Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electrostatically enhanced F⋯F interactions through hydrogen bonding, halogen bonding and metal coordination: an ab initio study†
Antonio
Bauzá
and
Antonio
Frontera
 *
*
Department of Chemistry Universitat de les Illes Balears, Crta. de Valldemossa km 7.5, 07122 Palma (Baleares), Spain. E-mail: toni.frontera@uib.es; Fax: +34 971 173426
First published on 4th July 2016
Abstract
In this manuscript the ability of hydrogen and halogen bonding interactions, as well as metal coordination to enhance F⋯F interactions involving fluorine substituted aromatic rings has been studied at the RI-MP2/def2-TZVPD level of theory. We have used 4-fluoropyridine, 4-fluorobenzonitrile, 3-(4-fluorophenyl)propiolonitrile and their respective meta derivatives as aromatic compounds. In addition, we have used HF and IF as hydrogen and halogen bond donors, respectively, and Ag(I) as the coordination metal. Furthermore, we have also used HF as an electron rich fluorine donor entity, thus establishing F⋯F interactions with the above mentioned aromatic systems. Moreover, a CSD (Cambridge Structural Database) search has been carried out and some interesting examples have been found, highlighting the impact of F⋯F interactions involving aromatic fluorine atoms in solid state chemistry. Finally, cooperativity effects between F⋯F interactions and both hydrogen and halogen bonding interactions have been analyzed and compared. We have also used Bader's theory of “atoms in molecules” to further describe the cooperative effects.
Introduction
Supramolecular chemists rely on in depth comprehension of noncovalent forces, which are pillars of modern chemistry. A proper understanding and intelligent utilization of them is essential to achieve progress in fields such as supramolecular chemistry,1 molecular recognition2 and materials science.3 One of the best known supramolecular forces that is ubiquitous in many chemical and biological environments is hydrogen bonding.4 A similar noncovalent force that shares strength and directionality features with hydrogen bonding is the halogen bonding interaction.5 A halogen bond (R–X⋯Y–Z, X = halogen, Z = electron rich atom, Z = any atom) occurs when “there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom in a molecular entity and a nucleophilic region in another, or the same, molecular entity”.6 Related to this, the ability of halogen atoms to interact with Lewis bases has been known for some time.7 For instance, the ability of iodo- and bromoperfluorocarbons to form noncovalent interactions with neutral and charged electron donors was reported by Resnati and co-workers, who remarked on the ability of haloperfluoroalkanes to participate as halogen-bond donor moieties in crystal engineering, unveiling the promising potential of halogen bonding interactions in supramolecular chemistry.8–16 Consequently, a series of studies using the Cambridge Structural Database (CSD) have been carried out in order to shed light on the impact of this interaction in crystal structures.17 The interest among the scientific community has expanded exponentially due to the recognition of its importance in biological systems and the design of new materials; leading to a plethora of theoretical and experimental studies devoted to this topic.18–20Related to this, fluorine–fluorine interactions have been considered as contentious noncovalent forces in the past, conversely to the heavier halogens, which are well-known to establish X⋯X interactions, showing a dual behaviour (both electron and donor acceptors).21
Owing to its low polarizability,22 which provokes the attractive dispersion contribution to be rather low, F⋯F interactions were skeptically considered as a stabilization source in solid state chemistry. However, there are a significant number of studies in the literature of C–F⋯F–C interactions that have been characterized both experimentally and theoretically as important contributors in energetic stabilization.23–27 Related to this, the exhaustive research on F⋯F interactions carried out by the group of Varadwaj and coworkers is also remarkable.28 They studied the possibility of establishing attractive intermolecular interactions between aromatic fluorine atoms in perfluorobenzene and several electron donor moieties. The energetic stability of the complexes studied was slightly favourable, and the authors concluded that, analogous to C–H⋯H–C interactions,29 in addition to the dispersion contribution the main stabilization force is attributed to the polarization term that is proportional to the atomic polarizabilities. Conversely, the electrostatic component is usually not attractive in regular F⋯F interactions due to the absence of a σ-hole in the fluorine atom. Herein, we study the possibility to electrostatically strengthen F⋯F interaction by using cooperative noncovalent forces.
In this study, our purpose is to investigate the ability of hydrogen and halogen bonding interactions, as well as metal coordination, to enhance F⋯F interactions involving aromatic fluorine atoms. In order to achieve that goal, we have used a series of fluoropyridine derivatives, attaching the fluorine atom in meta and para positions of the ring (Fig. 1). In addition, we have progressively increased the distance between the nitrogen and fluorine atoms in order to account for long range enhancing effects. We have used HF and IF as hydrogen and halogen bond donors, respectively, and Ag(I) as a transition metal coordinating atom (see Fig. 2 and 3). Furthermore, we have also used HF as an electron donor entity, thus establishing F⋯F interactions with the above mentioned aromatic moieties. Finally, cooperativity effects between the F⋯F interactions and either hydrogen or halogen bonding interactions have been analysed and compared. Moreover, a CSD (Cambridge Structural Database) search has been carried out and some interesting examples have been found, highlighting the impact of F⋯F interactions involving aromatic fluorine atoms in solid state chemistry. Finally, we have also used Bader's theory of “atoms in molecules” to further describe and rationalize the cooperative effects observed in the assemblies.
Theoretical methods
The energies of all complexes included in this study were computed at the RI-MP2/def2-TZVPD level of theory by means of the program TURBOMOLE version 7.0.30 The optimization of the molecular geometries has been performed imposing the Cs and C2v symmetry point groups unless otherwise noted. The interaction energies were calculated with correction for the basis set superposition error (BSSE) by using the Boys–Bernardi counterpoise technique.31 The MEP (Molecular Electrostatic Potential) calculations have been performed at the MP2/def2-TZVP level by means of the Gaussian09 calculation package.32 Frequency calculations have been performed at the RI-MP2/def2TZVPD level of theory. The Bader's “Atoms in molecules” theory has been used to study the interactions discussed herein by means of the AIMall calculation package.33 The calculations for wavefunction analyses were carried out at the MP2/def2-TZVP level of theory. In the ternary complexes where F⋯F and other interactions coexist, we have studied cooperativity effects by computing the cooperativity energy Ecoop using eqn (1).| Ecoop = ΔEBSSE(ABC) − ΔEBSSE(AB) − ΔEBSSE(AC) − ΔEBSSE(BC) | (1) |
Finally, we have explored the Cambridge Structural Database36 in order to find evidence of F⋯F interactions in systems involving aromatic rings. For the CSD search we have restricted the C–F⋯F angle to the range between 170 and 180 degrees. Moreover, only those structures with “no ions”, “no errors”, “no powder” and “no disorder” were considered.
Results and discussion
MEPS study
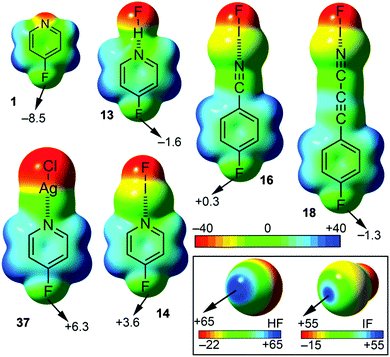
As a preliminary study, we have computed the molecular electrostatic potential (MEP) of compounds 1 to 6, 13 to 24 and 37 to 42 (see Table 1) to investigate the existence of a σ-hole in the F atom. Moreover, the MEP surface plots of some representative examples are shown in Fig. 4 and the MEP values are gathered in Table 1. From the inspection of the results several points arise. First, for compounds 1 to 6 a negative potential value ranging from −10 to −5 kcal mol−1 is found on the fluorine atom along the extension of the C–F bond, thus expecting non attractive interactions with electron rich entities. Second, for complexes 13 to 24 a different behaviour is observed depending on which interaction establishes the nitrogen atom of the aromatic moiety. For hydrogen bonding complexes (13, 15, 17, 19, 21 and 23) a negative potential value is also obtained for the fluorine atom along the C–F bond, ranging from −4 to −2 kcal mol−1. However, the MEP values are less negative than those obtained for compounds 1 to 6, thus expecting more attractive (or less repulsive) interaction with electron rich molecules. On the other hand, for halogen bonding complexes (14, 16, 18, 20, 22 and 24) two different tendencies are observed. First, in the para substituted complexes (14, 16 and 18) positive MEP values at the outermost part of the fluorine atom are obtained (compounds 14 and 16), ranging from 0 to +4 kcal mol−1. Thus, for these compounds a σ-hole on the F atom is generated and attractive interaction with electron donor moieties is expected. For complex 18 the σ-hole on the fluorine atom was not formed due to the presence of the CC triple bond that attenuates the effect of the halogen bond. Second, in the meta substituted complexes (20, 22 and 24) only 20 showed a positive MEP value over the fluorine atom. Finally, for Ag(I) compounds (37 to 42) a positive potential region located at the outermost region of the fluorine atom appeared, thus being electrostatically favoured the interaction with an electron rich entity. It is also worth noting that both IF and HF present a positive potential region located at the outermost region of the iodine and hydrogen atoms, respectively, thus highlighting their strong electron acceptor characteristics. Therefore the H-bonding and halogen bonding complexes are expected to be very favourable.| Compound | V s max | V s fluorinea |
|---|---|---|
| a Value measured at the extension of the C–F bond. | ||
| 1 | +23 | −8.52 |
| 2 | +28 | −5.77 |
| 3 | +28 | −5.65 |
| 4 | +25 | −9.41 |
| 5 | +28 | −6.27 |
| 6 | +27 | −6.26 |
| 13 | +31 | −1.57 |
| 14 | +38 | +3.57 |
| 15 | +33 | −1.63 |
| 16 | +38 | +0.32 |
| 17 | +30 | −2.63 |
| 18 | +38 | −1.25 |
| 19 | +31 | −3.01 |
| 20 | +38 | +1.88 |
| 21 | +31 | −2.70 |
| 22 | +33 | −0.50 |
| 23 | +32 | −3.32 |
| 24 | +32 | −1.87 |
| 37 | +41 | +6.27 |
| 38 | +38 | +3.76 |
| 39 | +36 | +1.45 |
| 40 | +41 | +4.51 |
| 41 | +38 | +2.95 |
| 42 | +35 | +0.75 |
Finally, for all three interactions (hydrogen bonding, halogen bonding and metal coordination), the MEP values become more negative on going from pyridine complexes (13, 14, 19, 20, 37 and 40) to their respective propiolonitrile analogues (17, 18, 23, 24, 39 and 42), due to the higher basicity of the former. In addition, the values obtained for the para substituted systems are more positive than the ones obtained for the meta substituted complexes, stressing the importance of resonance effects. Finally, a general trend with respect to the ability to generate a σ-hole on the fluorine atom can be establish, that is, hydrogen bonding < halogen bonding < Ag(I) coordination.
It is worth mentioning the relevance of these findings, since the formation of an N⋯I halogen bond in p-fluorocyanobenzene is able to provoke the formation of a σ-hole in the fluorine atom, thus inverting its electrostatic potential.
Energetic study
| Complex | ΔEBSSE | R | 102·ρ | N imag |
|---|---|---|---|---|
| 7 (1 + FH) | 0.19 | 3.028 | 0.27 | 0 |
| 8 (2 + FH) | 0.08 | 2.996 | 0.29 | 0 |
| 9 (3 + FH) | 0.08 | 3.001 | 0.29 | 0 |
| 10 (4 + FH) | 0.21 | 3.127 | 0.21 | 0 |
| 11 (5 + FH) | 0.09 | 2.984 | 0.30 | 0 |
| 12 (6 + FH) | 0.09 | 2.995 | 0.30 | 0 |
| 13 (1 + HF) | −11.80 | 1.659 | 5.50 | 0 |
| 14 (1 + IF) | −19.57 | 2.364 | 6.04 | 0 |
| 15 (2 + HF) | −8.33 | 1.805 | 3.31 | 0 |
| 16 (2 + IF) | −11.42 | 2.482 | 4.07 | 0 |
| 17 (3 + HF) | −7.73 | 1.825 | 3.12 | 0 |
| 18 (3 + IF) | −10.85 | 2.482 | 4.04 | 0 |
| 19 (4 + HF) | −11.46 | 1.667 | 5.39 | 0 |
| 20 (4 + IF) | −19.15 | 2.366 | 6.01 | 0 |
| 21 (5 + HF) | −8.15 | 1.810 | 3.26 | 0 |
| 22 (5 + IF) | −11.18 | 2.487 | 4.02 | 0 |
| 23 (6 + HF) | −7.62 | 1.828 | 3.09 | 0 |
| 24 (6 + IF) | −10.70 | 2.485 | 4.01 | 0 |
As it can be observed in Table 2, there is a progressive weakening in the interaction energy values obtained when the distance between the nitrogen and the fluorine atoms is enlarged (see for instance complexes 14, 16 and 18), in agreement with the MEP analysis discussed above. Finally, the para and meta substituted compounds exhibit similar binding energy values, thus indicating that the resonance effect is not crucial for the global stabilization of the complexes studied herein.
| Cmpd | ΔEBSSE | E coop | R e | R σB | ΔRe | ΔRσB |
|---|---|---|---|---|---|---|
| R e is the F⋯F equilibrium distance and RσB is the distance of N⋯X interactions. | ||||||
| 25 | −11.91 | −1.10 | 2.957 | 1.654 | 0.071 | 0.005 |
| 26 | −19.91 | −1.66 | 2.913 | 2.359 | 0.115 | 0.005 |
| 27 | −8.43 | −0.29 | 2.962 | 1.800 | 0.034 | 0.005 |
| 28 | −11.59 | −0.57 | 2.947 | 2.476 | 0.049 | 0.006 |
| 29 | −7.77 | −0.24 | 2.978 | 1.822 | 0.023 | 0.003 |
| 30 | −10.95 | −0.50 | 2.970 | 2.479 | 0.031 | 0.003 |
| 31 | −11.53 | −1.00 | 2.972 | 1.661 | 0.155 | 0.006 |
| 32 | −19.47 | −1.59 | 2.916 | 2.360 | 0.211 | 0.006 |
| 33 | −8.25 | −0.29 | 2.957 | 1.806 | 0.027 | 0.004 |
| 34 | −11.35 | −0.56 | 2.941 | 2.481 | 0.043 | 0.006 |
| 35 | −7.66 | −0.24 | 2.987 | 1.826 | 0.008 | 0.002 |
| 36 | −10.78 | −0.48 | 2.960 | 2.481 | 0.035 | 0.004 |
As suggested by one referee, we have further analysed the reinforcement of the F⋯F interactions in the ternary complexes by comparing the binding energies of the bimolecular complexes to those of the termolecular complexes (denoted as ΔΔE in Table 4), computing the energy of the termolecular considering that the H/X-bonded complex has been previously formed (denoted as ΔE(AB⋯C) in Table 4). This methodology has been successfully used in the analysis of cooperativity effects in F⋯O and I⋯N halogen bonding complexes.37 The F⋯F interactions (ΔE(A⋯B) values) in the bimolecular complexes are in all cases positive ranging from 0.08 kcal mol−1 to 0.21 kcal mol−1. In contrast the F⋯F interactions (ΔE(AB⋯C) values) in the termolecular complexes are in all cases negative ranging from −0.36 kcal mol−1 to −0.05 kcal mol−1. Therefore the F⋯F interactions enhance in the termolecular complexes changing from unfavourable (ΔE(A⋯B) values) in the bimolecular complexes to favourable in the termolecular ones (ΔE(AB⋯C) values). For example, the F⋯F halogen–halogen interaction energy is 0.19 kcal mol−1 in the bimolecular complex 7 (1⋯FH) and becomes −0.36 kcal mol−1 in the termolecular complex 27 (IF⋯1⋯FH) with an increase of 0.55 kcal mol−1 (ΔΔE = −0.55 kcal mol−1). In general, the F⋯F interaction energy is more enhanced in those termolecular complexes where the aromatic moiety participates in F–I⋯N halogen bonding interactions than in those involving H-bonding F–H⋯N interactions, in agreement with the cooperativity energies listed in Table 3.
| Complex | ΔE(A⋯B) | ΔE(AB⋯C) | ΔΔE |
|---|---|---|---|
| ΔΔE = ΔE(AB⋯C) – ΔE(A⋯B); ΔE(AB⋯C) = EABC − EAB − EC; ΔE(A⋯B) = EAB − EA − EB. | |||
| 25 (HF⋯1⋯FH) | 0.19 (1⋯FH) | −0.11 (13⋯FH) | −0.30 |
| 26 (IF⋯1⋯FH) | 0.19 (1⋯FH) | −0.36 (14⋯FH) | −0.55 |
| 27 (HF⋯2⋯FH) | 0.08 (2⋯FH) | −0.10 (15⋯FH) | −0.18 |
| 28 (IF⋯2⋯FH) | 0.08 (2⋯FH) | −0.18 (16⋯FH) | −0.26 |
| 29 (HF⋯3⋯FH) | 0.08 (3⋯FH) | −0.05 (17⋯FH) | −0.13 |
| 30 (IF⋯3⋯FH) | 0.08 (3⋯FH) | −0.11 (18⋯FH) | −0.19 |
| 31 (HF⋯4⋯FH) | 0.21 (4⋯FH) | −0.08 (19⋯FH) | −0.29 |
| 32 (IF⋯4⋯FH) | 0.21 (4⋯FH) | −0.34 (20⋯FH) | −0.55 |
| 33 (HF⋯5⋯FH) | 0.09 (5⋯FH) | −0.10 (21⋯FH) | −0.19 |
| 34 (IF⋯5⋯FH) | 0.09 (5⋯FH) | −0.19 (22⋯FH) | −0.28 |
| 35 (HF⋯6⋯FH) | 0.09 (6⋯FH) | −0.05 (23⋯FH) | −0.14 |
| 36 (IF⋯6⋯FH) | 0.09 (6⋯FH) | −0.15 (24⋯FH) | −0.24 |
AIM analysis
We have performed AIM analysis of all compounds studied in this work. It is well established that the charge density ρ at the critical points (CPs) that emerge upon complexation gives helpful information regarding the strength of the noncovalent interactions involved in the complexes. Indeed, they have been successfully used as a measure of the bond order in a variety of noncovalent interactions including anion–π, hydrogen bonding and halogen-bonding interactions. Therefore, the variation in the ρ value at CPs in ternary systems with respect to the isolated F⋯F and σB complexes can be used to analyse the mutual influence of both interactions. In Fig. 5, the F⋯F–σB complex 32 presents a ρ value of 6.12 × 10−2 a.u. at the bond CP that characterizes the halogen bonding interaction, which is greater than the value computed at the bond CP of complex 14 (6.04 × 10−2 a.u.). This result confirms that the halogen bond in 32 is reinforced with respect to complex 14. The same behaviour is observed for the values of ρ at the bond CP that characterizes the F⋯F interactions. Variation of the value from 0.27 × 10−2 a.u. for complex 7 to 0.36 × 10−2 a.u. for complex 32 also indicates a reinforcement of the F⋯F interactions. For the rest of the complexes of the study, similar conclusions can be extracted (see Table 5). In all F⋯F–σB complexes, the Δρ values are positive, thus indicating a reinforcement of both interactions, in agreement with the energetic and geometric results (vide supra). The variation of the ρ values at the bond CPs [ΔρBCP(F⋯F) and ΔρBCP(σB) values] can be used as a degree of strengthening of the interaction. For instance, the variation of the ρ value at the bond CP that characterized the F⋯F interactions in complex 32 is greater than in complex 31, as expected, considering the strong electron acceptor characteristics of the IF molecule.| Complex | ρ BCP(F⋯F) | ρ BCP(σB) | ΔρBCP(F⋯F) | ΔρBCP(σB) | N imag |
|---|---|---|---|---|---|
| 25 | 0.32 | 5.58 | 0.05 | 0.08 | 0 |
| 26 | 0.36 | 6.12 | 0.09 | 0.08 | 1 |
| 27 | 0.32 | 3.34 | 0.03 | 0.03 | 0 |
| 28 | 0.33 | 4.12 | 0.04 | 0.05 | 2 |
| 29 | 0.31 | 3.14 | 0.02 | 0.02 | 0 |
| 30 | 0.31 | 4.07 | 0.02 | 0.03 | 2 |
| 31 | 0.31 | 5.47 | 0.10 | 0.08 | 0 |
| 32 | 0.37 | 6.09 | 0.16 | 0.08 | 0 |
| 33 | 0.33 | 3.30 | 0.03 | 0.04 | 0 |
| 34 | 0.34 | 4.08 | 0.04 | 0.06 | 1 |
| 35 | 0.30 | 3.11 | 0.00 | 0.02 | 0 |
| 36 | 0.32 | 4.05 | 0.02 | 0.04 | 1 |
Effect of the metal coordination
It is well known that aromatic heterocyclic compounds are widely used as ligands in coordination chemistry.38 Therefore, we have analysed the influence of the N–metal coordination on the binding energy values involving the compounds used in this study (see Fig. 3). We have used AgCl as the metal coordination moiety. The energetic results are gathered in Table 6. As it can be observed, the binding energy values are favourable, ranging between −0.5 and −0.1 kcal mol−1. When compared to the related uncoordinated complexes (7 to 12, see ΔΔE values in Table 5) it can be observed a clear reinforcement of the F⋯F interaction, which ranges from 0.3 to 1.1 kcal mol−1.| Complex | ΔEBSSE | ΔΔEb | R | 102·ρ |
|---|---|---|---|---|
| a The charge density values at the bond CP gathered (102·ρ) have been calculated at the MP2/def2-TZVP level of theory. b ΔΔE, see footnote of Table 4. | ||||
| 43 | −0.48 | −0.67 | 2.890 | 0.38 |
| 44 | −0.33 | −0.41 | 2.924 | 0.35 |
| 45 | −0.22 | −0.30 | 2.952 | 0.33 |
| 46 | −0.46 | −0.67 | 2.890 | 0.38 |
| 47 | −0.27 | −0.36 | 2.802 | 0.21 |
| 48 | −0.13 | −0.22 | 2.802 | 0.21 |
These results are in agreement with the MEP analysis shown above, which anticipated a stronger binding for the Ag(I)–complexes. In addition, the F⋯F distances obtained are shorter than the ones obtained for the uncoordinated complexes, in agreement with the energetic and geometric results. Moreover, the values of the density at the bond CP are larger than the ones obtained for their uncoordinated analogues. Finally, it is worth mentioning that the interaction energies of all F⋯F complexes are energetically favourable, including complex 48 (HF⋯FC6H5–CC–CN⋯AgCl) where the Ag metal center is located distant from the F atom. Thus this is an interesting long range synergetic effect of the metal coordination on the F⋯F interactions.
CSD examples
We have examined the Cambridge Structural Database (CSD) in order to find experimental evidence that supports the calculations discussed above. We have found 497 hits exhibiting F⋯F contacts at distances that are less than the sum of their van der Waals radii. Three selected examples are shown in Fig. 6 including the CSD codes. First, in IPUSOC39 F⋯F interactions are established between an aromatic fluorine atom of the organic ligand and a pentafluorobenzonitrile molecule. Although the crystal packing is governed by an intricate combination of noncovalent interactions, F⋯F contacts are numerous, thus highlighting their importance in the solid state architecture. Second, in JUBKIA40 F⋯F interactions are established between two aromatic moieties (see Fig. 6) influencing the crystal packing. Finally, in DOMJOG41 the F⋯F interactions established between a CF3 group and an aromatic fluorine atom create a linear disposition where the discrete units of the crystal interact with each other. These examples highlight the importance of F⋯F in solid state chemistry, especially, in the chemistry of benzene derivatives.Conclusions
The results reported in this manuscript stress the importance of the mutual effects between noncovalent forces involving F⋯F interactions and hydrogen bonding, halogen bonding and metal coordination, which can lead to an electrostatic enhancement of F⋯F interactions and chiefly, to remarkable cooperativity effects. These effects are even more noticeable in systems where the nitrogen atom is forming part of the π-system of the ring. We have estimated the cooperativity effects energetically (Ecoop values), obtaining results that are in agreement with the geometric features of the complexes and the AIM analysis. Finally, in order to find experimental evidence that supports the calculations discussed above we have performed a search in the CSD (Cambridge Structural Database), and we have found 497 hits that exhibit F⋯F contacts, highlighting the impact of this interaction in solid state chemistry, particularly in fluorobenzene derivatives.Acknowledgements
We thank the MINECO of Spain (projects CTQ2014-57393-C2-1-P and CONSOLIDER INGENIO 2010 CSD2010-00065, FEDER funds). We thank the CTI (UIB) for computational facilities.References
- H. J. Schneider, Angew. Chem., Int. Ed., 2009, 48, 3924 CrossRef CAS PubMed.
- C. A. Hunter and J. K. M. Sanders, J. Am. Chem. Soc., 1990, 112, 5525 CrossRef CAS.
- W. J. Vickaryous, R. Herges and D. W. Jonhson, Angew. Chem., Int. Ed., 2004, 43, 5831 CrossRef CAS PubMed.
- S. J. Grabowski, Chem. Rev., 2011, 111, 2597 CrossRef CAS PubMed.
- P. Metrangolo and G. Resnati, Chem. – Eur. J., 2001, 7, 2511 CrossRef CAS.
- G. R. Desiraju, P. S. Ho, L. Kloo, A. C. Legon, R. Marquardt, P. Metrangolo, P. Politzer, G. Resnati and K. Rissanen, Pure Appl. Chem., 2013, 85, 1711 CrossRef CAS.
- P. Metrangolo and G. Resnati, Cryst. Growth Des., 2012, 12, 5835 CAS.
- P. Metrangolo, H. Neukirch, T. Pilati and G. Resnati, Acc. Chem. Res., 2005, 38, 386 CrossRef CAS PubMed.
- P. Metrangolo, G. Resnati, T. Pilati and S. Biella, Halogen Bonding: Fundamentals and Applications, Springer, Berlin, 2008 Search PubMed.
- P. Metrangolo and G. Resnati, Chem. – Eur. J., 2001, 7, 2511 CrossRef CAS.
- P. Metrangolo, F. Meyer, T. Pilati, G. Resnati and G. Terraneo, Angew. Chem., Int. Ed., 2008, 47, 6114 CrossRef CAS PubMed.
- R. Bertani, P. Metrangolo, A. Moiana, E. Perez, T. Pilati, G. Resnati, I. Rico-Lattes and A. Sassi, Adv. Mater., 2002, 14, 1197 CrossRef CAS.
- H. L. Nguyen, P. N. Horton, M. B. Hursthouse, A. C. Legon and D. W. Bruce, J. Am. Chem. Soc., 2003, 126, 16 CrossRef PubMed.
- C. Praesang, H. L. Nguyen, P. N. Horton, A. C. Whitwood and D. W. Bruce, Chem. Commun., 2008, 6164 RSC.
- C. Präsang, A. C. Whitwood and D. W. Bruce, Cryst. Growth Des., 2009, 9, 5319 Search PubMed.
- L. C. Roper, C. Prasang, V. N. Kozhevnikov, A. C. Whitwood, P. B. Karadakov and D. W. Bruce, Cryst. Growth Des., 2010, 10, 3710 CAS.
- P. Murrayrust and W. D. S. Motherwell, J. Am. Chem. Soc., 1979, 101, 4374 CrossRef CAS.
- P. Metrangolo, H. Neukirch, T. Pilati and G. Resnati, Acc. Chem. Res., 2005, 38, 386 CrossRef CAS PubMed.
- P. Politzer and J. S. Murray, ChemPhysChem, 2013, 14, 278 CrossRef CAS PubMed.
- P. Politzer, J. S. Murray and T. Clark, Phys. Chem. Chem. Phys., 2013, 15, 11178 RSC.
- M. Capdevila-Cortada and J. J. Novoa, CrystEngComm, 2015, 17, 3354 RSC.
- K. Reichenbächer, H. I. Süss and J. Hulliger, Chem. Soc. Rev., 2005, 34, 22 RSC.
- I. Alkorta and J. Elguero, Struct. Chem., 2004, 15, 117 CrossRef CAS.
- R. J. Baker, P. E. Colavita, D. M. Murphy, J. A. Platts and J. D. Wallis, J. Phys. Chem. A, 2012, 116, 1435 CrossRef CAS PubMed.
- S. R. Halper and S. M. Cohen, Inorg. Chem., 2005, 44, 4139 CrossRef CAS PubMed.
- M. Barceló-Oliver, C. Estarellas, A. García-Raso, A. Terrón, A. Frontera, D. Quinonero, I. Mata, E. Molins and P. M. Deyà, CrystEngComm, 2010, 12, 3758 RSC.
- V. A. Karnoukhova, I. V. Fedyanin and K. A. Lyssenko, Struct. Chem., 2016, 27, 17 CrossRef CAS; O. Brea, O. Mó, M. Yañez, I. Alkorta and J. Elguero, Chem. – Eur. J., 2015, 21, 1 CrossRef.
- A. Varadwai, P. R. Varadwaj and B.-Y. Jin, RSC Adv., 2016, 6, 19098 RSC.
- J. Echeverría, G. Alullón, D. Danovich, S. Shaik and S. Alvarez, Nat. Chem., 2011, 3, 323 CrossRef PubMed.
- R. Ahlrichs, M. Bär, M. Hacer, H. Horn and C. Kömel, Chem. Phys. Lett., 1989, 162, 165 CrossRef CAS.
- S. B. Boys and F. Bernardi, Mol. Phys., 1970, 19, 553–566 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision B.01, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- T. A. Keith, AIMAll (Version 13.05.06), TK Gristmill Software, Overland Park KS, USA, 2013 Search PubMed.
- I. Alkorta, F. Blanco, P. M. Deyà, J. Elguero, C. Estarellas, A. Frontera and D. Quiñonero, Theor. Chem. Acc., 2010, 126, 1 CrossRef CAS.
- A. Bauzá, D. Quiñonero, P. M. Deyà and A. Frontera, Theor. Chem. Acc., 2012, 131, 1219 CrossRef.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., 2016, B72, 171 Search PubMed.
- (a) W. Li, Y. Zeng, X. Zhang, S. Zheng and L. Meng, Phys. Chem. Chem. Phys., 2014, 16, 19282–19289 RSC; (b) N. Han, Y. Zeng, C. Sun, X. Li, Z. Sun and L. Meng, J. Phys. Chem. A, 2014, 118, 7058–7065 CrossRef CAS PubMed.
- A. Frontera, Coord. Chem. Rev., 2013, 257, 1716 CrossRef CAS.
- R. R. Fernandes, J. Lasri, M. F. C. G. da Silva, A. M. F. Palavra, J. A. L. da Silva, J. J. R. F. da Silva and A. J. L. Pombeiro, Adv. Syn. Cat., 2011, 353, 1153 CrossRef CAS.
- D. Britton, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1992, 48, 2040 CrossRef.
- J. R. Wolstenhulme, A. Cavell, M. Gredičak, R. W. Driver and M. D. Smith, Chem. Commun., 2014, 50, 13585 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Cartesian coordinates of the optimized complexes. See DOI: 10.1039/c6cp03862j |
| This journal is © the Owner Societies 2016 |