 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Exploration of the phase diagram of liquid water in the low-temperature metastable region using synthetic fluid inclusions†
C.
Qiu
a,
Y.
Krüger‡
a,
M.
Wilke§
b,
D.
Marti
ac,
J.
Rička
a and
M.
Frenz
*a
aInstitute of Applied Physics, University of Bern, Sidlerstrasse 5, 3012 Bern, Switzerland. E-mail: martin.frenz@iap.unibe.ch
bGeoForschungsZentrum Potsdam, Telegrafenberg, 14473 Potsdam, Germany
cDepartment of Photonics Engineering, Technical University of Denmark, Frederiksborgvej 399, Himmelev, 4000, Roskilde, Denmark
First published on 1st September 2016
Abstract
We present new experimental data of the low-temperature metastable region of liquid water derived from high-density synthetic fluid inclusions (996–916 kg m−3) in quartz. Microthermometric measurements include: (i) prograde (upon heating) and retrograde (upon cooling) liquid–vapour homogenisation. We used single ultrashort laser pulses to stimulate vapour bubble nucleation in initially monophase liquid inclusions. Water densities were calculated based on prograde homogenisation temperatures using the IAPWS-95 formulation. We found retrograde liquid–vapour homogenisation temperatures in excellent agreement with IAPWS-95. (ii) Retrograde ice nucleation. Raman spectroscopy was used to determine the nucleation of ice in the absence of the vapour bubble. Our ice nucleation data in the doubly metastable region are inconsistent with the low-temperature trend of the spinodal predicted by IAPWS-95, as liquid water with a density of 921 kg m−3 remains in a homogeneous state during cooling down to a temperature of −30.5 °C, where it is transformed into ice whose density corresponds to zero pressure. (iii) Ice melting. Ice melting temperatures of up to 6.8 °C were measured in the absence of the vapour bubble, i.e. in the negative pressure region. (iv) Spontaneous retrograde and, for the first time, prograde vapour bubble nucleation. Prograde bubble nucleation occurred upon heating at temperatures above ice melting. The occurrence of prograde and retrograde vapour bubble nucleation in the same inclusions indicates a maximum of the bubble nucleation curve in the ϱ–T plane at around 40 °C. The new experimental data represent valuable benchmarks to evaluate and further improve theoretical models describing the p–V–T properties of metastable water in the low-temperature region.
1. Introduction
The present work is a contribution to the research aimed at the understanding of the anomalies of water, in particular those observed in supercooled metastable water at temperatures below the ice–liquid coexistence curve.1,2 For example, isothermal compressibility κT exhibits a minimum at 46 °C and increases upon further lowering the temperature, instead of monotonically decreasing as in “normal” liquids. In the supercooled region, the increase becomes quite rapid and κT may even diverge at around 228 K (−45 °C).3 (Unfortunately this would happen in the experimentally inaccessible region below the ice nucleation limit.) Several scenarios have been proposed to explain the strange behaviour of the thermodynamic response functions (see e.g. the review by Pallares et al.4), the first was the so-called ‘stability limit conjecture’ of Speedy.5Speedy and Angell3 observed that the behaviour of the response functions in the supercooled region resembles the behaviour of κT when approaching the spinodal line, where κT ∝ ∂ϱ/∂p|T → ∞. The liquid–vapour spinodal delimits the portions of the thermodynamic p–ϱ–T surface where fluid water can exist in a stable or metastable state. The liquid branch of the spinodal starts at the critical point and runs to negative pressures (tensile stress) with decreasing temperature. However, to explain the divergences in the supercooled water at atmospheric pressure, it would have to run through a pressure minimum and then increase again, as shown in Fig. 1a, to reach zero pressure at around 228 K. Speedy suggested that such re-entrant behaviour is indeed possible, because of the well-known density anomaly of water, the density maximum found at 4.0 °C when moving along an isobar at atmospheric pressure. When moving along isochores p(T|ϱ) with different densities ϱ, one finds the corresponding pressure minima, whose loci form the so-called temperature of maxima density (TMD) line, the dotted line in Fig. 1. The TMD line extends to the negative pressure region where liquid water is in a stretched metastable state. If the TMD line hits the spinodal, then it must be in a spinodal pressure minimum, as can be shown by a simple thermodynamic argument. Thus, the spinodal is re-entrant, possibly reaching zero pressure at the desired temperature of −45 °C.
 | ||
| Fig. 1 Two models proposed for the stability limit of metastable liquid water. (a) The “stability limit conjecture”5 postulates a single continuous spinodal curve that exhibits a pressure minimum and returns to positive pressures at low temperatures. The TMD line remains negatively sloped in the metastable region. The p–T diagram additionally displays the liquid–solid (L + S) and liquid–vapour (L + V) equilibrium curves. At low temperatures, the spinodal is hidden behind the Ticenr curve indicating homogeneous ice nucleation. (b) The model proposed by Poole et al.6 displays a TMD line that bends over to a positive slope in the metastable region and does not intersect the spinodal. As a consequence of this, the spinodal remains positively sloped and extends to higher negative pressures with decreasing temperature. The model postulates a second critical point (cp2) terminating the HDA–LDA coexistence line (HDA = high-density amorphous ice, LDA = low-density amorphous ice). Note, the liquid–solid coexistence curve and the ice nucleation curve are not shown. | ||
Speedy's stability limit conjecture was soon challenged, but direct experimental verification remains impossible because in stretched water spontaneous bubble nucleation occurs way before reaching the spinodal, and in the supercooled region the accessible range is limited by ice nucleation. However, a strong indication against Speedy's conjecture came from molecular dynamic simulations using realistic water models. Poole and coworkers6 found evidence that with decreasing density the TMD line in the p–T representation bends to a positive slope and never meets the spinodal, as indicated in Fig. 1b. Thus, the simulated liquid spinodal keeps monotonically decreasing with decreasing temperature. In this scenario, another reason for the anomalies of supercooled water must be sought, for example a Widom line7 emanating from a novel critical point associated with two states of amorphous ice, or a novel state of low-density liquid.8 Moreover, the number of simulations and possible scenarios has increased,4 but the debate still suffers from the lack of experimental data.
The major problem that frustrates experimental studies in the metastable region of liquid water is heterogeneous nucleation of the vapour bubble. Different experimental techniques, such as Berthelot tubes, shock waves, or acoustic cavitation have been used to approach the liquid-spinodal (see the report by Caupin and Herbert9 and references therein), but by far the highest negative pressures of up to −140 MPa have been achieved by (pseudo-)isochoric cooling of synthetic fluid inclusions in quartz crystals (e.g. Zheng et al.10). Due to their microscopic size and strong hydrogen bonds between water and quartz, spontaneous heterogeneous nucleation of both the vapour and solid phases is strongly hampered and the water remains in a stretched metastable state down to large negative pressures. A disadvantage of the fluid inclusion approach, however, is that the fluid pressure cannot be directly measured, but has to be calculated from an adequate equation of state that provides a reasonable extrapolation into the metastable region (e.g. IAPWS-9511).
Previous applications of fluid inclusions in this field have focused on the measurements of spontaneous retrograde bubble nucleation temperatures Tvapnr to determine the maximum negative pressures along different fluid isochores.10,12,13 The density of the water in the inclusions as well as the corresponding liquid-isochores has been calculated from an equation of state, based on the measurements of the liquid–vapour homogenisation temperature Th. Because spontaneous nucleation of the vapour bubble was a prerequisite for the measurements of both Th and Tvapnr, these studies were restricted to inclusion densities lower than 943 kg m−3, corresponding to homogenisation temperatures greater than 120 °C. At higher densities, spontaneous nucleation usually fails to occur, particularly in small inclusions.
A different approach to determine fluid pressures inside the inclusions has been used by Alvarenga et al.14 and recently by Pallares et al.4,15 They measured Brillouin scattering of metastable liquid fluid inclusions at different temperatures and internal pressures and compared the data with reference spectra measured along the liquid–vapour equilibrium curve. From the frequency shift with respect to the reference spectra, they calculated the sound velocity in the stretched water, and finally the pressure inside the inclusions.
In the present study, we report new microthermometric data from synthetic high-density water inclusions that, in most cases, do not show spontaneous vapour bubble nucleation upon cooling. Single ultrashort laser pulses were used to stimulate bubble nucleation in the metastable liquid,16 a precondition for subsequent measurements of the liquid–vapour homogenisation temperature Th (L + V → L), and thus for an accurate determination of the water density in the inclusions. (The reader who is not familiar with this technique may consult Fig. 1 in ref. 17.) Besides prograde homogenisation temperatures Th (upon heating) we also report retrograde liquid–vapour homogenisation temperatures Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r measured upon cooling, ice nucleation temperatures Ticenr both in the presence and absence of the vapour bubble (L + V → S and L → S), final ice melting temperatures Ticem, most of them measured in the absence of a vapour bubble (L + S → L), and some measurements of spontaneous, retrograde and prograde vapour bubble nucleation Tvapnr and Tvapnp (L → L + V), respectively. The IAPWS-95 formulation of Wagner and Pruss11 was used in this study to calculate water densities and simultaneously serves as a reference to compare with our data. We note that IAPWS-95 assumes a negative slope of the TMD line throughout the metastable region, which results in a similar trend of the spinodal as proposed in Speedy's “stability-limit conjecture”.5
r measured upon cooling, ice nucleation temperatures Ticenr both in the presence and absence of the vapour bubble (L + V → S and L → S), final ice melting temperatures Ticem, most of them measured in the absence of a vapour bubble (L + S → L), and some measurements of spontaneous, retrograde and prograde vapour bubble nucleation Tvapnr and Tvapnp (L → L + V), respectively. The IAPWS-95 formulation of Wagner and Pruss11 was used in this study to calculate water densities and simultaneously serves as a reference to compare with our data. We note that IAPWS-95 assumes a negative slope of the TMD line throughout the metastable region, which results in a similar trend of the spinodal as proposed in Speedy's “stability-limit conjecture”.5
2. Materials and methods
2.1. Synthetic fluid inclusions
Synthetic fluid inclusions were produced according to the principles described by Sterner and Bodnar18 and Bodnar and Sterner.19 A total of twelve gold capsules were prepared containing pre-fractured quartz prisms and deionized (air-saturated) water. Hydrothermal syntheses of the inclusions were performed in an internally heated pressure vessel (IHPV) at temperatures between 420 and 520 °C and at pressures ranging from 710 to 860 MPa using argon as the pressure medium. Run durations were between 46 and 96 hours. After the hydrothermal runs the quartz prisms were removed from the gold capsules and cut into slices. The slices were ground and polished on both sides to a final thickness of 250 to 300 μm. The synthesized fluid inclusions were usually flat without negative crystal faces and inclusion sizes were typically below 500 μm3 (Fig. 2). Water densities of the inclusions were precisely determined by microthermometric measurements (cf. Section 2.4.2) and ranged from 996 to 916 kg m−3. In most of the samples, the inclusions were in a monophase liquid state at room temperature and did not show spontaneous bubble nucleation upon further cooling. | ||
| Fig. 2 Assemblage of synthetic fluid inclusions in quartz formed during hydrothermal experiments along a healed crack. | ||
A detailed description of the preparation and synthesis procedures used to produce the fluid inclusions in this study can be found in Section S2.1 in the ESI.†
2.2. Experimental setup
The experimental setup is built around an upright microscope (Olympus BX51) and consists of: (i) a heating/freezing stage, (ii) a femtosecond laser system providing amplified ultrashort laser pulses, and (iii) a Raman spectrometer. A simplified scheme of the setup is shown in Fig. 3 and a more detailed version is provided in Fig. S1 (ESI†). | ||
| Fig. 3 Simplified scheme of the experimental setup used for this study. See the text for details. DM: dichroic mirror. | ||
(i) The heating/freezing stage (Linkam THMSG 600) is mounted on the microscope and allows for microthermometric measurements in the temperature range between −180 and 600 °C. Temperature calibrations of the stage were performed using synthetic H2O and H2O–CO2 fluid inclusion standards for the triple point of water (0.0 °C), the triple point of CO2 (−56.6 °C), and the critical point of CO2 (31.4 °C20). Based on these calibrations, we estimate the accuracy of the temperature measurements at ±0.1 °C around 0 °C, at ±0.2 °C below −20 °C and above 40 °C and at ±0.3 °C above 100 °C. The precision (reproducibility) determined from replicate measurements is ±0.05 °C. Further details are provided in Section S2.2 (ESI†).
(ii) An amplified femtosecond (fs) laser system (Coherent) was used to stimulate vapour bubble nucleation in the metastable liquid state of the inclusions by means of single ultrashort laser pulses. For a detailed description of the setup we refer to the ESI† and to Krüger et al.16 The experimental setup allows us to stimulate vapour bubble nucleation in selected fluid inclusions at different temperatures under microscopic observation. Subsequent microthermometric measurements can be performed without moving the sample.
(iii) Confocal Raman spectroscopy was used to identify the ice phase in the inclusions in order to determine ice nucleation temperatures. In pure water inclusions the phase transition is not visible from microscopic observations unless a vapour bubble is present that becomes strongly compressed upon ice nucleation. The fiber-coupled Raman setup consists of a 500 mW diode-pumped solid-state single mode laser with 532 nm wavelength and 1 MHz bandwidth (Torus, Laser Quantum), two dichroic mirrors, a spectrometer (Horiba iHR550M CORE 3) and a back-illuminated CCD camera (Horiba SYNAPSE BIVS); for Raman excitation 60 mW was used. Again, a detailed description of the Raman setup is given in the ESI.† The setup allows us to measure Raman spectra at different temperatures under simultaneous observation of the focus position of the excitation beam relative to the fluid inclusions.
2.3. Microthermometric measurements
Using the synthetic pure water inclusions we measured prograde and retrograde liquid–vapour homogenisation temperatures Th and Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r, respectively, ice nucleation temperatures Ticenr, and ice melting temperatures Ticem. Moreover, in low density (ϱ < 925 kg m−3) inclusions, we measured the temperatures of spontaneous bubble nucleation, not only the common retrograde Tvapnr (upon cooling), but also the prograde bubble nucleation Tvapnp, upon heating of inclusions that previously underwent an ice nucleation and melting loop.
r, respectively, ice nucleation temperatures Ticenr, and ice melting temperatures Ticem. Moreover, in low density (ϱ < 925 kg m−3) inclusions, we measured the temperatures of spontaneous bubble nucleation, not only the common retrograde Tvapnr (upon cooling), but also the prograde bubble nucleation Tvapnp, upon heating of inclusions that previously underwent an ice nucleation and melting loop.
The temperatures of the different phase transitions were measured at very low heating and cooling rates respectively to ensure that the sample was in thermal equilibrium with the heating and cooling block of the stage. When approaching the expected phase transition, i.e., for the final 1 or 2 centigrade the temperature was changed in 0.1 centigrade steps with intervals of 10 to 20 seconds in between. The different phase transitions are illustrated in Fig. 4 in a schematic representation of the p–T diagram of water. The phase diagram displays the liquid–vapour (L + V) and liquid–solid (L + S) equilibrium curves, the bubble–nucleation (cavitation) curve (Tvapnr and Tvapnp), the ice nucleation curve (Ticenr) and three liquid-isochores passing through a pressure minimum.
After Th measurements the inclusions were cooled to room temperature and vapour bubble nucleation was induced again by means of a femtosecond laser pulse. Subsequently, the inclusions were further cooled to measure the retrograde homogenisation temperature Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r following the same procedure as described for the Th measurements. But unlike prograde homogenisation, retrograde homogenisation can only be observed in high-density inclusions, in which Th
r following the same procedure as described for the Th measurements. But unlike prograde homogenisation, retrograde homogenisation can only be observed in high-density inclusions, in which Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r is higher than Ticenr, the temperature of ice nucleation. Fig. 4 shows that the isochores of such high-density inclusions intersect the liquid–vapour equilibrium curve (L + V) twice, at Th and Th
r is higher than Ticenr, the temperature of ice nucleation. Fig. 4 shows that the isochores of such high-density inclusions intersect the liquid–vapour equilibrium curve (L + V) twice, at Th and Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r, respectively. The reproducibility of the measured Th and Th
r, respectively. The reproducibility of the measured Th and Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r values was routinely checked by duplicate measurements alternately measuring prograde and retrograde homogenisation.
r values was routinely checked by duplicate measurements alternately measuring prograde and retrograde homogenisation.
In high-density inclusions featuring retrograde liquid–vapour homogenisation, ice nucleation occurs at positive pressures, while in low-density metastable liquid inclusions nucleation of ice takes place at negative pressures. Since these low-density inclusions do not exhibit retrograde homogenisation, we could also use them to measure Ticenr at saturation vapour pressure. To do so, we stimulated vapour bubble nucleation in the metastable liquid and cooled the inclusions along the liquid–vapour equilibrium curve (see Fig. 4). Upon ice nucleation the vapour bubble disappeared again due to the volume expansion of ice relative to liquid water (L + V → S; Fig. 6c).
The nucleation of ice results in an abrupt pressure increase in the inclusions. The pressure in the ice Ih state can be calculated using the equation of state by Feistel and Wagner,22 but since pressure in the inclusions is not measurable, we refrain from exact calculations. To assess the magnitude of the pressure, a rough estimate using Table 11 in ref. 22 is sufficient: in the inclusions with the lowest density of 922 kg m−3 ice nucleates at −30.5 °C (see Fig. 9) and the resulting pressure remains below 0.1 MPa (because the density of inclusions is the same as the density of ice at nearly zero pressure at the given temperature). However, in inclusions with ϱ > 942 kg m−3 the pressure becomes larger than 200 MPa after ice nucleation, exceeding thereby the range of validity of the ice Ih equation of state. Such high internal pressures can cause irreversible volume changes of the inclusions. Therefore, we routinely re-measured prograde homogenisation temperatures after Ticenr and subsequent Ticem measurements to check for a potential decrease of the water densities. In the case of irreversible volume changes, the initially measured Ticenr values did not reproduce in duplicate measurements but were systematically higher.
The inclusions with the lowest water densities analysed in this study (Th > 140 °C) allowed us to measure the ice melting temperature under three-phase conditions (see Fig. 7b). For this purpose, we used the femtosecond laser pulses to stimulate vapour bubble nucleation during melting at −0.1 °C in the presence of liquid water and ice (L + S → L + S + V). In the inclusion shown in Fig. 7b, the final ice melting was measured between 0.0 and +0.1 °C (L + V + S → L + V) as expected for pure water inclusions.
2.4. Basics of data analysis
In the first place, we used the IAPWS-95 formulation to calculate the density of the liquid phase at saturation pressure, i.e., along the liquid–vapour equilibrium curve. At this point, we note that in addition to the IAPWS-95 formulation, Wagner and Pruss also reported a polynomial function that can be used alternatively to calculate liquid-densities along the liquid–vapour curve (eqn (2.6), Section 2.3.111). However, since this polynomial is an empirical fit to experimental data above 0 °C, it covers only the stable part of the liquid–vapour curve and should not be extrapolated into the supercooled metastable region. A comparison of the two modes of calculation revealed increasing divergence of the liquid densities below −20 °C, with lower densities resulting from IAPWS-95.
The IAPWS-95 formulation is considered to exhibit ‘reasonable’ behaviour when extrapolated into the metastable liquid region (Section 7.3.211). It allows us to extrapolate the liquid-isochores into the metastable region and to calculate the liquid-spinodal. Similar to the ‘stability limit conjecture’,5 IAPWS-95 assumes a negative slope of the TMD line in the metastable region, and, as a consequence, the spinodal turns up towards positive pressures at low temperatures.
Although IAPWS-95 does not provide any information about the solid states of water, Wagner and Pruss gave a polynomial function describing the p–T trend of the ice melting curve (Ih) (eqn (2.16), Section 2.4.111) derived from Wagner et al.23 The curve is based on a fit to experimental data, including a few measurements of Henderson and Speedy,24 providing ice melting temperatures in the metastable region down to −22.8 MPa. Due to the lack of experimental data at higher tensile stress, we extrapolated the ice melting curve further into the metastable region for comparison with our new ice melting data.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) obs depends not only on the density but also on the size of the inclusions as demonstrated by Fall et al.25 This means that besides Th
obs depends not only on the density but also on the size of the inclusions as demonstrated by Fall et al.25 This means that besides Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) obs an additional volume measurement is required to determine the density of the inclusions. For practical reasons, we do not measure the volume V of the inclusions (cf. Stoller et al.26). Instead, we determine the volume of the spherical vapour bubble based on radius measurements r(T) at known temperatures (see Section 2.4.4. below). For the calculations of Th∞ (and thus ϱbulk) and the inclusion volume V we used the thermodynamic model of Marti et al.17 that accounts for the effect of surface tension on liquid–vapour equilibria in isochoric systems. The model relies on the minimisation of the Helmholtz energy of the system derived from the IAPWS-95 formulation, and predicts the thermodynamic state, the vapour bubble radius, the density and pressure of the liquid and vapour phases at a given temperature, and the volume and bulk density of the system. For inclusions analysed in this study, the temperature differences between Th
obs an additional volume measurement is required to determine the density of the inclusions. For practical reasons, we do not measure the volume V of the inclusions (cf. Stoller et al.26). Instead, we determine the volume of the spherical vapour bubble based on radius measurements r(T) at known temperatures (see Section 2.4.4. below). For the calculations of Th∞ (and thus ϱbulk) and the inclusion volume V we used the thermodynamic model of Marti et al.17 that accounts for the effect of surface tension on liquid–vapour equilibria in isochoric systems. The model relies on the minimisation of the Helmholtz energy of the system derived from the IAPWS-95 formulation, and predicts the thermodynamic state, the vapour bubble radius, the density and pressure of the liquid and vapour phases at a given temperature, and the volume and bulk density of the system. For inclusions analysed in this study, the temperature differences between Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) obs and Th∞ were up to 1.8 °C. A schematic p–T diagram illustrating the effect of surface tension on liquid–vapour homogenisation is shown in Fig. S2 (ESI†).
obs and Th∞ were up to 1.8 °C. A schematic p–T diagram illustrating the effect of surface tension on liquid–vapour homogenisation is shown in Fig. S2 (ESI†).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) obs and Ticenr. The solid curve represents the best fit of the radius measurements and predicts the theoretical evolution of the bubble radius for an inclusion volume of 210 μm3. In contrast, Fig. 8b shows another ‘low-density’ inclusion, in which the bubble becomes spherical only at radii smaller than 1.7 μm, i.e., at temperatures above 60 °C and below −20 °C, respectively. At temperatures in between the measured bubble radii are systematically larger than the theoretical radii predicted for an inclusion volume of 410 μm3, which indicates deformation of the bubble due to the confining inclusion walls. We performed these radius analyses for all inclusions in this study in order to eliminate a potential source of error in the determination of the inclusion volume V, and thus Th∞, Th
obs and Ticenr. The solid curve represents the best fit of the radius measurements and predicts the theoretical evolution of the bubble radius for an inclusion volume of 210 μm3. In contrast, Fig. 8b shows another ‘low-density’ inclusion, in which the bubble becomes spherical only at radii smaller than 1.7 μm, i.e., at temperatures above 60 °C and below −20 °C, respectively. At temperatures in between the measured bubble radii are systematically larger than the theoretical radii predicted for an inclusion volume of 410 μm3, which indicates deformation of the bubble due to the confining inclusion walls. We performed these radius analyses for all inclusions in this study in order to eliminate a potential source of error in the determination of the inclusion volume V, and thus Th∞, Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r∞, and the bulk density ϱbulk. The accuracy of the radius measurements is estimated to be ±0.15 μm. The precision of the measurements is ±0.05 μm reflecting the deviation of the measured bubble radii from the best-fit curve of the theoretical radius evolution.
r∞, and the bulk density ϱbulk. The accuracy of the radius measurements is estimated to be ±0.15 μm. The precision of the measurements is ±0.05 μm reflecting the deviation of the measured bubble radii from the best-fit curve of the theoretical radius evolution.
 | ||
Fig. 9
ϱ–T diagram illustrating the results of the fluid inclusion measurements. Ticenr: ice nucleation in the absence of the vapour phase (L → S). Ticem: final ice melting in the absence of the vapour phase (L + S → L). Th∞ and Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r∞: prograde and retrograde liquid–vapour homogenisation (L + V → L). Tvapnr and Tvapnp: spontaneous retrograde and prograde vapour bubble nucleation (L → L + V). The results of previous experimental studies are plotted for comparison. L + V: liquid–vapour equilibrium curve (IAPWS-95). L + S: liquid–solid equilibrium curve.23 Dashed line: liquid-spinodal (IAPWS-95). Grey solid nearly horizontal lines: pseudo-isochore curves. Dash-dotted line: density of ice at zero pressure.22 r∞: prograde and retrograde liquid–vapour homogenisation (L + V → L). Tvapnr and Tvapnp: spontaneous retrograde and prograde vapour bubble nucleation (L → L + V). The results of previous experimental studies are plotted for comparison. L + V: liquid–vapour equilibrium curve (IAPWS-95). L + S: liquid–solid equilibrium curve.23 Dashed line: liquid-spinodal (IAPWS-95). Grey solid nearly horizontal lines: pseudo-isochore curves. Dash-dotted line: density of ice at zero pressure.22 | ||
3. Results
A graphical representation of the results obtained in this study is shown in Fig. 9 and displays Th∞, Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r∞, Ticenr (only L → S), Ticem, Tvapnr and Tvapnp as a function of density. The reason for using a ϱ–T representation instead of the more familiar p–T diagram is that we have an accurate measure for the density of the inclusions via Th
r∞, Ticenr (only L → S), Ticem, Tvapnr and Tvapnp as a function of density. The reason for using a ϱ–T representation instead of the more familiar p–T diagram is that we have an accurate measure for the density of the inclusions via Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) obs and the bubble radius r(T), whereas the pressure inside the inclusions needs to be calculated by extrapolation of the IAPWS-95 formulation into the metastable region. The diagram displays additional experimental data from previous studies10,12,13,29–31 for comparison with our new data. The liquid–vapour equilibrium curve (L + V) and the liquid-spinodal were derived from IAPWS-95,11 and the liquid–solid equilibrium curve (L + S) was derived from the polynomial function given by Wagner et al.23 Finally, the diagram displays three pseudo-isochores (with slight negative slopes) that intersect the liquid–vapour equilibrium curve at Th∞ values of 50, 90 and 150 °C (with bulk densities of 988.0, 965.3 and 917.0 kg m−3, respectively). Numerical values of our experimental data are reported in Tables 1–3.
obs and the bubble radius r(T), whereas the pressure inside the inclusions needs to be calculated by extrapolation of the IAPWS-95 formulation into the metastable region. The diagram displays additional experimental data from previous studies10,12,13,29–31 for comparison with our new data. The liquid–vapour equilibrium curve (L + V) and the liquid-spinodal were derived from IAPWS-95,11 and the liquid–solid equilibrium curve (L + S) was derived from the polynomial function given by Wagner et al.23 Finally, the diagram displays three pseudo-isochores (with slight negative slopes) that intersect the liquid–vapour equilibrium curve at Th∞ values of 50, 90 and 150 °C (with bulk densities of 988.0, 965.3 and 917.0 kg m−3, respectively). Numerical values of our experimental data are reported in Tables 1–3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) obs and Th
obs and Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r
r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) obs, (retrograde) ice nucleation Ticenr, and final ice melting Ticem of 26 fluid inclusions. Temperature accuracies are estimated based on the calibration of the heating/freezing stage. Vapour bubble radii r(T) refer to a temperature of 20 °C and were derived from theoretical radius curves fitted to the measured values. Th∞, Th
obs, (retrograde) ice nucleation Ticenr, and final ice melting Ticem of 26 fluid inclusions. Temperature accuracies are estimated based on the calibration of the heating/freezing stage. Vapour bubble radii r(T) refer to a temperature of 20 °C and were derived from theoretical radius curves fitted to the measured values. Th∞, Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r∞ and the volume V of the inclusions were calculated using the model of Marti et al.17 Due to the small size of the inclusions, the uncertainty of the bubble radius measurements (±0.15 μm) results in large relative errors of the inclusion volumes. However, the effect of the volume error on Th∞ and Th
r∞ and the volume V of the inclusions were calculated using the model of Marti et al.17 Due to the small size of the inclusions, the uncertainty of the bubble radius measurements (±0.15 μm) results in large relative errors of the inclusion volumes. However, the effect of the volume error on Th∞ and Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r∞ is small. The overall uncertainty of Th∞ and Th
r∞ is small. The overall uncertainty of Th∞ and Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r∞ given in the table comprises the error relating to the temperature accuracy of the stage, the error resulting from the volume uncertainty, and an additional uncertainty associated with the vapour bubble collapse (see Section S2.4.2, ESI). The density of water at Ticenr and at Ticem was determined from Th∞ by applying a correction for the temperature-dependent volume change of the quartz host. Note that the densities assigned to Ticem were determined from re-measurements of Th
r∞ given in the table comprises the error relating to the temperature accuracy of the stage, the error resulting from the volume uncertainty, and an additional uncertainty associated with the vapour bubble collapse (see Section S2.4.2, ESI). The density of water at Ticenr and at Ticem was determined from Th∞ by applying a correction for the temperature-dependent volume change of the quartz host. Note that the densities assigned to Ticem were determined from re-measurements of Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) obs performed after ice melting to account for potential volume increases of the inclusions resulting from ice nucleation. In particular, high-density inclusions with Th∞ < 90 °C were often subject to irreversible volume changes, and thus, the densities at Ticem did not relate to the initial Th∞ values reported in the table. For low-density inclusions that did not stretch due to ice nucleation Table 1 reports only the lowest Ticenr values measured in each inclusion considering these minimum temperatures as the closest approximation to homogeneous ice nucleation
obs performed after ice melting to account for potential volume increases of the inclusions resulting from ice nucleation. In particular, high-density inclusions with Th∞ < 90 °C were often subject to irreversible volume changes, and thus, the densities at Ticem did not relate to the initial Th∞ values reported in the table. For low-density inclusions that did not stretch due to ice nucleation Table 1 reports only the lowest Ticenr values measured in each inclusion considering these minimum temperatures as the closest approximation to homogeneous ice nucleation
| Inclusion |
T
h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) obs (L + V → L) (°C) obs (L + V → L) (°C) |
T
h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) obs (L + V → L) (°C) obs (L + V → L) (°C) |
T icenr (L → S) ±0.2 °C | T icem (L + S → L) ±0.1 °C | r(@20 °C) ±0.15 μm | T h∞ (°C) |
T
h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r∞ (°C) r∞ (°C) |
V (μm3) | ϱ bulk(Th∞) (kg m−3) | ϱ(Ticenr) (kg m−3) | ϱ(Ticem) (kg m−3) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 13-2-3 | 28.7 ± 0.1 | −12.4 ± 0.1 | −43.3 | 0.4 | 0.61 | 30.18+0.46−0.32 | −13.63−0.39+0.29 | 450−260+400 | 995.55−0.14+0.10 | 997.75−0.13+0.09 | 995.23−0.12+0.10 |
| 13-2-2 | 29.8 ± 0.1 | −13.2 ± 0.1 | −43.3 | 0.4 | 0.46 | 31.64+0.72−0.46 | −14.70−0.60+0.38 | 160−110+230 | 995.10−0.23+0.15 | 997.34−0.21+0.13 | 994.53−0.18+0.11 |
| 13-3-3 | 36.4 ± 0.2 | −16.8 ± 0.1 | −43.0 | 0.6 | 0.70 | 37.67+0.45−0.37 | −17.80−0.29+0.23 | 330−170+260 | 993.05−0.16+0.14 | 995.44−0.15+0.12 | 993.37−0.15+0.12 |
| 13-3-4 | 45.0 ± 0.2 | −21.5 ± 0.2 | −42.4 | 1.0 | 0.55 | 46.42+0.58−0.43 | −22.55−0.37+0.37 | 90−60+100 | 989.57−0.25+0.19 | 992.22−0.23+0.17 | 989.86−0.23+0.17 |
| 2-2-6 | 49.2 ± 0.2 | −23.2 ± 0.2 | −42.1 | 1.1 | 0.99 | 50.09+0.31−0.29 | −23.84−0.28+0.26 | 430−160+250 | 987.96−0.14+0.13 | 990.70−0.13+0.12 | 985.90−0.12+0.11 |
| 14-1-4 | 51.4 ± 0.2 | −24.2 ± 0.2 | −42.3 | 1.2 | 0.51 | 52.85+0.62−0.46 | −25.22−0.49+0.37 | 70−30+70 | 986.68−0.29+0.22 | 989.51−0.28+0.20 | 987.87−0.27+0.20 |
| 14-1-3 | 61.8 ± 0.2 | −28.6 ± 0.2 | −41.1 | 1.8 | 0.61 | 63.00+0.47−0.39 | −29.39−0.37+0.32 | 60−30+60 | 981.59−0.25+0.21 | 984.68−0.26+0.18 | 981.45−0.24+0.20 |
| 2-1-3 | 71.8 ± 0.2 | −31.9 ± 0.2 | −40.4 | 2.1 | 0.91 | 72.64+0.32−0.29 | −32.42−0.27+0.25 | 160−70+90 | 976.21−0.19+0.17 | 979.55−0.18+0.16 | 978.51−0.18+0.16 |
| 2-1-3c | 74.5 ± 0.2 | −32.9 ± 0.2 | −40.1 | 2.1 | 0.91 | 75.34+0.31−0.30 | −33.42−0.27+0.25 | 150−60+90 | 974.61−0.18+0.18 | 978.02−0.18+0.17 | 977.00−0.17+0.17 |
| 8-1-4 | 79.9 ± 0.2 | −34.9 ± 0.2 | −39.7 | 2.3 | 1.60 | 80.44+0.24−0.23 | −35.21−0.22+0.22 | 690−180+230 | 971.49−0.15+0.15 | 975.06−0.16+0.12 | 973.87−0.14+0.14 |
| 2-1-4c | 81.6 ± 0.2 | −35.1 ± 0.2 | −39.8 | 2.2 | 1.20 | 82.26+0.27−0.26 | −35.48−0.24+0.23 | 280−90+120 | 970.35−0.17+0.16 | 973.94−0.16+0.16 | 972.94−0.16+0.16 |
| 8-1-1 | 88.8 ± 0.2 | −36.8 ± 0.2 | −38.5 | 2.8 | 1.05 | 89.52+0.28−0.27 | −37.19−0.24+0.23 | 160−60+80 | 965.62−0.19+0.18 | 969.37−0.17+0.19 | 968.24−0.18+0.17 |
| 8-1-7 | 91.7 ± 0.2 | Not observed | −37.7 | 3.0 | 2.04 | 92.42+0.28−0.27 | — | 150−60+70 | 963.66−0.19+0.18 | 967.49−0.19+0.18 | 966.55−0.19+0.17 |
| 8-2-7 | 94.8 ± 0.2 | n.o. | −37.3 | 3.1 | 1.35 | 95.38+0.25−0.25 | — | 310−90+100 | 961.61−0.18+0.17 | 965.51−0.17+0.17 | 963.50−0.17+0.17 |
| 10-5-6 | 111.5 ± 0.3 | n.o. | −36.1 | 4.4 | 1.14 | 112.14+0.36−0.36 | — | 140−50+60 | 949.31−0.28+0.28 | 953.62−0.27+0.27 | 952.74−0.27+0.27 |
| 10-5-4 | 113.5 ± 0.3 | n.o. | −35.9 | 4.5 | 1.18 | 114.11+0.37−0.35 | — | 150−50+60 | 947.78−0.29+0.27 | 952.13−0.28+0.27 | 951.26−0.27+0.26 |
| 4-2-6 | 115.2 ± 0.3 | n.o. | −36.1 | 4.6 | 1.07 | 115.86+0.38−0.36 | — | 100−40+50 | 946.41−0.30+0.28 | 950.81−0.29+0.28 | 949.94−0.29+0.27 |
| 4-2-4 | 120.9 ± 0.3 | n.o. | −35.2 | 5.1 | 1.19 | 121.50+0.36−0.35 | — | 130−50+60 | 941.46−0.30+0.28 | 946.42−0.29+0.28 | 945.58−0.29+0.27 |
| 4-1-3 | 126.7 ± 0.3 | n.o. | −34.1 | 5.3 | 1.12 | 127.32+0.37−0.35 | — | 100−30+50 | 937.09−0.31+0.30 | 941.77−0.30+0.29 | 940.94−0.30+0.29 |
| 7-7-10 | 127.4 ± 0.3 | n.o. | −34.4 | 5.4 | 1.20 | 127.99+0.36−0.35 | — | 130−40+50 | 936.53−0.30+0.29 | 941.21−0.30+0.28 | 940.40−0.29+0.28 |
| 4-1-7 | 132.1 ± 0.3 | n.o. | −33.1 | 5.7 | 1.80 | 132.53+0.33−0.32 | — | 400−90+100 | 932.67−0.28+0.28 | 937.44−0.27+0.27 | 936.65−0.27+0.27 |
| 4-1-6 | 135.2 ± 0.3 | n.o. | −32.3 | 6.0 | 1.11 | 135.82+0.37−0.36 | — | 90−30+40 | 929.83−0.32+0.31 | 934.66−0.31+0.30 | 933.89−0.31+0.30 |
| 4-1-1 | 137.8 ± 0.3 | n.o. | −32.1 | 6.1 | 1.82 | 138.22+0.33−0.32 | — | 380−90+100 | 927.71−0.29+0.29 | 932.60−0.28+0.28 | 931.84−0.28+0.28 |
| 4-1-4 | 141.9 ± 0.3 | n.o. | −31.4 | 6.4 | 1.52 | 142.38+0.34−0.33 | — | 210−60+70 | 924.00−0.31+0.30 | 928.98−0.30+0.29 | 928.23−0.30+0.29 |
| 6-2-4 | 148.0 ± 0.3 | n.o. | −30.7 | 6.7 | 1.86 | 148.41+0.33−0.32 | — | 360−80+100 | 918.49−0.30+0.30 | 923.60−0.30+0.29 | 922.88−0.29+0.30 |
| 6-2-1 | 149.7 ± 0.3 | n.o. | −30.5 | 6.8 | 1.55 | 150.17+0.34−0.33 | — | 201−53+65 | 916.85−0.32+0.31 | 922.00−0.31+0.30 | 921.28−0.31+0.30 |
| Inclusion | T icenr (L + V → S) ±0.2 °C | T h∞ (°C) | V (μm3) | r(Ticenr) ±0.15 μm |
|---|---|---|---|---|
| 7-2-1 | −37.8 | 145.75+0.33−0.33 | 250−60+80 | 1.36 |
| 7-6-1 | −38.1 | 145.70+0.32−0.32 | 420−90+100 | 1.64 |
| 7-6-6 | −38.3 | 146.10+0.37−0.35 | 80−30+40 | 0.94 |
| 7-6-5 | −38.3 | 146.14+0.35−0.34 | 120−40+50 | 1.06 |
| 7-6-3 | −38.1 | 146.21+0.33−0.32 | 360−80+100 | 1.52 |
| 7-6-9 | −38.5 | 146.45+0.33−0.33 | 250−60+80 | 1.39 |
| 7-6-7 | −38.5 | 147.12+0.38−0.36 | 70−30+30 | 0.90 |
| 7-2-2 | −37.9 | 147.14+0.33−0.32 | 280−70+80 | 1.42 |
| 7-6-4 | −38.4 | 147.14+0.34−0.33 | 270−70+80 | 1.40 |
| 6-2-4 | −38.4 | 148.31+0.33−0.32 | 360−80+100 | 1.59 |
| 6-2-7 | −38.7 | 148.47+0.36−0.35 | 100−30+40 | 1.02 |
| 6-2-5 | −39.0 | 148.62+0.37−0.36 | 70−20+30 | 0.90 |
| 6-2-6 | −39.1 | 148.87+0.36−0.35 | 100−30+40 | 1.03 |
| 6-3-8 | −39.5 | 149.71+0.35−0.34 | 140−40+50 | 1.12 |
| 6-3-9 | −39.6 | 150.01+0.35−0.34 | 140−40+50 | 1.14 |
| 6-2-3 | −38.7 | 150.21+0.37−0.36 | 70−30+30 | 0.91 |
| 6-2-2 | −38.8 | 151.04+0.38−0.36 | 60−20+30 | 0.87 |
| 6-3-13 | −39.6 | 150.99+0.35−0.34 | 120−40+50 | 1.10 |
| 6-2-1 | −38.9 | 150.92+0.35−0.34 | 130−40+50 | 1.13 |
| 4-2-4 | −39.2 | 121.50+0.36−0.35 | 130−50+60 | 0.86 |
| 4-2-6 | −39.1 | 115.86+0.38−0.36 | 100−40+50 | 0.72 |
| 8-2-7 | −39.2 | 95.38+0.25−0.25 | 310−90+100 | 0.55 |
| 10-5-6 | −38.2 | 112.14+0.36−0.36 | 140−50+60 | 0.71 |
| Inclusion | T vapnr (L → L + V) ±0.2 °C | T vapnp (L → L + V) ±0.1 °C | r(20 °C) ±0.15 μm | T h∞ (°C) | V (μm3) | ϱ bulk(Th∞) (kg m−3) | ϱ(Tvapnr) (kg m−3) | ϱ(Tvapnp) (kg m−3) |
|---|---|---|---|---|---|---|---|---|
| 7-6-2 | 53.7 | 29.6 | 1.86 | 145.21+0.33−0.32 | 370−80+100 | 921.43−0.30+0.30 | 924.50−0.29+0.29 | 925.17−0.29+0.29 |
| 7-2-1 | 55.9 | 22.7 | 1.64 | 145.75+0.33−0.33 | 250−60+80 | 920.94−0.31+0.30 | 923.96−0.30+0.29 | 924.74−0.31+0.49 |
| 7-6-1 | 59.7 | 22.8 | 1.94 | 145.70+0.32−0.32 | 420−90+100 | 920.99−0.30+0.29 | 923.89−0.29+0.28 | 924.91−0.29+0.29 |
| 7-6-10 | 53.8 | 29.6 | 1.82 | 145.72+0.33−0.32 | 350−80+100 | 920.97−0.30+0.30 | 924.05−0.29+0.29 | 924.72−0.29+0.29 |
| 7-6-6 | 64.7 | 17.2 | 1.13 | 146.10+0.37−0.35 | 80−30+40 | 920.62−0.34+0.32 | 923.39−0.33+0.31 | 924.69−0.33+0.31 |
| 7-6-5 | 65.5 | 17.1 | 1.28 | 146.14+0.35−0.34 | 120−40+50 | 920.58−0.32+0.31 | 923.32−0.31+0.30 | 924.66−0.31+0.30 |
| 7-6-3 | 63.3 | 17.3 | 1.85 | 146.21+0.33−0.32 | 360−80+100 | 920.52−0.30+0.30 | 923.33−0.29+0.29 | 924.59−0.29+0.29 |
| 7-6-9 | 62.2 | 23.8 | 1.63 | 146.45+0.33−0.33 | 250−60+80 | 920.30−0.31+0.30 | 923.15−0.30+0.29 | 924.21−0.30+0.29 |
| 7-6-8 | 52.9 | 26.2 | 2.15 | 146.57+0.32−0.32 | 560−110+130 | 920.19−0.29+0.29 | 923.32−0.28+0.28 | 924.05−0.29+0.28 |
| 7-6-7 | 62.9 | 18.1 | 1.06 | 147.12+0.38−0.36 | 70−30+30 | 919.68−0.35+0.33 | 922.53−0.33+0.32 | 923.76−0.34+0.32 |
| 7-2-2 | 61.8 | 24.3 | 1.70 | 147.14+0.33−0.32 | 280−70+80 | 919.66−0.31+0.30 | 922.55−0.30+0.29 | 923.59−0.30+0.29 |
| 7-6-4 | 68.8 | 16.7 | 1.68 | 147.14+0.34−0.33 | 270−70+80 | 919.66−0.31+0.30 | 922.33−0.30+0.29 | 923.77−0.30+0.29 |
| 6-2-4 | 64.4 | n.o. | 1.88 | 148.31+0.33−0.32 | 360−80+100 | 918.58−0.30+0.30 | 921.43−0.29+0.29 | — |
| 6-2-7 | 64.8 | 20.1 | 1.20 | 148.47+0.36−0.35 | 100−30+40 | 918.43−0.33+0.32 | 921.27−0.32+0.31 | 922.50−0.32+0.31 |
| 6-2-5 | 54.4 | 29.1 | 1.07 | 148.62+0.37−0.36 | 70−20+30 | 918.29−0.35+0.33 | 921.44−0.34+0.32 | 922.14−0.34+0.32 |
| 6-2-6 | 59.1 | 29.5 | 1.20 | 148.87+0.36−0.35 | 100−30+40 | 918.06−0.33+0.32 | 921.08−0.32+0.31 | 921.91−0.32+0.31 |
| 6-3-8 | 69.8 | 19.5 | 1.38 | 149.71+0.35−0.34 | 140−40+50 | 917.28−0.32+0.31 | 920.00−0.31+0.30 | 921.40−0.31+0.31 |
| 6-3-9 | 65.9 | 18.2 | 1.38 | 150.01+0.35−0.34 | 140−40+50 | 917.00−0.32+0.32 | 919.85−0.31+0.30 | 921.16−0.31+0.31 |
| 6-2-3 | 65.2 | 22.6 | 1.09 | 150.21+0.37−0.36 | 70−30+30 | 916.81−0.35+0.33 | 919.69−0.34+0.32 | 920.87−0.34+0.32 |
| 6-2-2 | 72.8 | 16.8 | 1.01 | 151.04+0.38−0.36 | 60−20+30 | 916.03−0.36+0.34 | 918.70−0.35+0.33 | 920.25−0.35+0.33 |
| 6-3-13 | 71.8 | n.o. | 1.29 | 150.99+0.35−0.34 | 120−40+50 | 916.41−0.33+0.32 | 919.10−0.32+0.31 | — |
| 6-2-1 | 72.8 | 12.6 | 1.35 | 150.92+0.35−0.34 | 130−40+50 | 916.15−0.33+0.32 | 918.81−0.32+0.31 | 920.46−0.32+0.31 |
3.1. Liquid–vapour homogenisation data
Fig. 9 displays the Th∞ and Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r∞ data of prograde and retrograde liquid–vapour homogenisation, respectively, which by definition are plotted on the liquid–vapour equilibrium curve (L + V) and hence determine the bulk density of the water in the inclusions. The densities ϱbulk derived from prograde liquid–vapour homogenisation are used here as reference values to characterise the inclusions. They range from 996 to 916 kg m−3. By applying a correction to ϱbulk for the temperature-dependent volume change of the quartz host, we obtained ϱ(T), the density of an inclusion at any other temperature, namely at Ticenr, Ticem, Tvapnp and Tvapnr. Retrograde liquid–vapour homogenisation was observed only in high-density inclusions with ϱbulk greater than 965 kg m−3, corresponding to Th∞ values below 90 °C. The lowest Th
r∞ data of prograde and retrograde liquid–vapour homogenisation, respectively, which by definition are plotted on the liquid–vapour equilibrium curve (L + V) and hence determine the bulk density of the water in the inclusions. The densities ϱbulk derived from prograde liquid–vapour homogenisation are used here as reference values to characterise the inclusions. They range from 996 to 916 kg m−3. By applying a correction to ϱbulk for the temperature-dependent volume change of the quartz host, we obtained ϱ(T), the density of an inclusion at any other temperature, namely at Ticenr, Ticem, Tvapnp and Tvapnr. Retrograde liquid–vapour homogenisation was observed only in high-density inclusions with ϱbulk greater than 965 kg m−3, corresponding to Th∞ values below 90 °C. The lowest Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r∞ value determined in this study was −37.2 °C.
r∞ value determined in this study was −37.2 °C.
3.2. Ice nucleation data
In high-density inclusions with Th∞ < 90 °C, ice nucleation commonly resulted in irreversible volume changes, and thus, Ticenr systematically increased in replicate measurements. This effect did not occur in low-density inclusions, in which case, Ticenr was typically reproduced within ±0.5 °C. In Table 1 and Fig. 9, we report only the lowest Ticenr value measured in each inclusion considering these minimum temperatures as the closest approximation to homogeneous ice nucleation. Apart from a slight scatter, the data points (eight spoked asterisks) shown in Fig. 9 indicate a linear increase of Ticenr with decreasing density in the range covered in this study. (Sorry for the lack of data points just below the saturation curve.) For a more detailed view of the ice nucleation data on an enlarged scale we refer to Fig. S4 (ESI†). We report an ice nucleation line (L → S) derived from a linear fit to our data:| ϱ(Ticenr) = −5.8481T + 743.53 |
The ice nucleation line intersects the liquid–vapour equilibrium curve at −38.2 °C, providing an estimate of the expected ice nucleation temperature Ticenr at saturation pressure (L + V → S). In comparison to this, the real Ticenr measurements performed in the presence of the vapour bubble (not shown in Fig. 9) yielded slightly lower values, ranging between −37.8 and −39.6 °C (see Fig. S5, ESI†). Although these measurements suggest a potential effect of the vapour bubble on ice nucleation temperatures, we found only a weak correlation between Ticenr and the bubble radius r at Ticenr (see Fig. S6, ESI†). Replicate measurements of individual inclusions reproduced, with few exceptions, within ±0.5 °C. Again, Table 2 and Fig. S5 and S6 (ESI†) report the lowest Ticenr (L + V → S) values measured in each inclusion.
At −36.2 °C, Fig. 9 and Fig. S4 (ESI†) additionally show an intersection of the ice nucleation line with the liquid-spinodal predicted by IAPWS-95 (dashed curve). As a result, ice nucleation temperatures measured in low-density inclusions (ϱ(Ticenr) < 955 kg m−3) are plotted on the left hand side of the spinodal i.e., within the IAPWS-95 defined region of the unstable liquid. Therefore, IAPWS-95 cannot provide any pressures at Ticenr for these low-density inclusions, which is another reason for presenting our data in a ϱ–T diagram. It should be noted that we did not observe any indication for a change in the state of liquid water above Ticenr. Moreover, we found that the application of femtosecond laser pulses in the doubly metastable region, even close to Ticenr, resulted in vapour bubble nucleation but never stimulated ice nucleation. We therefore conclude that ice nucleation in fluid inclusions is not triggered by bubble nucleation as it was observed by Barrow et al.34 in Berthelot tube experiments.
3.3. Final ice melting data
The results of final ice melting measurements (dots) illustrated in Fig. 9 indicate an increase of Ticem with decreasing density ϱ(Ticem). For comparison, the diagram also displays a reference ice-melting curve derived by extrapolation of eqn (2.16)11 into the metastable region. Again, the initial p–T curve was converted to a ϱ–T curve using the IAPWS-95 formulation. Our fluid inclusion data show significant deviations from the extrapolated Ticem curve, which will be discussed in detail in Section 4.3. At densities around 970 kg m−3, we also observed a slight step in our ice melting data, which is supposed to have no physical meaning. Repeated measurements of these inclusions revealed significant variations of Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) obs after Ticem measurements that were not linked to corresponding changes in Ticem. Therefore, we do not consider these data reliable. At saturation pressure, i.e., in the presence of the vapour bubble, Ticem was measured at 0.0 ± 0.1 °C, which is in agreement with pure water inclusions. The maximum temperature of the final ice melting was measured to be +6.8 °C, which corroborates a previously reported temperature of +6.5 °C observed by Roedder33 in metastable fluid inclusions.
obs after Ticem measurements that were not linked to corresponding changes in Ticem. Therefore, we do not consider these data reliable. At saturation pressure, i.e., in the presence of the vapour bubble, Ticem was measured at 0.0 ± 0.1 °C, which is in agreement with pure water inclusions. The maximum temperature of the final ice melting was measured to be +6.8 °C, which corroborates a previously reported temperature of +6.5 °C observed by Roedder33 in metastable fluid inclusions.
3.4. Spontaneous bubble nucleation data
In the present study, spontaneous vapour bubble nucleation (or cavitation) occurred only in inclusions with Th∞ > 145 °C. Replicate measurements of Tvapnr and Tvapnp revealed a reproducibility within ±3 °C for individual inclusions. A comparison of different inclusions of similar densities (i.e., similar Th∞ values), however, revealed larger variations of up to 10 °C, though we found no correlation with the inclusion volume in the range of inclusion sizes we were looking at. In Table 3 and Fig. 9, we report the lowest Tvapnr values measured in each inclusion and the highest values of Tvapnp, respectively. Retrograde bubble nucleation was observed 80 °C to 90 °C below Th∞, far away from the predicted liquid-spinodal. Tvapnp can be observed only in a narrow range of inclusion densities ϱbulk between 921.4 and 916.1 kg m−3, corresponding to Th∞ values between 145.2 and 150.9 °C. At lower bulk densities, i.e., higher Th∞ prograde bubble nucleation cannot be measured anymore because in these inclusions the ice phase does not fully compensate the bubble volume, and as a consequence ice melting occurs solely at saturation pressure and not at negative pressures in the metastable region. The temperature difference between Tvapnr and Tvapnp tends to decrease with increasing density (decreasing Th∞). An interpolation of our data suggests that Tvapnr and Tvapnp become equal at around 40 °C. In the present study, the highest Th∞ value of an inclusion that did not show spontaneous bubble nucleation was determined to be 142.4 °C (924.0 kg m−3).In addition, Fig. 9 also displays some Tvapnr data from previous studies.10,12,13,29,31 For the calculation of the corresponding ϱ(Tvapnr) from these studies we treated Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) obs equal to Th∞ since they only report Th
obs equal to Th∞ since they only report Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) obs values. In fact in inclusions with ϱ < 930 kg m−3, the relative difference in ϱbulk resulting from the difference between Th
obs values. In fact in inclusions with ϱ < 930 kg m−3, the relative difference in ϱbulk resulting from the difference between Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) obs and Th∞ is less than 0.05%, and thus, is negligible. In contrast to our own data, the Tvapnr values reported in ref. 10, 29 and Table 1 of ref. 13 show a much larger scatter by several tens of degrees for different inclusions of similar densities. We therefore display only the lowest of these values in Fig. 9. The locus of the nucleation data points in the ϱ–T plane appears to form a bubble nucleation (cavitation) curve that exhibits a maximum at around 40 °C (see Discussion in Section 4.4).
obs and Th∞ is less than 0.05%, and thus, is negligible. In contrast to our own data, the Tvapnr values reported in ref. 10, 29 and Table 1 of ref. 13 show a much larger scatter by several tens of degrees for different inclusions of similar densities. We therefore display only the lowest of these values in Fig. 9. The locus of the nucleation data points in the ϱ–T plane appears to form a bubble nucleation (cavitation) curve that exhibits a maximum at around 40 °C (see Discussion in Section 4.4).
4. Discussion
4.1. Evaluation of saturation liquid densities in the supercooled region
Retrograde liquid–vapour homogenisation temperatures provide information on the ϱ–T trend of the liquid–vapour equilibrium curve in the supercooled region. To evaluate our data, we calculated Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r∞ based on Th
r∞ based on Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r
r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) obs and the reference bubble radius r(20 °C) of the inclusions. The corresponding (prograde) Th∞ values were calculated accordingly from Th
obs and the reference bubble radius r(20 °C) of the inclusions. The corresponding (prograde) Th∞ values were calculated accordingly from Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) obs using the same reference bubble radius. In this way, we obtained two independent densities for the same inclusion. In Fig. 10, we compare the Th∞–Th
obs using the same reference bubble radius. In this way, we obtained two independent densities for the same inclusion. In Fig. 10, we compare the Th∞–Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r∞ pairs obtained from high-density inclusions with a reference curve derived from IAPWS-95. To make the isochoric IAPWS-95 curve (dashed line) comparable to our fluid inclusion data, we applied a correction for the temperature-dependent volume change of the quartz host, which shifts the predicted Th∞–Th
r∞ pairs obtained from high-density inclusions with a reference curve derived from IAPWS-95. To make the isochoric IAPWS-95 curve (dashed line) comparable to our fluid inclusion data, we applied a correction for the temperature-dependent volume change of the quartz host, which shifts the predicted Th∞–Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r∞ curve to the left (solid line). The starting points of the two curves are defined by the temperatures at which Th∞ equals Th
r∞ curve to the left (solid line). The starting points of the two curves are defined by the temperatures at which Th∞ equals Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r∞ (open circles). In the isochoric system this is at 4.0 °C, i.e. at the temperature of maximum density (TMD), whereas in the case of a quartz-confined system, the starting point is at 6.15 °C.35 According to IAPWS-95 both curves end at −39.6 °C (vertical dash-dotted line), representing the temperature at which the liquid-spinodal intersects the liquid–vapour curve in the supercooled region. We recall that IAPWS-95 does not provide information on ice nucleation. In reality, ice nucleation in the presence of the vapour bubble (L + V → S) was observed close to the alleged spinodal crossing at temperatures in the grey shaded area between −37.8 and −39.6 °C.
r∞ (open circles). In the isochoric system this is at 4.0 °C, i.e. at the temperature of maximum density (TMD), whereas in the case of a quartz-confined system, the starting point is at 6.15 °C.35 According to IAPWS-95 both curves end at −39.6 °C (vertical dash-dotted line), representing the temperature at which the liquid-spinodal intersects the liquid–vapour curve in the supercooled region. We recall that IAPWS-95 does not provide information on ice nucleation. In reality, ice nucleation in the presence of the vapour bubble (L + V → S) was observed close to the alleged spinodal crossing at temperatures in the grey shaded area between −37.8 and −39.6 °C.
Fig. 10 demonstrates that the Th∞–Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r∞ data obtained in this study are in excellent agreement with IAPWS-95 (also see Fig. S7, ESI†), and thus, confirm the results of the IAPWS-5 formulation with respect to saturation liquid densities in the supercooled region. This finding suggests that the IAPWS-95 isochores for densities larger than 970 kg m−3, as drawn through the metastable region from Th
r∞ data obtained in this study are in excellent agreement with IAPWS-95 (also see Fig. S7, ESI†), and thus, confirm the results of the IAPWS-5 formulation with respect to saturation liquid densities in the supercooled region. This finding suggests that the IAPWS-95 isochores for densities larger than 970 kg m−3, as drawn through the metastable region from Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r∞ to Th∞, through the temperature of maximum density, are essentially correct (the pseudo-isochore p(T|ϱ = 970 kg m−3) is included in Fig. 12b). However, our data discussed in the following paragraphs indicate that the validity of the IAPWS-95 extrapolation becomes questionable when diving deeper into the stretched metastable region.
r∞ to Th∞, through the temperature of maximum density, are essentially correct (the pseudo-isochore p(T|ϱ = 970 kg m−3) is included in Fig. 12b). However, our data discussed in the following paragraphs indicate that the validity of the IAPWS-95 extrapolation becomes questionable when diving deeper into the stretched metastable region.
4.2. Low-temperature trend of the liquid-spinodal
In Section 3.2, we have demonstrated that ice nucleation temperatures measured in low-density inclusions (ϱ(Ticenr) < 955 kg m−3) are plotted in the region where IAPWS-95 predicts an unstable thermodynamic state for liquid water. Therefore, our new experimental data clearly disprove the validity of the extrapolation of the IAPWS-95 formulation into the doubly metastable region at densities much below 970 kg m−3. Of course, our finding does not disprove Speedy's conjecture of a re-entrant liquid-spinodal.5 However, to explain the anomalies at −45 °C, the actual spinodal would have to be much more sloped in the ϱ–T representation than the IAPWS-95 spinodal (see Fig. 9) or cross to the positive pressure region through an ill-defined, possibly non-existent, extrapolation of the binodal at densities below the density of ice at zero pressure.4.3. Ice melting curve
In Fig. 11, we have a closer look at the comparison of our ice melting data with the reference curve derived by extrapolation of eqn (2.16) in ref. 11 into the metastable region. The solid curve represents a weighed polynomial fit to our data, namely| ϱ(Ticem) = −0.01279T4 + 0.1368T3 − 0.06136T2 − 9.637T + 1000 |
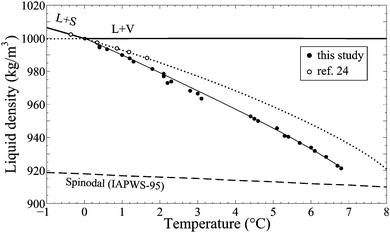 | ||
| Fig. 11 ϱ–T diagram illustrating the deviation of our experimental ice melting data (dots) from the extrapolated reference curve23 (dotted line). Solid line: weighted fit to our data. | ||
 | ||
| Fig. 12 (a) Enlarged detail of the ϱ–T phase diagram of water displaying the cavitation (bubble nucleation) curve ϱcav(T) (thick grey curve) derived from experimental data of this study and previous studies as well as the final ice melting data (this study). The cavitation curve exhibits a maximum at around 40 °C. Besides the thermodynamic spinodal (dashed line, IAPWS-95), the diagram features two kinetic spinodals for δT = 0 and +0.1 nm40 (lower and upper grey dash-dotted curves, respectively). The two TMD lines (IAPWS-95 and Pallares et al.15) have negative slopes. As a guide to the eye the diagram displays two pseudo-isochores (solid, nearly horizontal grey lines) at Th∞ of 143 and 151 °C, respectively. (b) p–T representation of the water phase diagram derived from (a). The pseudo-isochores (solid grey curves) exhibit a pressure minimum, while the cavitation curve pcav(T) (thick grey line) is only gently curved. The two kinetic spinodals for δT = 0 and +0.1 nm40 (lower and upper grey dash-dotted curves, respectively) are illustrated as well. | ||
Data points that we regarded as less reliable (see Section 3.1) were weighted low for curve fitting. The deviations from the reference increase with increasing Ticem, i.e., with decreasing density ϱ(Ticem) and are significantly larger than the uncertainty of the temperature measurements. At the present stage of research, it is hard to tell if these deviations mainly indicate the limits of the extrapolation or if effects associated with the confinement of the ice–liquid system in a microscopic inclusion need to be considered.
One effect that definitely plays a role is elastic deformation of the inclusion walls under large tensile stress, which results in a volume decrease and thus an increase of the water density. To account for the difference between our data and the extrapolated curve we would have to assume elastic volume changes of up to 1.8% which seem too much considering the maximum tensile stress of −130 MPa at Ticem in the lowest density inclusions (according to IAPWS-95). However, Burnley and Davis36 have demonstrated that elastic volume changes of fluid inclusions depend not only on the magnitude of differential stress but also on the size and the shape of the inclusions, the thickness of the inclusion walls and the orientation of the inclusions within an anisotropic crystal.
The effect of surface tension at the ice–liquid interface might also have an impact on ice melting temperatures. Actually, one would expect that the surface tension affects ice melting in a similar way as it affects the liquid–vapour homogenisation temperature. (recall Section 2.4.2. and ref. 17). However, for high density inclusions with Ticem near zero our data approach the results of Henderson and Speedy24 obtained in a macroscopic Berthelot tube. This may indicate that the surface tension at the ice–water interface is sufficiently small (estimates vary from 0.04 mN m−1 by van Oss et al.37 to about 30 mN m−1 by Hardy,38 compared to 75 mN m−1 for water), and the size of the inclusions is sufficiently large to neglect the effect of surface tension. On the other hand, interfaces with the host material may also play a role.
Setting aside, for now, the question of the validity of the melting curve extrapolation, we conclude the discussion of ice melting by pointing out the following observation: the lowest melting points nearly approach the IAPWS-95 spinodal, but nevertheless, the stretched ice melts into a homogeneous stretched liquid. This is yet another indication for the limitation of the IAPWS-95 extrapolation into the deeply stretched region. In the next section, we shall have a closer look at this finding.
4.4. Interpretation of spontaneous vapour bubble nucleation
Inspecting Fig. 12a, one finds that our measurements of the retrograde bubble nucleation temperatures Tvapnr as a function of inclusion density ϱ confirm the general trend observed in previous studies: the loci of the lowest retrograde nucleation temperatures determined for different inclusion densities appear to lay close to a “cavitation curve” Tcav(ϱ) that decreases monotonically with increasing ϱ, until no nucleation is observed for densities larger than 930 kg m−3. In particular, we confirm the data point denoted by Azouzi et al.,31 which was obtained in a particularly thorough investigation of a nice (cylindrical) inclusion with a density of 928 kg m−3. An exception in the general trend is a singular inclusion reported by Zheng et al.,10 which exhibited a record Tvapnr of 40 °C, despite its relatively small nominal density of about 910 kg m−3. When converting the ϱ–T data into p–T, one obtains the frequently cited record cavitation pressure of −140 MPa. (Zheng et al. used the EOS of Haar et al. for this.39 Pressures shown in Fig. 12b have been recalculated using the IAPWS-95 formulation, which gives about −150 MPa.) However, a microphotograph in Zheng's thesis29 shows that the respective inclusion was exceptionally large and very flat. If this inclusion is close to the sample surface, the large area of the thin quartz wall cannot resist high negative pressures at 40 °C and will be elastically curved into the inclusion, resulting in a comparatively large density increase. Note that in comparison to the inclusions analyzed in this study, an extra density increase of about 2% would be necessary to place the record data point into the gap between our retro- and prograde data. In view of this uncertainty, we suggest to use the second lowest temperature obtained by Zheng29 in another inclusion from the same sample for this density, the data point enclosed in parenthesis in Fig. 12a and b.A new finding of our study is the existence of prograde vapour bubble nucleation (cavitation) that occurs upon heating of metastable liquid inclusions above the ice melting temperature. The prograde Tvapnp(ϱ) appears to slightly increase with increasing density, in contrast to the decreasing trend of retrograde Tvapnr(ϱ). This finding suggests to associate the pro- and retrograde values with a single cavitation curve ϱcav(T) which is not monotonic, but rather curved, exhibiting a maximum at around 40 °C. In our study we never reached this maximum, lacking samples with bulk densities in a small interval between 924 and 921 kg m−3; therefore, the gap. The thick grey line in Fig. 12a represents a suggestion for the ϱcav(T) curve. The nucleation data for low density inclusions at high temperatures10 are taken into account in a simplified fashion by requiring the ϱcav(T) to end at the critical point.
The formation of the cavitation curve is illustrated in Fig. 12b, where we translated all ϱ–T values into p–T using the IAPWS-95 formulation. The extrapolation of IAPWS-95 deep into the metastable region is questionable, but still the (pseudo-) isochores are qualitatively correct, exhibiting a pressure minimum close to the TMD line recently determined by Pallares et al.15 Thus, our data are consistent with the following picture: when moving along an pseudo-isochore p(T|ϱ), irrespective of the temperature increase or decrease, cavitation will occur at a temperature T where the pressure p(T|ϱ) approaches a certain critical value pcav, which depends on the temperature. In Fig. 12b the thick grey line is the proposed pcav(T) curve obtained by IAPWS-95 transformation of ϱcav(T) from Fig. 12a. While pseudo-isochores shown in Fig. 12b are curved, exhibiting a minimum at the TMD line, the cavitation curve pcav(T) is a monotonic, gently curved function of temperature. Because of the difference in curvature, an isochore p(T|ϱ) can intersect the cavitation curve pcav(T) twice, or not at all. Unfortunately, it is not clear from our data whether or not pcav(T) exhibits a pressure minimum at low temperatures. If so, the pressure minimum would be at a temperature below the intersection with the TMD line predicted by IAPWS-95.
In Fig. 12a and b we plot one of the existing theoretical proposals for the cavitation curve, namely the “kinetic spinodal” by Kiselev and Ely for comparison.40 The two kinetic spinodals were calculated based on IAPWS-95, but in addition took into account density fluctuations and the effect of the curvature of the liquid–vapour interface. The two grey dash-dotted curves, thus correspond to two values of the curvature parameter Tolman length (δT). Apparently, a kinetic spinodal with a Tolman length between 0 and 0.1 nm would coincide with our proposal at high temperatures, but at temperatures around the maximum of the ϱcav(T) curve the differences become large. This could be due to the failure of the extrapolation of IAPWS-95 deep into the metastable region. Notice that the form of the two kinetic spinodals resembles the form of the re-entrant IAPWS-95 spinodal. In fact, it has been proposed (Caupin,41 Caupin and Stroock42) to exploit the cavitation data to obtain information on the p–T trend of the spinodal. Looking at our cavitation data from this point of view, we confirm our conclusion from ice nucleation data in Section 3.2: the actual spinodal, if re-entrant at all, must have its pressure minimum at a much lower temperature than that predicted by IAPWS-95 and a steeper negative slope at low temperatures.
However, we feel that one should not over-interpret the cavitation data and their theories. Unfortunately, there is still an unanswered question about the origin of the large difference in cavitation pressures obtained in microscopic inclusions and macroscopic Berthelot tubes or by the acoustical technique.42 Thus, one should remain open to a substantial revision of the theories of cavitation in the stretched state of water. Perhaps one should take explicitly into account the temperature dependence of the strength of the hydrogen bond,43 which plays a role in the cohesion of water as well as in adhesion to the host material.
5. Conclusion
We presented a comprehensive fluid inclusion study that combines microthermometric measurements of homogenisation temperatures, both prograde Th and retrograde Th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r, ice nucleation and melting temperatures (Ticenr, Ticem) as well as spontaneous bubble nucleation temperature upon cooling (Tvapnr) and for the first time, prograde bubble nucleation temperature upon heating (Tvapnp). The set of our quartz samples provides a sufficiently broad range of inclusion densities to explore the low-temperature metastable region of liquid water. We compared the results of our study with the predictions of the IAPWS-95 formulation of the equation of state and the major findings are:
r, ice nucleation and melting temperatures (Ticenr, Ticem) as well as spontaneous bubble nucleation temperature upon cooling (Tvapnr) and for the first time, prograde bubble nucleation temperature upon heating (Tvapnp). The set of our quartz samples provides a sufficiently broad range of inclusion densities to explore the low-temperature metastable region of liquid water. We compared the results of our study with the predictions of the IAPWS-95 formulation of the equation of state and the major findings are:
On the one hand, we confirm IAPWS-95 concerning saturation liquid-densities in the supercooled region. Thus, IAPWS-95 seems to be appropriate for inclusion densities larger than 970 kg m−3.
On the other hand, we disproof the low-temperature trend of the liquid-spinodal predicted by IAPWS-95, due to inconsistency with ice nucleation data in the doubly metastable region. This finding is further supported by our ice melting data, demonstrating that ice melts into a homogeneous liquid even for low densities with a melting point close to the predicted spinodal. Such inclusions exhibit prograde vapour bubble nucleation upon heating, in addition to the well-known retrograde bubble nucleation upon cooling. The resulting bubble nucleation (or cavitation) curve is not monotonic in the ϱ–T representation, but exhibits a maximum at around 40 °C. (Is this related with the compressibility minimum?) The cavitation pressure curve pcav(T) obtained therefrom using IAPWS-95, is monotonic and only slightly curved.
Our findings do not determine whether the spinodal is re-entrant or not. However, looking at the ϱ–T data in Fig. 9, which are not affected by the questionable EOS extrapolation, one closing remark seems to be appropriate: liquid water with a density of 921 kg m−3 remains in a homogeneous state during cooling down to a temperature of −30.5 °C, where it is transformed into ice whose density corresponds to zero pressure. Is this finding compatible with any of the proposed scenarios?
List of abbreviations
| L | Liquid phase |
| S | Solid phase |
| V | Vapour phase |
| Qtz | Quartz host |
| TMD | Temperature of maximum density |
| T | Temperature (°C) |
| V | Volume (μm3) |
| p | Pressure (MPa) |
| ϱ | Density (kg m−3) |
| obs | Observed (measured) value |
| h | Liquid–vapour homogenisation |
| n | Nucleation |
| m | Melting |
| ∞ | Hypothetical value for infinitely large volumes |
| p | Phase transition occurring upon heating |
| r | Phase transition occurring upon cooling |
Funding
This work was supported by the Swiss National Science Foundation [grant number 200021-140777/1].Acknowledgements
We acknowledge the use of the HP-GeoMatS Lab of the Geo.X partners GFZ and Universität Potsdam for pure water fluid inclusion synthesis.References
- C. A. Angell, Water: a comprehensive treatise, Plenum, New York, 1982, vol. 7 Search PubMed.
- E. W. Lang and H.-D. Ludemann, Angew. Chem., Int. Ed. Engl., 1982, 21, 315–388 CrossRef.
- R. J. Speedy and C. A. Angell, J. Chem. Phys., 1976, 65, 851 CrossRef CAS.
- G. Pallares, M. El Mekki Azouzi, M. A. Gonzalez, J. L. Aragones, J. L. F. Abascal, C. Valeriani and F. Caupin, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 7936–7941 CrossRef CAS PubMed.
- R. J. Speedy, J. Phys. Chem., 1982, 86, 982–991 CrossRef CAS.
- P. H. Poole, F. Sciortino, U. Essmann and H. E. Stanley, Nature, 1992, 360, 324–328 CrossRef CAS.
- B. Widom, in Phase transitions and criticcal phenomena, ed. C. Domb and M. S. Green, Academic Press, 1972, vol. 7 Search PubMed.
- C. A. Angell, Nat. Mater., 2014, 13, 673–675 CrossRef CAS PubMed.
- F. Caupin and E. Herbert, C. R. Phys., 2006, 7, 1000–1017 CrossRef CAS.
- Q. Zheng, D. J. Durben, G. H. Wolf and C. A. Angell, Science, 1991, 254, 829–832 CAS.
- W. Wagner and A. Pruss, J. Phys. Chem. Ref. Data, 2002, 31, 387–535 CrossRef CAS.
- J. L. Green, D. J. Durben, G. H. Wolf and C. A. Angell, Science, 1990, 249, 649–652 CAS.
- K. I. Shmulovich, L. Mercury, R. Thiéry, C. Ramboz and M. El Mekki, Geochim. Cosmochim. Acta, 2009, 73, 2457–2470 CrossRef CAS.
- A. D. Alvarenga, M. Grimsditch and R. J. Bodnar, J. Chem. Phys., 1993, 98, 8392–8396 CrossRef CAS.
- G. Pallares, M. A. Gonzalez, J. L. F. Abascal, C. Valeriani and F. Caupin, Phys. Chem. Chem. Phys., 2016, 18, 5896–5900 RSC.
- Y. Krüger, P. Stoller, J. Rička and M. Frenz, Eur. J. Mineral., 2007, 19, 693–706 CrossRef.
- D. Marti, Y. Krüger, D. Fleitmann, M. Frenz and J. Rička, Fluid Phase Equilib., 2012, 314, 13–21 CrossRef CAS.
- S. M. Sterner and R. J. Bodnar, Geochim. Cosmochim. Acta, 1984, 48, 2659–2668 CrossRef CAS.
- R. J. Bodnar and S. M. Sterner, in Hydrothermal experimental techniques, ed. G. C. Ulmer and H. L. Barnes, J. Wiley & Sons, 1987, pp. 423–457 Search PubMed.
- G. Morrison, J. Phys. Chem., 1981, 85, 759–761 CrossRef CAS.
- R. H. Goldstein and T. J. Reynolds, in Society for Sedimentary Geology, 1994, p. 94.
- R. Feistel and W. Wagner, J. Phys. Chem. Ref. Data, 2006, 35, 1021–1047 CrossRef CAS.
- W. Wagner, A. Saul and A. Pruss, J. Phys. Chem. Ref. Data, 1994, 23, 515 CrossRef CAS.
- S. J. Henderson and R. J. Speedy, J. Phys. Chem., 1987, 91, 3069–3072 CrossRef CAS.
- A. Fall, J. D. Rimstidt and R. J. Bodnar, Am. Mineral., 2009, 94, 1569–1579 CrossRef CAS.
- P. Stoller, Y. Krüger, J. Rička and M. Frenz, Earth Planet. Sci. Lett., 2007, 253, 359–368 CrossRef CAS.
- P. D. Desai, in HTMIAC Special Report 40, 1990.
- F. Spadin, D. Marti, R. Hidalgo-Staub, J. Rička, D. Fleitmann and M. Frenz, Clim. Past, 2015, 11, 905–913 CrossRef.
- Q. Zheng, PhD thesis, Department of Chemistry, Purdue University, 1991.
- H. Kanno and K. Miyata, Chem. Phys. Lett., 2006, 422, 507–512 CrossRef CAS.
- M. E. M. Azouzi, C. Ramboz, J.-F. Lenain and F. Caupin, Nat. Phys., 2013, 9, 38–41 CrossRef CAS.
- S. J. Henderson and R. J. Speedy, J. Phys. Chem., 1987, 17, 3062–3068 CrossRef.
- E. Roedder, Science, 1967, 155, 1413–1417 CAS.
- M. S. Barrow, P. R. Williams, H.-H. Chan, J. C. Dore and M.-C. Bellissent-Funel, Phys. Chem. Chem. Phys., 2012, 14, 13255–13261 RSC.
- D. Marti, Y. Krüger and M. Frenz, in ECROFI, Granada (Spain), 2009, pp. 4–5.
- P. C. Burnley and M. K. Davis, Can. Mineral., 2004, 42, 1369–1382 CrossRef CAS.
- C. J. Van Oss, R. F. Giese, R. Wentzek, J. Norris and E. M. Chuvilin, J. Adhes. Sci. Technol., 1992, 6, 503–516 CrossRef CAS.
- S. C. Hardy, Philos. Mag., 1977, 35, 471–484 CrossRef CAS.
- L. Haar, J. Gallagher and G. S. Kell, National Bureau of Standard-National Research Council Steam Tables, McGraw-Hill, NewYork, 1985 Search PubMed.
- S. B. Kiselev and J. F. Ely, Phys. A, 2001, 299, 357–370 CrossRef CAS.
- F. Caupin, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2005, 71, 1–5 CrossRef PubMed.
- F. Caupin and A. Stroock, Adv. Chem. Physics, Liq., 2013, 152, 51–80 CAS.
- R. C. Dougherty, J. Chem. Phys., 1998, 109, 7372–7378 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cp04250c |
| ‡ Current address: Institute of Environmental Physics, University of Heidelberg, Im Neuenheimer Feld 229, 69102 Heidelberg, Germany. |
| § Current address: Institute of Earth and Environmental Sciences, University of Potsdam, Karl-Liebknecht-Str. 24-25, 14476 Potsdam-Golm, Germany. |
| This journal is © the Owner Societies 2016 |






