO42 | ||
|---|---|---|---|---|---|---|
| B3LYP | B97-3 | B3LYP | B97-3 | B3LYP | ||
| 0 | IS | 6.16(6.16) | 5.86(5.86) | 6.13(6.13) | 5.85(5.85) | 6.59(6.59) |
| 1 | IS | 7.64(1.49) | 7.49(1.10) | 7.59(1.44) | 7.43(1.04) | 8.26(1.80) |
| 1 | HS | 7.92(1.77) | 7.75(1.36) | 7.88(1.73) | 7.71(1.32) | — |
| 2 | IS | 11.65(−0.65) | 12.24(−0.54) | 11.09(−1.21) | 11.11(−1.67) | 10.87(−2.05) |
| 2 | HS | 10.48(−1.82) | 10.38(−2.40) | 10.41(−1.89) | 10.28(−2.50) | — |
A closed-shell configuration is energetically favoured for a neutral F0-centre on the (100) surface, with both electrons occupying the oxygen vacancy, i.e. V×O, and the Mn2+ ion maintaining an open-shell d5 valence configuration. We denote this as intermediate-spin (IS), as a high-spin (HS) configuration would unpair the vacancy electrons and low-spin (LS) configuration would have the Mn d5 electrons paired together, both of which are energetically unfavourable. For Mnsurf– and Mnsub–MgO, Ef(F0) is 6.14(5.85) ± 0.02 eV for the B3LYP(B97-3) XC functional, which is ∼0.4 eV lower than that of the pure MgO(100) surface,42 as shown in Fig. 4.
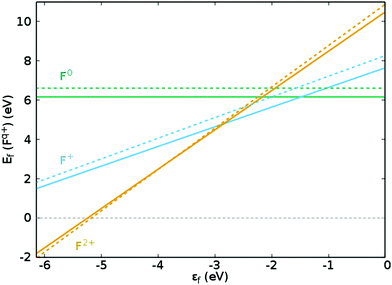 | ||
| Fig. 4 E f for Fq+-centres using the B3LYP XC functional, with solid lines representing Mnsurf–MgO, with εf ranging from the VBM (−6.15 eV) through to vacuum level (0 eV), and dashed lines representing the same defects on a pristine MgO surface, as calculated in Table 4 from the work of Downing et al.42 Dashed green, blue and yellow lines show Ef when the defect charge is q = 0, 1 and 2, respectively (i.e. F0, F+ and F2+). | ||
In order to explore the effect of exchange coupling between the vacancy-trapped electrons and the Mn 3d-states for an F+-centre, we compare a parallel and anti-parallel alignment of the VO and Mn 3d electrons. As can be seen in Table 4, IS (anti-parallel) alignment gives Ef(F+) of 7.64 eV for Mnsurf, with a very similar result for Mnsub, and Ef(F+) is ∼0.27 eV greater for the HS (parallel) configuration. The position of εf has significant effect on Ef(F+): when below −1.48 eV, Ef(F0) > Ef(F+) for both Mn-doped surfaces. Additionally, Ef(F+Mn–MgO) is at least 0.35 eV lower than Ef(F+MgO), indicating that an Mn-dopant facilitates formation of an F+-centre (Fig. 4).
Ionisation of a second electron to form an F2+-centre could also be from the oxygen vacancy or the Mn dopant, which could potentially lead to doubly-charged (a)  or (b)
or (b)  defects, or alternatively (c) sharing of the holes between both extrinsic and intrinsic defects, i.e.:
defects, or alternatively (c) sharing of the holes between both extrinsic and intrinsic defects, i.e.:
 | (15) |
 , irrespective of Mn position or XC functional. We again note that Ef(F2+) for Mn–MgO is generally lower than for MgO, in the favoured HS state, though not when εf drops towards the VBM. Perhaps more pertinently, Ef(F+) > Ef(F2+) for Mnsurf when εf is lower than −2.84(−2.89) eV, and F2+-centre formation is exothermic when εf is lower than −5.21(−5.14) eV, i.e. p-type, when using the B3LYP(B97-3) XC functional (Fig. 4).
, irrespective of Mn position or XC functional. We again note that Ef(F2+) for Mn–MgO is generally lower than for MgO, in the favoured HS state, though not when εf drops towards the VBM. Perhaps more pertinently, Ef(F+) > Ef(F2+) for Mnsurf when εf is lower than −2.84(−2.89) eV, and F2+-centre formation is exothermic when εf is lower than −5.21(−5.14) eV, i.e. p-type, when using the B3LYP(B97-3) XC functional (Fig. 4).
As illustrated in Fig. 5, the IS VIP for the F0-centre with an Mnsurf-dopant is found to be 2.62 eV whereas the HS VIP is 2.94 eV. Referring to our prior comment that HS corresponds to parallel alignment of the vacancy electrons and the Mn 3d states, we can re-affirm the larger Ef(F+) for a HS configuration is based on the greater stability of the highest occupied molecular orbital (HOMO) when compared to an anti-parallel (IS) configuration. The VIP of the oxygen vacancy is similar for Mnsub: 2.51 and 2.92 eV for the IS and HS configuration, respectively. The upwards shift of the HOMO for an anti-parallel alignment, which is between 0.3 and 0.4 eV, gives a reasonable approximation to the exchange coupling energy, whereas the position of the parallel aligned VO state at ∼−2.9 eV is only slightly lower than the −3.05 eV calculated previously for an F0-centre on an MgO(100) surface.42 The increased stability of the parallel states is in agreement with previous observations for ferromagnetic Mn–O and Mn–C coupling in doped MgO,25 though higher concentrations of Mn dopants would result in strong Mn–Mn anti-parallel coupling.26,30
 | ||
| Fig. 5 Intrinsic defect levels, relative to vacuum, for F-centres on the Mnsurf–MgO(100) surface. The colour scheme of the atoms is the same as Fig. 3, with an additional white circle to show an surface oxygen vacancy. The colour scheme of the electronic transitions matches the origin of electrons: the valence band is O 2p states, with the F0 and F+ defect states positioned in the band gap. F0HS and F0IS are used to denote parallel and anti-parallel alignment, respectively, of the oxygen vacancy electrons with the Mn 3d states. The energies presented are calculated with the B3LYP XC functional, and the adiabatic ionisation potential (AIP) was calculated as 1.48, 1.76 and 2.84 eV for the F0IS, F0HS and F+ defect states, respectively. | ||
Using the lower energy IS F+-centre as our starting point, we also calculated the energy required to remove a second electron from the VO site to form a HS F2+-centre. The VIP is 4.53 eV for Mnsurf with a very similar result of 4.52 eV for Mnsub, very close to the previously calculated 2nd VIP of an MgO F-centre (4.55 eV). Coupled with Mulliken analysis, we conclude that the second electron is also ionised from the oxygen vacancy, which means that the highest Mn 3d states must be below −4.5 eV, implying that they are stabilised by the presence of the VO when compared to the pristine surface, for which the VIP was 4.2 to 4.4 eV (Section 4.1).
5 CO2 adsorption on Mn-doped MgO(100) surface
Within the reaction process of CO2 to CH3OH conversion, CO2 is bound to a surface oxygen vacancy and undergoes hydrogenation, leading to the formation of CH3OH and the removal of the surface defect; CO is then used to regenerate the defect sites via its transformation to CO2, completing the catalytic cycle.73 Such a reaction cycle is transferable and highly tunable via modification of the surface as we have recently shown for F-centres on MgO.42,43 As a fundamental step in this topically relevant reaction, a number of CO2 adsorption processes were considered here for the Mn–MgO(100) surfaces investigated in Section 4. The CO2 adsorbate was aligned with the O–C–O axis along either the Cartesian x- or z-axes of the system, “parallel” or “perpendicular” to the surface.42 A third CO2 adsorbate orientation was included for Mnsurf, with the O–C–O axis aligned along the y-axis of the model. The adsorption energy, Eads, for the CO2 molecule was defined as:| Eads(CO2) = E(CO2–MnMgO) − E(CO2) − E(MnMgO), | (16) |
5.1 O5c site
The structures for chemisorbed CO2 parallel to the Mnsurf–MgO(100) surface are shown in Fig. 6(a) and (b), whilst CO2 chemisorption parallel to the Mnsub–MgO surface is shown in Fig. 6(c). The CO2 physisorbed perpendicular to the O5c site is not shown as significant interactions were not seen, which was expected, based on previous results for MgO,42 and the data presented in the ESI† shows that the interaction is negligible. In experiment, van der Waals interactions that are absent from our calculations would, perhaps, allow the CO2 to be physisorbed near the Mn–MgO surface.For the x- and y-axis aligned adsorbates, a carbonate species forms, and Eads(CO2) on the O5c site ranges between −0.66 and −0.86 eV, which is similar to that observed for MgO;42 full results are included in the ESI.† We note for Mnsurf that stronger CO2 adsorption is seen for y-axis alignment, compared to x-axis, which can be correlated with the longer Mn–O bond of 2.29 Å between the (100) surface and the CO2 in the latter system. Mulliken analysis shows that there is partial withdrawal of 0.5 e from the O5c and neighbouring Mg atoms to the adsorbate; however the Mn-dopant retains its d5 configuration, which hinders charge transfer.
Comparison of the different XC functionals shows consistently stronger binding when using B97-3, with the interactions around 10% stronger than for B3LYP. This result is consistent with the parameterisation of this XC functional towards thermochemical processes such as chemisorption, supporting stronger electron localisation.
5.2 F0-centre
For a neutral oxygen vacancy, the z-axis (vertically) aligned CO2 dissociates to produce a defect-free surface and a CO molecule, as shown in Fig. 7(a). Table 5 shows that this process is strongly exothermic (∼−3 eV) due to the healing of the oxygen vacancy. Strong adsorption also occurs when CO2 is aligned parallel to the surface, with Eads(CO2) ≈ −2 eV. The strong binding results in a bent CO2 geometry, as seen previously for F0-centre on MgO;42 however healing of the oxygen vacancy does not occur in this particular case. Regardless of the position of the Mn dopant, the CO2 rotates so that the central C atom is directed towards a surface Mg, rather than downwards into the vacancy as seen for undoped MgO.42 It is noted that, in the y-axis orientation on Mnsurf–MgO, the CO2 is able to rotate towards either an Mn or Mg atom, however, the latter is favoured.| CO2 axis | B3LYP | B97-3 | |
|---|---|---|---|
| Mnsurf | x | −2.00 | −1.74 |
| y | −2.25 | −1.98 | |
| z | −3.13 | −2.80 | |
| Mnsub | x | −2.24 | −1.98 |
| z | −3.20 | −2.87 | |
| Mg42 | x | −2.36 | — |
| z | −3.52 | — | |
5.3 F+-centre
Due to the energetic competition between the IS and HS F+-centres, both were considered as adsorption sites for CO2 (Table 6), which resulted in a range of adsorption strengths. Eads(CO2) is greatest for IS Mnsurf–MgO with the adsorbate aligned along the x-axis of the surface (−2.00 eV). Fig. 8(a) shows that the CO2 has dissociated, with one O healing the surface vacancy and the remaining CO coordinated to a surface Mg. Mulliken analysis shows that the electron previously trapped at the F+-centre has reduced the Mn2+ cation to Mn+, which has a spin-density of 3.90 e; further calculations would be necessary to determine if an entirely LS configuration is preferable for the Mn cation. For the same system with a HS configuration, no dissociation is observed and instead the vacancy-trapped electron transfers to the CO2, hence the smaller Eads(CO2). The second greatest Eads(CO2) is with the CO2 aligned in the y-axis of an IS-configured surface (−1.38 eV): the CO2 remains parallel to the surface but the C bonds directly to the Mn, with the two atoms separated by 2.14 Å compared to 2.61 Å for the HS equivalent as shown in Fig. 8(c) and (d), respectively. The spin-density is 4.08 e in the former case, which is ∼0.76 e lower than for HS (4.76 e) and again indicates that the vacancy electron has moved to the Mn.| CO2 axis | B3LYP | B97-3 | |
|---|---|---|---|
| Mnsurf | x | −2.00(−0.62) | −1.64(−0.48) |
| y | −1.38(−0.80) | −1.06(−0.64) | |
| z | −0.90(−1.12) | −0.76(−0.97) | |
| Mnsub | x | −0.64(−0.85) | −0.49(−0.69) |
| z | −0.99(−1.18) | −0.85(−1.05) | |
| Mg42 | x | −0.71 | — |
| z | −1.11 | — | |
In contrast to the above, Eads(CO2) for z-axis aligned CO2 on the Mnsurf is stronger for HS than IS, with no chemisorption, which indicates that exchange coupling benefits physisorption. The resulting bent CO2 remains perpendicular to the surface for both systems [Fig. 8(e) and (f)], with the terminal O only ∼0.7 Å above the surface plane, but the difference in Eads(CO2) between HS and IS is 0.22 eV. Similar differences are seen for the Mnsub systems [Fig. 7(b)], where Eads(CO2) for HS is consistently ∼0.2 eV greater than for IS. Mulliken analysis of the CO2 when x-axis aligned on Mnsub–MgO shows that the spin-density is 0.69 and 0.84 e when using IS and HS configurations, respectively, which correlates charge transfer with adsorption energy; we also note that the majority of this spin density is located on the central C atom.
5.4 F2+-centre
E ads(CO2) on HS F2+-centres is presented in Table 7, with similar structures observed for CO2 adsorbed on Mnsub– and Mnsurf–MgO [Fig. 7(c)]. Bonding is marginally exothermic for z-axis aligned CO2, with the lack of bend in the adsorbate suggesting that no significant charge transfer occurs, which is consistent with results for undoped MgO42 and confirmed by Mulliken analysis. CO2 adsorption is endothermic when the molecule is aligned with the x- or y-axis; as separation of the CO2 and surface would be more favourable, this is a local minimum. Electron density transfers from the Mn atom to the CO2 to form a CO2−/Mn3+ surface complex, with greater charge transfer when the Mn interacts with a terminal O (x-axis) rather than the C atom (y-axis), which correlates with the more endothermic nature of the x-axis aligned adsorption (0.90 eV compared to 0.50 eV).| CO2 axis | B3LYP | B97-3 | |
|---|---|---|---|
| Mnsurf | x | 0.90 | 1.14 |
| y | 0.50 | 0.00 | |
| z | −0.09 | −0.11 | |
| Mnsub | x | 0.24 | 0.52 |
| z | −0.12 | −0.13 | |
| Mg42 | x | 0.11 | — |
| z | 0.04 | — | |
6 Summary and conclusions
We have performed embedded-cluster QM/MM calculations for Mn-doped MgO, studying bulk and (100) surface systems, as well as testing the reactivity of the doped material using CO2 adsorption as a representative catalytic process. Low concentration Mn-doping is endothermic for isovalent bulk defects, irrespective of a low or high p(O2) reference environment, with the Mn 3d levels positioned in the band gap ∼4.2 eV below the vacuum level, however, Mn-doping is energetically favourable in higher oxidation states, especially under p-type conditions. The formation of isovalent Mn-doped (100) MgO surfaces is marginally exothermic at high p(O2), with subsurface doping being exothermic; however higher oxidation states become more favourable in p-type conditions.Once present, an isovalent Mn-dopant aids the formation of oxygen vacancies, i.e. F-centres, on the (100) surface: F+-centres particularly benefit from an exchange-coupling mechanism between the trapped electrons and the Mn 3d-states, with a parallel aligned electron 0.32 eV lower in energy than an anti-parallel aligned electron when using the B3LYP XC functional. In general, formation energies for F0 and F2+-centres indicate their prevalence in n- and p-type environments, respectively, in broad agreement with findings for undoped MgO; in future work we will extend this analysis to Mn-dopants in higher oxidation states.
When a CO2 probe molecule is adsorbed on the pristine and defective Mn–MgO(100) surface, the interaction between substrate and adsorbate is dependent on both defect charge and adsorbate orientation. Strongest adsorption is for CO2 over an F0-centre, with the CO2 dissociating when aligned perpendicular to the surface so that the oxygen vacancy can be filled by one of the oxygen atoms from the CO2 molecule. We also observe strong binding between F+-centres and CO2 when the Mn-dopant is positioned at the surface, with interaction strengths less varied when the Mn is subsurface. In general, positioning of an Mn-dopant subsurface implicitly affects CO2 adsorption through exchange coupling effects, whereas Mn dopants on the surface strongly influence the chemical reactivity, with distinctly different interactions compared to MgO. In general our results justify further investigation of the doping of stable alkaline-earth metal oxides with transition metal species, which we will continue in our future work with an emphasis on catalytic reactivity: it has been previously noted that high-valence dopants activate molecular adsorbates for other transition metal dopants in rocksalt oxides78 and clearly we need to investigate this further for Mn–MgO.
Acknowledgements
The authors are grateful to David Scanlon and John Buckeridge for discussions associated with this work. AJL acknowledges the Ramsay Memorial Trust and University College London for the provision of a Ramsay Fellowship. CAD acknowledges the Molecular Modelling and Materials Science Industrial Doctorate Centre (M3S IDC) and the Science and Technology Facilities Council (STFC) for funding. AJL, TWK, PS, AAS and CRAC acknowledge funding from EPSRC grants EP/IO30662/1 and EP/K038419/1. The authors acknowledge the use of the ARCHER high-performance computing facilities, and associated support services, via our membership of the UK HPC Materials Chemistry Consortium (EP/L000202).References
- S. Wang, S. Yan, X. Ma and J. Gong, Energy Environ. Sci., 2011, 4, 3805–3819 CAS.
- S. Choi, J. H. Drese and C. W. Jones, ChemSusChem, 2009, 2, 796–854 CrossRef CAS PubMed.
- H. A. Al-Abadleh and V. H. Grassian, Surf. Sci. Rep., 2003, 52, 63–161 CrossRef CAS.
- D. P. Woodruff, Chem. Rev., 2013, 113, 3863–3886 CrossRef CAS PubMed.
- C. Noguera, Physics and Chemistry at Oxide Surfaces, Cambridge University Press, 1996 Search PubMed.
- A. J. Logsdail, D. O. Scanlon, C. R. A. Catlow and A. A. Sokol, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 155106 CrossRef.
- A. J. Logsdail, D. Mora-Fonz, D. O. Scanlon, C. R. A. Catlow and A. A. Sokol, Surf. Sci., 2015, 642, 58–65 CrossRef CAS.
- G. Pacchioni, ChemPhysChem, 2003, 4, 1041–1047 CrossRef CAS PubMed.
- K. Honkala, Surf. Sci. Rep., 2014, 69, 366–388 CrossRef CAS.
- H. Hattori, Appl. Catal., A, 2001, 222, 247–259 CrossRef CAS.
- J. Goniakowski and C. Noguera, Surf. Sci., 1995, 340, 191–204 CrossRef CAS.
- G. Pacchioni and H. Freund, Chem. Rev., 2013, 113, 4035–4072 CrossRef CAS PubMed.
- D. K. Kanan and E. A. Carter, J. Phys. Chem. C, 2012, 116, 9876–9887 CAS.
- R. Hammami, A. Dhouib, S. Fernandez and C. Minot, Catal. Today, 2008, 139, 227–233 CrossRef CAS.
- N. Nilius and H.-J. Freund, Acc. Chem. Res., 2015, 48, 1532–1539 CrossRef CAS PubMed.
- E. W. McFarland and H. Metiu, Chem. Rev., 2013, 113, 4391–4427 CrossRef CAS PubMed.
- N. Nilius, J. Phys.: Condens. Matter, 2015, 27, 303001 CrossRef PubMed.
- A. Cimino and V. Indovina, J. Catal., 1974, 33, 493–496 CrossRef CAS.
- P. Koidl and K. W. Blazey, J. Phys. C: Solid State Phys., 1976, 9, L167 CrossRef CAS.
- R. Mariscal, J. Soria, M. Pena and J. Fierro, J. Catal., 1994, 147, 535–543 CrossRef CAS.
- M. Abramishvili, Z. Akhvlediani, M. Galustashvili, G. Dekanozishvili, T. Kalabegishvili, V. Kvatchadze and V. Tavkhelidze, J. Mod. Phys., 2011, 2, 6759 Search PubMed.
- M. Königstein, F. Cora and C. R. A. Catlow, J. Solid State Chem., 1998, 137, 261–275 CrossRef.
- H. Gripenberg, S. Seetharaman and L. Staffansson, Chem. Scr., 1979, 13, 162–164 CAS.
- K. D. Heath, W. C. Mackrodt, V. R. Saunders and M. Causà, J. Mater. Chem., 1994, 4, 825–829 RSC.
- V. Sharma, G. Pilania and J. E. Lowther, AIP Adv., 2011, 1, 032129 CrossRef.
- L.-J. Shi, Phys. Lett. A, 2010, 374, 1292–1296 CrossRef CAS.
- S. Azzaza, M. El-Hilo, S. Narayanan, J. J. Vijaya, N. Mamouni, A. Benyoussef, A. El Kenz and M. Bououdina, Mater. Chem. Phys., 2014, 143, 1500–1507 CrossRef CAS.
- S. Meskine, A. Boukortt, R. Hayn and A. Zaoui, Phys. Status Solidi B, 2014, 251, 845–849 CrossRef CAS.
- P. Wu, G. Cao, F. Tang and M. Huang, Comput. Mater. Sci., 2014, 86, 180–185 CrossRef CAS.
- P. Panigrahi, T. Hussain, C. M. Araujo and R. Ahuja, J. Phys.: Condens. Matter, 2014, 26, 265801 CrossRef PubMed.
- P. Panigrahi, C. M. Araujo, T. Hussen and R. Ahuja, Sci. Technol. Adv. Mater., 2014, 15, 035008 CrossRef.
- N. A. Richter, S. Sicolo, S. V. Levchenko, J. Sauer and M. Scheffler, Phys. Rev. Lett., 2013, 111, 045502 CrossRef PubMed.
- F. Kröger and H. Vink, Solid State Phys., 1956, 3, 307–435 Search PubMed.
- E. Scorza, U. Birkenheuer and C. Pisani, J. Chem. Phys., 1997, 107, 9645–9658 CrossRef CAS.
- P. V. Sushko, A. L. Shluger and C. R. A. Catlow, Surf. Sci., 2000, 450, 153–170 CrossRef CAS.
- P. Sushko, J. Gavartin and A. Shluger, J. Phys. Chem. B, 2002, 106, 2269–2276 CrossRef CAS.
- Y. Wang, H. N. Nguyen and T. N. Truong, Chem. – Eur. J., 2006, 12, 5859–5867 CrossRef CAS PubMed.
- E. Florez, P. Fuentealba and F. Mondragon, Catal. Today, 2008, 133, 216–222 CrossRef.
- C. Qin, Chem. Phys. Lett., 2008, 460, 457–460 CrossRef CAS.
- G. Pacchioni and H.-J. Freund, Chem. Rev., 2013, 113, 4035–4072 CrossRef CAS PubMed.
- K. Kwapien, J. Paier, J. Sauer, M. Geske, U. Zavyalova, R. Horn, P. Schwach, A. Trunschke and R. Schlögl, Angew. Chem., Int. Ed., 2014, 53, 8774–8778 CrossRef CAS PubMed.
- C. A. Downing, A. A. Sokol and C. R. A. Catlow, Phys. Chem. Chem. Phys., 2014, 16, 184–195 RSC.
- C. A. Downing, A. A. Sokol and C. R. A. Catlow, Phys. Chem. Chem. Phys., 2014, 16, 21153–21156 RSC.
- S. Metz, J. Kästner, A. A. Sokol, T. W. Keal and P. Sherwood, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2014, 4, 101–110 CrossRef CAS.
- J. Kaestner, J. M. Carr, T. W. Keal, W. Thiel, A. Wander and P. Sherwood, J. Phys. Chem. A, 2009, 113, 11856–11865 CrossRef PubMed.
- P. Sherwood, A. H. de Vries, M. F. Guest, G. Schreckenbach, C. R. A. Catlow, S. A. French, A. A. Sokol, S. T. Bromley, W. Thiel, A. J. Turner, S. Billeter, F. Terstegen, S. Thiel, J. Kendrick, S. C. Rogers, J. Casci, M. Watson, F. King, E. Karlsen, M. Sjovoll, A. Fahmi, A. Schafer and C. Lennartz, J. Mol. Struct.: THEOCHEM, 2003, 632, 1–28 CrossRef CAS.
- M. Valiev, E. J. Bylaska, N. Govind, K. Kowalski, T. P. Straatsma, H. J. J. V. Dam, D. Wang, J. Nieplocha, E. Apra, T. L. Windus and W. A. de Jong, Comput. Phys. Commun., 2010, 181, 1477–1489 CrossRef CAS.
- J. D. Gale, J. Chem. Soc., Faraday Trans., 1997, 93, 629–637 RSC.
- J. D. Gale and A. L. Rohl, Mol. Simul., 2003, 29, 291–341 CrossRef CAS.
- J. Gale, Z. Kristall., 2005, 220, 552–554 CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. T. Lee, W. T. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- P. J. Stephens, F. J. Devlin, C. F. Chablowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- T. W. Keal and D. J. Tozer, J. Chem. Phys., 2005, 123, 121103 CrossRef PubMed.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- M. Dolf, U. Wedig, H. Stoll and H. Preuss, J. Chem. Phys., 1987, 86, 866–872 CrossRef.
- P. Fuentealba, L. Vonszentpaly, H. Preuss and H. Stoll, J. Phys. B: At., Mol. Opt. Phys., 1985, 18, 1287–1296 CrossRef CAS.
- G. V. Lewis and C. R. A. Catlow, J. Phys. C: Solid State Phys., 1985, 18, 1149–1161 CrossRef CAS.
- W. Jost, J. Chem. Phys., 1933, 1, 466–475 CrossRef CAS.
- A. E. Hughes and B. Henderson, Color Centers in Simple Oxides, in Point Defects in Solids: General and Ionic Crystals, ed. J. H. Crawford and L. M. Slifkin, Springer US, Boston, MA, 1972, pp. 381–490 Search PubMed.
- F. Stavale, X. Shao, N. Nilius, H.-J. Freund, S. Prada, L. Giordano and G. Pacchioni, J. Am. Chem. Soc., 2012, 134, 11380–11383 CrossRef CAS PubMed.
- Y. Cui, X. Shao, S. Prada, L. Giordano, G. Pacchioni, H.-J. Freund and N. Nilius, Phys. Chem. Chem. Phys., 2014, 16, 12764–12772 RSC.
- A. Walsh, J. Buckeridge, C. R. A. Catlow, A. J. Jackson, T. W. Keal, M. Miskufova, P. Sherwood, S. A. Shevlin, M. B. Watkins, S. M. Woodley and A. A. Sokol, Chem. Mater., 2013, 25, 2924–2926 CrossRef CAS.
- D. R. Lide, CRC Handbook of Chemistry and Physics, CRC Press, Boca Raton, Florida, 84th edn, 2003 Search PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- R. O. Jones and O. Gunnarsson, Rev. Mod. Phys., 1989, 61, 689–746 CrossRef CAS.
- T. Jaouen, G. Jézéquel, G. Delhaye, B. Lépine, P. Turban and P. Schieffer, Appl. Phys. Lett., 2010, 97, 232104 CrossRef.
- R. Whited, C. J. Flaten and W. C. Walker, Solid State Commun., 1973, 13, 1903–1905 CrossRef CAS.
- R. D. Shannon, Acta Crystallogr., 1976, 32, 751–767 CrossRef.
- L. N. Kantorovich, A. L. Shluger, P. V. Sushko, J. Gunster, P. Stracke, D. W. Goodman and V. Kempter, Faraday Discuss., 1999, 114, 173–194 RSC.
- H. H. Kung, Transition metal oxides: surface chemistry and catalysis, Elsevier, 1989, vol. 45 Search PubMed.
- D. O. Scanlon, C. W. Dunnill, J. Buckeridge, S. A. Shevlin, A. J. Logsdail, S. M. Woodley, C. R. A. Catlow, M. J. Powell, R. G. Palgrave, I. P. Parkin, G. W. Watson, T. W. Keal, P. Sherwood, A. Walsh and A. A. Sokol, Nat. Mater., 2013, 12, 798–801 CrossRef CAS PubMed.
- S. A. French, A. A. Sokol, S. T. Bromley, C. R. A. Catlow, S. C. Rogers, F. King and P. Sherwood, Angew. Chem., Int. Ed., 2001, 40, 4437–4440 CrossRef CAS.
- A. Sokol, S. Bromley, S. French, C. Catlow and P. Sherwood, Int. J. Quantum Chem., 2004, 99, 695–712 CrossRef CAS.
- S. French, A. Sokol, S. Bromley, C. Catlow and P. Sherwood, Top. Catal., 2003, 24, 161–172 CrossRef CAS.
- S. Bromley, S. French, A. Sokol, C. Catlow and P. Sherwood, J. Phys. Chem. B, 2003, 107, 7045–7057 CrossRef CAS.
- C. Catlow, S. French, A. Sokol and J. Thomas, Philos. Trans. R. Soc., A, 2005, 363, 913–936 CrossRef CAS PubMed.
- Y. Cui, X. Shao, M. Baldofski, J. Sauer, N. Nilius and H.-J. Freund, Angew. Chem., Int. Ed., 2013, 52, 11385–11387 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional data table with CO2 adsorption energies for pristine Mn–MgO(100) surfaces. See DOI: 10.1039/c6cp04622c |
| This journal is © the Owner Societies 2016 |




