Open Access Article
Open Access ArticlePhotoionization access to cyclodextrin-encapsulated resveratrol phenoxy radicals and their repair by ascorbate across the phase boundary
Christoph
Kerzig
and
Martin
Goez
*
Martin-Luther-Universität Halle-Wittenberg, Institut für Chemie, Kurt-Mothes-Str. 2, D-06120 Halle (Saale), Germany. E-mail: martin.goez@chemie.uni-halle.de
First published on 24th June 2016
Abstract
Repair reactions of phenoxy radicals by co-antioxidants are key parts of radical scavenging cascades in nature. Yet, kinetic and mechanistic studies of such repairs are scarce, particularly at biologically relevant interfaces. For the popular red-wine polyphenol resveratrol, we present the first example of repairing a cyclodextrin-complexed phenoxy radical by a water soluble co-antioxidant (ascorbate), a reaction of practical importance given the fact that both antioxidants and cyclodextrins are large-scale food additives. To prepare the phenoxy radical from its parent compound inside the cavities of native or hydroxypropyl-substituted α- and β-cyclodextrins, we employed laser photoionization with UV-A (355 nm), which does not rely on additional reagents, and therefore leaves the repair completely undisturbed. A global fit of the intensity dependence pinpoints the cyclodextrin influences on the biphotonic resveratrol ionization as a shift of the ground-state absorption spectrum and a longer life of the first excited state due to the suppression of the geometrical isomerization by the rigid containers, whereas the actual electron ejection from an upper excited state is almost medium-independent. The exchange of the phenoxy radical between the cyclodextrin interior and the aqueous bulk is immeasurably slow on the timescale of its repair by the ascorbate monoanion. Kinetic H/D isotope effects and activation entropies identify the repair at the cyclodextrin–water interface as a concerted proton–electron transfer with no mechanistic difference to homogeneous aqueous solution. The activation enthalpies reveal a steric repulsion between ascorbate and cyclodextrin that indicates a deeper embedding of the less hydrophilic phenoxy radical in the macrocycle compared to the parent compound, with the observed structure–rate relationships explainable on the basis of the cavity diameter and depth.
1 Introduction
Resveratrol (ResOH; for the structure, see Fig. 1b) is a red-wine polyphenol whose antioxidant properties are thought to be responsible for an astonishing variety of health benefits,1 kindling continuously growing interest in its uses both for medical treatment2 and as a dietary supplement.3 To overcome the poor water solubility of this lipophilic “wonder molecule”, which inhibits its oral uptake, cyclodextrins are receiving increasing attention as its carriers.4–12 An alternative way of increasing the bioavailability of ResOH is the addition of co-antioxidants,13 which themselves might not be as effective as ResOH in scavenging free radicals but can reconvert the resulting phenoxy radical ResO˙ to ResOH, the most important hydrophilic co-antioxidant for this purpose being ascorbate HAsc− (vitamin C). Elucidating the kinetic and mechanistic aspects of these so-called repair reactions is essential for understanding radical-scavenging cascades as they occur in nature.14Although several reports have addressed radical scavenging by cyclodextrin-complexed ResOH,7,9,15–17 nothing whatsoever is known about the repair of ResO˙ at the water–cyclodextrin interface. Even for α-tocopherol (vitamin E), the best-investigated lipophilic antioxidant, kinetic and mechanistic studies on the repair of its phenoxy radical have focused exclusively on homogeneous solutions14,18–23 and model membranes.19–24 This lack of information concerning reactions across the water–cyclodextrin phase boundary—despite the widespread use of both cyclodextrins and antioxidants in food processing—prompted us to examine them using HAsc− as the repairing agent for ResO˙.
To this end, we have employed our recently developed25 conversion of ResOH into ResO˙ without the participation of any auxiliary reagent through laser photoionization with UV-A (355 nm) to generate the radical inside the cavities of native and modified cyclodextrins (for the structures, see Fig. 1a) and to study its subsequent reaction with HAsc−, which can only reside in the aqueous phase. As we will show, the cyclodextrin containers influence both the photoionization and the repair reaction decisively through the geometric constraints they impose, but they affect neither the quantum yield of the actual ionization step nor the repair mechanism, and their different effects on radical scavenging by ResOH and on the repair of ResO˙ by HAsc− can be traced back to a deeper embedding of the more hydrophobic phenoxy radical in their interior.
2 Results and discussion
2.1 Complexation of resveratrol with cyclodextrins and properties of the inclusion complexes
The promising increase of both solubility and stability of ResOH upon its complexation by cyclodextrins has stimulated studies of its inclusion in these cavities by a variety of methods (phase solubility,4,6,7,9 fluorescence,6,8,11 enzymatic oxidation,6 HPLC,5 isothermal titration calorimetry,10 NMR,12 and circular dichroism12). However, these investigations often gave substantially divergent results even when similar experimental procedures were used: e.g., values between 1600 M−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 18
4 and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1
000 M−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 were reported for the complexation constant of ResOH by HP β-CD. These discrepancies prompted us to (re)determine the binding constants of ResOH to all cyclodextrins employed in this work spectrophotometrically. This well-established method26 does not seem to have been applied to these systems although in the case of ResOH it is much more sensitive than fluorescence. From measurements of the fluorescence quantum yields, in conjunction with the absorption and luminescence spectra, we further obtained the excited-state lifetimes, which reflect how the encapsulation affects the photoinduced cis–trans isomerization.
6 were reported for the complexation constant of ResOH by HP β-CD. These discrepancies prompted us to (re)determine the binding constants of ResOH to all cyclodextrins employed in this work spectrophotometrically. This well-established method26 does not seem to have been applied to these systems although in the case of ResOH it is much more sensitive than fluorescence. From measurements of the fluorescence quantum yields, in conjunction with the absorption and luminescence spectra, we further obtained the excited-state lifetimes, which reflect how the encapsulation affects the photoinduced cis–trans isomerization.
In each case, a plot of the reciprocal absorbance change at the selected wavelength as a function of the reciprocal cyclodextrin concentration (Benesi–Hildebrand plot) gives a straight line with no discernible deviations (see the insets of Fig. 2). This identifies the complexation stoichiometry as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in agreement with previous studies of ResOH in cyclodextrins.4–12 The linear regressions have high correlation coefficients (0.990 < R2 < 0.999) demonstrating that the UV absorption is well suited for investigating the cyclodextrin complexation of ResOH; their parameters yield the complexation constants, which are displayed in each respective subplot of Fig. 2 and also summarized in Table 1. These constants suggest two trends. First, the wider β-cyclodextrin cavity can accommodate ResOH in a less constrained manner than the tighter one of an α-cyclodextrin; second, the extension of the effective cavity depth by HP substitution significantly helps binding this guest molecule.
1, in agreement with previous studies of ResOH in cyclodextrins.4–12 The linear regressions have high correlation coefficients (0.990 < R2 < 0.999) demonstrating that the UV absorption is well suited for investigating the cyclodextrin complexation of ResOH; their parameters yield the complexation constants, which are displayed in each respective subplot of Fig. 2 and also summarized in Table 1. These constants suggest two trends. First, the wider β-cyclodextrin cavity can accommodate ResOH in a less constrained manner than the tighter one of an α-cyclodextrin; second, the extension of the effective cavity depth by HP substitution significantly helps binding this guest molecule.
| Cyclodextrin | K /M−1 | Φ f | τ /ps | eaq˙− yielde | ε 410 (ResO˙)/(M−1 cm−1) | k repair |
|---|---|---|---|---|---|---|
a At 297 K.
b Association constant of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex of ResOH with the respective cyclodextrin.
c Fluorescence quantum yield of ResOH relative to the value in homogeneous aqueous solution, 0.0039.
d Excited-state lifetime of ResOH obtained by a Strickler–Berg analysis.
e Relative yield of eaq˙− in the 355 nm photoionization of ResOH at 400 mJ cm−2 (corresponding to the highest laser intensity used).
f Rate constant for the repair reaction of ResO˙ by HAsc− (eqn (2)) with the value in homogeneous aqueous solution, 7.1 × 107 M−1 s−1, as a reference. 1 complex of ResOH with the respective cyclodextrin.
c Fluorescence quantum yield of ResOH relative to the value in homogeneous aqueous solution, 0.0039.
d Excited-state lifetime of ResOH obtained by a Strickler–Berg analysis.
e Relative yield of eaq˙− in the 355 nm photoionization of ResOH at 400 mJ cm−2 (corresponding to the highest laser intensity used).
f Rate constant for the repair reaction of ResO˙ by HAsc− (eqn (2)) with the value in homogeneous aqueous solution, 7.1 × 107 M−1 s−1, as a reference.
|
||||||
| — | — | 1 | 28 | 1 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1 |
| α-CD | 850 | 4.5 | 160 | 3.1 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
0.37 |
| β-CD | 1650 | 2.1 | 70 | 2.3 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.45 |
| HP α-CD | 1800 | 7.1 | 250 | 4.0 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.25 |
| HP β-CD | 7950 | 4.2 | 140 | 3.4 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
0.27 |
Geometrical isomerization shortens the S1 lifetime of excited ResOH to ~28 ps,28,30 resulting in a very low fluorescence quantum yield.27,28 To compensate for the associated sensitivity loss, we increased the excitation slit of our spectrometer to 5 nm, reduced the scan speed to 200 nm per minute, and averaged three independent measurements. Because we observed that a threefold repetition of the experiment on the same sample already led to a detectable presence of the unwanted cis isomer under these conditions, we used fresh solution for each of the three measurements.
By this methodology, we were further able to keep the absorbances of our samples below 0.15 to avoid inner filter effects, which are the most likely causes of the discrepancies reported in ref. 6 between the ResOH–cyclodextrin complexation constants determined by fluorescence in strongly absorbing solutions (A ≈ 0.6) and by other methods. Rather than using extremely small ResOH concentrations to that end, we excited in the vicinity of the minimum at the left edge of the spectra in Fig. 2. Excitation at either 250 nm or 260 nm gave very similar quantum yields (0.0039 ± 3%). The average value is in good agreement with that obtained by Deak et al.27
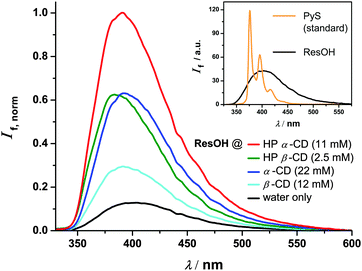
Next, we extended these investigations to the complexed molecule. A quantitative (≥99%) complexation of ResOH would require unreasonably high concentrations of our cyclodextrins (for β-CD even far above the solubility limit). Instead, we standardized all systems to 95% complexation, which requires the cyclodextrin concentrations listed in Fig. 3. The remaining contribution of free ResOH is easily removed by subtracting the fluorescence spectrum recorded in the absence of cyclodextrins after weighting it with a factor of 0.05; scaling the result with 1/0.95 then yields the pure spectrum of complexed ResOH. Both corrections are seen to be so small as not to impede the reliability.
Furthermore, we excited at the isosbestic point of free ResOH and its respective complexed form (250 nm for the two α-cyclodextrins and 252 nm for the two β-cyclodextrins; see Fig. 2), where minute adjustments of the ResOH concentration sufficed to keep the absorbances of all solutions identical. As an advantage over previous investigations,6,8,11 the described procedure allows an immediate quantification of the cyclodextrin influences simply by comparing the fluorescence spectra.
The main plot of Fig. 3 collates the relative fluorescence spectra of differently complexed and uncomplexed ResOH. Whereas the spectral shape and the position of the maximum are hardly affected by the cyclodextrins, the emission becomes considerably stronger upon complexation. The fluorescence quantum yields Φf obtained by integration are listed in Table 1. They approximately double when the container is tightened (from a β-cyclodextrin to the α-analogue) or deepened (from an unsubstituted cyclodextrin to its HP-substituted counterpart); a similar increase is observed when free ResOH becomes encapsulated by β-CD. It seems natural to ascribe these effects to an impeded twist of the resveratrol skeleton by the cyclodextrin cavity, which decelerates the radiationless deactivation through cis–trans isomerization, and thereby lengthens the life of the excited state and thus increases the emission probability.
The excited-state lifetime τ is the ratio of Φf and the rate constant of fluorescence kf. The latter can in turn be calculated from the absorption and emission spectra and the refractive index n using the well-known Strickler–Berg equation.31 For a vanishing Stokes shift and a mirror-image symmetry of the two spectra, this equation simplifies to a proportionality between kf and the integrated absorption spectrum times n2 times the squared energy of the 0–0 transition.32 Although these conditions are approximately met by our systems, a much better reliability than for the absolute values of kf is expected for the relative values, i.e., relative to the rate constant in homogeneous aqueous solution, 1.4 × 108 s−1, which directly follows from the known τ28,30 and our measured Φf. The lifetimes τ so obtained are included in Table 1. Within 10%, τ and Φf show the same relationships with the cyclodextrin structures, which identifies the change in the oscillator strength of the transition as a minor factor; even the parameter n2 remains constant within 1% when going from water to a cyclodextrin interior.32
The observed trend is in line with the reported quadrupling of the fluorescence lifetime of trans-stilbene, i.e., the structural parent of ResOH, upon complexation with α-CD.33 A further consistency test is provided by the successful fits of the photoionization yields with τ as fixed parameters (see the next section).
2.2 Generation of the resveratrol radical
Our recently developed strategy of photoionizing ResOH using a 355 nm laser pulse25 generates the ResO˙ radical exactly at the location of its precursor, and therefore appears to be particularly promising for the cyclodextrin-complexed systems. We have already demonstrated the feasibility of this concept for ResOH in anionic micelles.25 In the same way, an intense laser pulse on cyclodextrin-encapsulated ResOH brings about photoionization on a large scale. This is established by the characteristic absorption of eaq˙−, which fades away within a few microseconds and can be removed completely by the selective electron scavenger N2O34 (Fig. 4a, inset).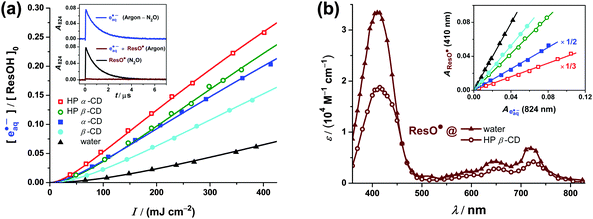 | ||
Fig. 4 Influence of cyclodextrins on the near-UV (355 nm) photoionization of an aqueous solution of 6 × 10−5 M ResOH ([ResOH]0) at pH 6.5. (a) Main plot: intensity dependence of eaq˙− formation in homogeneous aqueous solution (black data and fit function) and in the presence of cyclodextrins (coloured data and fit functions). Cyclodextrin concentrations are the same as in Fig. 3. Fit functions, 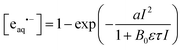 . Global best-fit parameter B0, 5.91 × 104 cm3 M mJ−1 s−1; constants τ from Table 1. Medium/constant parameters ε (in M−1 cm−1)/best-fit parameters a (in 106 cm4 mJ−2); water/800/0.65, α-CD/2100/12.3, β-CD/1440/3.52, HP α-CD/1720/21.0, and HP β-CD/1370/8.74. Inset: representative absorption traces illustrating the separation of ResO˙ and eaq˙− absorption signals by difference measurements in Ar- and N2O-saturated solution with all other experimental parameters (2.5 mM HP β-CD; laser intensity, 359 mJ cm−2) unmodified. (b) Main plot: calibrated absorption spectrum of ResO˙ in water (filled triangles) and encapsulated by HP β-CD (open circles). Inset: ResO˙ absorptions at the spectral maximum (410 nm) against the eaq˙− absorptions at a reference wavelength (824 nm) in homogeneous solution and in the presence of cyclodextrins at different laser intensities (same color coding as in the main plot of (a)); the respective slopes of the regression lines are used for calibration of the ResO˙ spectra (for clarity, two datasets have been rescaled). For further explanations, see the text. . Global best-fit parameter B0, 5.91 × 104 cm3 M mJ−1 s−1; constants τ from Table 1. Medium/constant parameters ε (in M−1 cm−1)/best-fit parameters a (in 106 cm4 mJ−2); water/800/0.65, α-CD/2100/12.3, β-CD/1440/3.52, HP α-CD/1720/21.0, and HP β-CD/1370/8.74. Inset: representative absorption traces illustrating the separation of ResO˙ and eaq˙− absorption signals by difference measurements in Ar- and N2O-saturated solution with all other experimental parameters (2.5 mM HP β-CD; laser intensity, 359 mJ cm−2) unmodified. (b) Main plot: calibrated absorption spectrum of ResO˙ in water (filled triangles) and encapsulated by HP β-CD (open circles). Inset: ResO˙ absorptions at the spectral maximum (410 nm) against the eaq˙− absorptions at a reference wavelength (824 nm) in homogeneous solution and in the presence of cyclodextrins at different laser intensities (same color coding as in the main plot of (a)); the respective slopes of the regression lines are used for calibration of the ResO˙ spectra (for clarity, two datasets have been rescaled). For further explanations, see the text. | ||
The main plot of Fig. 4a displays the intensity dependence of the eaq˙− yield, relative to the initial ResOH concentration, in the four cyclodextrins investigated in this work and in homogeneous aqueous solution as a reference. Exploiting an enhanced sensitivity of our detection system at 824 nm,25 we observed eaq˙− at this wavelength and removed a very small contribution of ResO˙ to the absorption signal by difference experiments, as shown in the inset of Fig. 4a. For the measurements on complexed ResOH, we again chose the cyclodextrin concentrations so as to bind 95% of our substrate (for comparison, see Section 2.1.2 and Fig. 3). As we found from the difference spectra in Ar- and N2O-saturated solutions, the broad band of eaq˙− peaking at about 720 nm in phosphate-buffered homogeneous aqueous solution25 remains unchanged and unshifted by the addition of millimolar concentrations of our cyclodextrins; hence, we were able to use our recently determined molar absorption coefficient (ε824(eaq˙−) = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 M−1 cm−1)25 for obtaining the eaq˙− concentrations.
900 M−1 cm−1)25 for obtaining the eaq˙− concentrations.
The upward curvatures in the low-intensity regime and the negative intercepts of the straight-line extrapolations to zero intensity characterize the ionizations as biphotonic35 regardless of the environment of ResOH. However, the cyclodextrin complexation increases the eaq˙− yield from ResOH by a factor between 2 and 4, as is evidenced by the main plot of Fig. 4a. All dependence on the intensity I can be fitted excellently with a two-parameter model,  , which we have developed for the ionization of an excited state replenished by a fast photostationary pre-equilibrium,36 a condition that is almost perfectly met by ResOH owing to its short excited-state lifetime τ.25In addition, because of the composition of b36 all five data sets can be fitted simultaneously with a global, medium-independent parameter B0, from which each specific value of b is derived by multiplication with the predetermined ground-state molar absorption coefficient ε of ResOH at the excitation wavelength and with τ, both in the respective environment. We regard the success of this fit—with a as the only adjustable parameter for each data set—as incontrovertible evidence for the biphotonic ionization mechanism via the S1 state of ResOH (*ResOH) according to25,28
, which we have developed for the ionization of an excited state replenished by a fast photostationary pre-equilibrium,36 a condition that is almost perfectly met by ResOH owing to its short excited-state lifetime τ.25In addition, because of the composition of b36 all five data sets can be fitted simultaneously with a global, medium-independent parameter B0, from which each specific value of b is derived by multiplication with the predetermined ground-state molar absorption coefficient ε of ResOH at the excitation wavelength and with τ, both in the respective environment. We regard the success of this fit—with a as the only adjustable parameter for each data set—as incontrovertible evidence for the biphotonic ionization mechanism via the S1 state of ResOH (*ResOH) according to25,28
 | (1) |
Fig. 4b focuses on the absorption spectrum of ResO˙. All these measurements were carried out in N2O-saturated solution, where the strongly absorbing eaq˙− are instantaneously (i.e., within the duration of our laser pulses) converted into non-absorbing hydroxyl radicals. The latter are in turn scavenged by the cyclodextrins to give carbon centered radicals, which are non-absorbing in our detection range;37 these diffusion controlled reactions are completed within nanoseconds at our cyclodextrin concentrations. On a longer timescale, these radicals are ultimately repaired by ResOH, which is also the only sink of the hydroxyl radicals in the absence of cyclodextrins. However, at low ResOH concentrations used in our experiments, none of these secondary processes play any role for the concentrations of ResO˙ immediately after the laser flash, on which Fig. 4b is based.
The inset of Fig. 4b compares the post-flash absorbances of ResO˙ (at its absorption maximum, 410 nm) and eaq˙− (without N2O and at its monitoring wavelength, 824 nm, where ResO˙ is almost non-absorbing) throughout our range of laser intensities. The strict proportionality of the signals clearly indicates that both species, ResO˙ and eaq˙−, are produced by the same elementary reaction step not only in homogeneous solution, but also in the presence of cyclodextrins. The initial product of this photoionization is the resveratrol radical cation, which is known to undergo instantaneous deprotonation in aqueous and other homogeneous media to give ResO˙ as the only observable resveratrol-derived radical form.25,28 Based on the absence of any additional signals, we conclude that this is case in the cyclodextrin systems as well. Hence, the absorption spectrum of cyclodextrin-complexed ResO˙ can be calibrated in a straightforward way against the eaq˙− absorption as the reference; to reduce the errors of the single-point measurements, we used the slopes of the regression lines in the inset of the figure. The molar absorption coefficients so obtained are compiled in Table 1.
The main plot of Fig. 4b finally juxtaposes the calibrated absorption spectra of ResO˙ in homogeneous solution and complexed by HP β-CD, which we chose as an example because that cyclodextrin has the highest complexation constant for the starting material ResOH while for all four cyclodextrins investigated in this work the spectra of encapsulated ResO˙ are very similar (see the molar absorption coefficients in Table 1). The complexation retains the spectral shape, with only slight red shifts of the maxima at about 410, 650 and 720 nm, but decreases the molar absorption coefficients by about 40%. This parallels the observations for ground-state ResOH (for comparison, see Fig. 2 and ref. 11) with an even more pronounced hypochromic effect, and therefore clearly indicates a complexation of ResO˙ by all cyclodextrins used in this study.
In the presence of cyclodextrins, the described photoionization approach has a double preference for generating ResO˙ in encapsulated form: (i) because of the complexation of the parent molecule ResOH combined with the fact that the radical is born at the very location of its precursor and (ii) because the photoionization of complexed ResOH is more efficient than that of free ResOH.
2.3 Repair reaction of the resveratrol radical by ascorbate
So far, the photoionization approach to cyclodextrin-encapsulated phenoxy radicals has been limited to the use of UV-C light (266 nm), and the few available reports have only been concerned with the generation of the radicals38,39 or their reaction with the container.40 More directly related to the present work are two studies on how the co-antioxidant HAsc− interacts with α-tocopherol phenoxy radicals prepared by UV-B (308 nm) photoionization in model membranes.19,20For all experiments addressing the repair reaction (eqn (2)),
| ResO˙ + HAsc− → ResOH + Asc˙− | (2) |
Under these conditions, ResO˙ alone disappears only on a timescale as long as hundreds of microseconds in homogeneous aqueous solution,25 and even more slowly in the presence of our cyclodextrins. While the complexity of this decay41 prohibited its use for studying the association/dissociation equilibrium between ResO˙ and the cyclodextrins, its low rate permitted the undisturbed observation of the repair by ascorbate.
To eliminate the intense electron absorption, we worked with N2O-saturated solutions (see Section 2.2), thereby instantaneously and quantitatively transforming eaq˙− into the non-absorbing hydroxyl radicals. As opposed to Section 2.2, in our experiments with HAsc− (concentrations, several mM) a significant amount of these hydroxyl radicals is already scavenged directly by HAsc−; the cyclodextrin-based radicals are also repaired by HAsc−; and the secondary formation of ResO˙ is totally suppressed. Hence, all absorptions above 500 nm arise solely from ResO˙ produced by the photoionization flash. For selectively monitoring its further fate, we used its red absorption band, averaging the two maxima at 650 and 720 nm to improve the signal-to-noise ratio.
Because the laser flash preferentially ionizes ResOH residing within a cyclodextrin (see Section 2.2) and generates the radical ResO˙ at the location of that precursor, the initial post-flash fraction of complexed ResO˙ exceeds that of complexed ResOH and lies between 98% (β-CD) and 99% (HP α-CD). The differences between the curves of Fig. 5 could thus be caused by a continuum of possibilities between two extreme situations that (a) the repair reaction according to eqn (2) occurs not only in homogeneous solution but also—with a substantial deceleration—at the cyclodextrin–water interface, and the exchange of ResO˙ between cyclodextrin and water is too slow to play any role; or (b) the repair is only possible in the aqueous phase while the decomplexation of ResO˙ is rate-limiting.
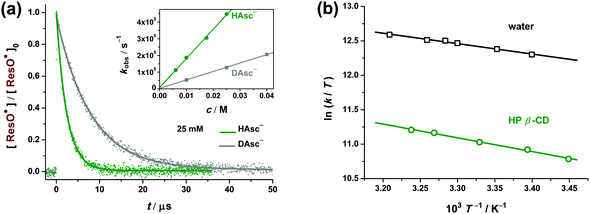
To determine the position within this mechanistic spectrum, we first varied the HAsc− concentration between 5 and 25 mM. In the two limiting cases, the observed rate constant kobs would have to be concentration-proportional (a) or completely concentration-independent (b); in-between, one would expect some non-linear concentration dependence. All the plots of kobs as a function of [HAsc−] were linear with negligible intercepts (an example is given in the inset of Fig. 6a), establishing case (a) as well as confirming that radical–radical recombinations are unimportant under our conditions.
For further corroboration, we also carried out experiments at constant [HAsc−] but with lower cyclodextrin concentrations, that is, with a significant fraction of ResO˙ present in uncomplexed form. Case (a) demands independent decays of complexed (index c) and free (index f) ResO˙ with their respective pseudo first-order rate constants, i.e., an integrated rate law nc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−kct) + nf
exp(−kct) + nf![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−kft), with the rate constants kc and kf and the initial mole fractions nc and nf. The decays observed in these experiments were perfectly reproducible by this superposition, with kc approximated by the rate constants obtained at twice the cyclodextrin concentrations given in Fig. 5 (except for β-CD, where the solubility limit allowed an increase by a factor of 1.5 only) and kf measured without cyclodextrin, and with the mole fractions calculated from the results of Sections 2.1 and 2.2.
exp(−kft), with the rate constants kc and kf and the initial mole fractions nc and nf. The decays observed in these experiments were perfectly reproducible by this superposition, with kc approximated by the rate constants obtained at twice the cyclodextrin concentrations given in Fig. 5 (except for β-CD, where the solubility limit allowed an increase by a factor of 1.5 only) and kf measured without cyclodextrin, and with the mole fractions calculated from the results of Sections 2.1 and 2.2.
Another mechanistic complication would arise from binding of HAsc− to the cyclodextrins. In one report, an association constant between HAsc− and α-CD of 112 M−1 was inferred indirectly from kinetic measurements in a multicomponent system.42 Because we considered such a complexation unlikely, given the less polar interior of the cyclodextrin and the delocalized negative charge of the guest,43 we recorded absorption spectra of HAsc− in the presence of different cyclodextrin concentrations. We observed no changes even with 20 mM α-CD, where the above equilibrium constant would predict 69% of HAsc− to be encapsulated. In contrast, a control experiment in methanol/water (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), i.e., in a medium of comparable polarity to that presumed for the cyclodextrin interior,44 gave an unambiguous red shift of the narrow absorption band by ~4 nm. These findings rule out a noticeable degree of HAsc− complexation in the measurements of this work.
1, v/v), i.e., in a medium of comparable polarity to that presumed for the cyclodextrin interior,44 gave an unambiguous red shift of the narrow absorption band by ~4 nm. These findings rule out a noticeable degree of HAsc− complexation in the measurements of this work.
From the experiments of this section, we can thus conclude that the cyclodextrin–water interface substantially decelerates the repair reaction: the second-order rate constants (krepair), which follow from the fit parameter kobs and the HAsc− concentration, decrease by a factor of 2–4, depending on the cyclodextrin (see Table 1).
The obviously stronger cyclodextrin effects on ResO˙ scavenging by the co-antioxidant HAsc− compared to radical scavenging by the antioxidant ResOH prompted us to check whether the repair according to eqn (2) undergoes a mechanistic change when ResO˙ is complexed. Thermodynamically, a concerted process and a sequence of rate-determining electron transfer followed by fast proton transfer are feasible for the proton-coupled electron transfer (for details of this class of reactions, see ref. 47 and 48) of reaction (2).
Our recent study25 on the H/D kinetic isotope effect (KIE) of this reaction in homogeneous solution gave a KIE of 3.9, consistent with the one-step process. To extend this to cyclodextrin-complexed ResO˙, we chose HP β-CD because this host exhibits the strongest complexation of ResOH, the largest effect on the ResO˙ absorption and almost the maximum reduction of krepair, making it the most likely of the four cyclodextrins for a mechanistic turnaround, if the latter were responsible for the observed rate decrease. Fig. 6a compares the ResO˙ repair kinetics in H2O and D2O (where all hydroxylic protons in the reactants are replaced by deuterons) with all other experimental parameters identical. The KIE obtained from Fig. 6a, 3.5, is very similar to the KIE that we measured in homogeneous aqueous solution, 3.9,25 indicating that the repair mechanism does not deviate from a one-step process upon cyclodextrin complexation of ResO˙.
These findings narrow down the mechanistic possibilities to a hydrogen atom transfer (HAT) or a concerted proton–electron transfer (CPET). Both convert the linear fragment O⋯H–O into O–H⋯O, yet with different orbital participation. The hydrogen atom (HAT) or the proton (CPET) travel along the σ orbitals of the old and new bond, but the CPET additionally relies on a π type overlap of the heteroatom lone pairs for the simultaneous shift of the electron.48,49 This discrepancy of geometric constraints is tantamount to retaining or losing the free rotation around the σ bonds, and therefore must be reflected by a zero (HAT) or a strongly negative (CPET) contribution to the activation entropy. In both cases, this is overlaid with the same, small and positive, vibrational contribution arising mainly from the frequency differences between the H–O stretching mode in the hydrogen-bonded starting state and the symmetrical O⋯H⋯O stretching mode in the activated complex, such that in total a small positive activation entropy results for HAT and a significantly negative one for CPET.20
To obtain the activation parameters, we carried out temperature dependent measurements of the ResO˙ repair rate both in homogeneous aqueous solution and at the cyclodextrin–water interface (Fig. 6b). For this purpose, HP β-CD is again the most suitable of our cyclodextrins because its room-temperature complexation constant is already the largest by far, yet decreases only by 20% over the temperature interval of the experiments,8 as opposed to a factor of 2 for unsubstituted β-CD,5 and its high water solubility allowed a trebling of its concentration, thereby ensuring quantitative (>99%) complexation of ResO˙ at all temperatures.
The Eyring plots of Fig. 6b are linear in both media. The activation parameters were calculated from the regression lines and are summarized in Table 2. The negative activation entropies ΔS‡ are consistent with the CPET process for reaction (2). In any case, the numerically and extremely similar (to within 3%) values of ΔS‡ clearly establish that the complexation does not entail a mechanistic change, whereas the appreciable difference in the activation enthalpies ΔH‡ points to the steric repulsion23 between the cyclodextrin and (non-complexable) HAsc− as the reason for the deceleration by the macrocycle.
Different localizations of ResOH and its phenoxy radical ResO˙ within the containers provide a natural rationalization of the disparate kinetic effects. For ResOH, NMR experiments4 have shown the centre of its olefinic bond approximately to coincide with the centre of the cyclodextrin, regardless of whether the latter is native or HP-substituted. In consequence, the reactive single phenolic group extends sufficiently far into the aqueous bulk so as to allow almost unrestricted scavenging of radicals ˙OR (Fig. 7a). However, once this group has been converted into a phenoxy radical, which is significantly less hydrophilic than is −OH because of its inability to function as a hydrogen-bond donor,50 the guest ResO˙ is drawn deeper into the hydrophobic interior of the host such that the access of the co-antioxidant HAsc− is hindered by its interactions with the cyclodextrin rim (Fig. 7b). This model also explains why a β-cyclodextrin with its wider diameter causes only a slightly smaller deceleration of the ResO˙ repair than does the corresponding α-cyclodextrin whereas HP substitution, i.e., a dilation of the container in the direction of the long axis of ResO˙, has a significantly larger effect.
 | ||
| Fig. 7 Structures of the complexed species explaining the observed effects of cyclodextrins on radical scavenging by ResOH and on the repair of ResO˙. (a) Confirmed structure4 of the ResOH cyclodextrin complex (ResOH@CD) allowing unhindered radical scavenging. (b) Most likely structure, owing to a higher guest hydrophobicity of complexed ResO˙ (ResO˙@CD), in which the phenoxy group is shielded by the cyclodextrin cavity, impeding access of co-antioxidant HAsc− from the aqueous phase. For further details, see the text. | ||
3 Experimental section
All chemicals were obtained commercially in the highest available purity and used as received (trans-resveratrol, >98%, TCI; sodium ascorbate, ≥99%, Roth; sodium hydrogen phosphate, ≥99%, Fluka; sodium dihydrogen phosphate, >98%, VEB Laborchemie Apolda; α-cyclodextrin, >98%, TCI; β-cyclodextrin, >99%, TCI; (2-hydroxypropyl)-β-cyclodextrin, molecular weight 1460 g mol−1, Aldrich; (2-hydroxypropyl)-α-cyclodextrin, molecular weight 1180 g mol−1, Aldrich; sodium pyrene-1-sulfonate, ≥97%, Aldrich; 2-aminopyridine, ≥99%, Aldrich; N,N-dimethylamino-m-nitrobenzene, 98%, Alfa Aesar; methanol, ≥99.9%, Aldrich).The solvent was either ultrapure Millipore Milli-Q water (specific resistance, 18.2 MΩ cm) or D2O (99.9% deuteration, Deutero GmbH). The ResOH-containing solutions were always freshly prepared before experiments and stored in the dark to avoid degradation. To ensure the presence of (fully protonated) ResOH only, all measurements were carried out at pH 6.5,25 using a phosphate buffer (general buffer concentration, 6.7 mM) prepared by mixing stock solutions of sodium hydrogen phosphate and sodium dihydrogen phosphate.
Steady-state absorption and fluorescence spectra were recorded using a Shimadzu UV-2102 spectrophotometer and a Perkin Elmer LS 50B spectrometer. Control experiments without ResOH established the absence of absorption or luminescence signals from the cyclodextrins. The fluorescence quantum yields were determined in deaerated solutions to avoid oxygen quenching of the excited standard pyrene-1-sulfonate29 (the fluorescence of ResOH is insensitive to oxygen). The fluorescence spectrometer was calibrated with the standards 2-aminopyridine and N,N-dimethylamino-m-nitrobenzene.51 The sensitivity factors so obtained were used to correct all emission spectra.
The laser flash photolysis setup featured a frequency-tripled Nd:YAG laser (Continuum Surelite-III, 355 nm, 5 ns pulse width) for excitation and optical detection of the intermediates. A detailed description has been given elsewhere.52 All solutions were purged with argon (5.0, Air Liquide) or N2O (5.0, Air Liquide) for 30 minutes before and for the whole duration of the laser flash photolysis experiments. To avoid the oxidation of the co-antioxidant ascorbate by molecular oxygen,53 the required amount of sodium ascorbate was added in solid form to the already degassed solutions immediately before starting the laser experiments.
Unless otherwise noted, all experiments were carried out at room temperature (297 ± 1 K).
4 Conclusions
These experiments have demonstrated the wealth of information that is accessible by the selective in situ generation of the resveratrol phenoxy radical at the precise location of its parent compound, such as within a cyclodextrin cavity, through UV-A photoionization. Because no auxiliary reagent is involved in its production, the repair reaction by ascorbate—a key step for the bioavailability of the famous antioxidant resveratrol—can be observed in isolation, providing unequivocal answers about the factors that govern it. This approach clearly paves the way for investigations of the interactions between other antioxidants across the phase boundaries of microheterogeneous systems.Acknowledgements
Financial support provided by the Fonds der Chemischen Industrie to C. K. (PhD scholarship) is gratefully acknowledged.References
- B. B. Aggarwal and S. Shishodia, Resveratrol in health and disease, CRC Press, Boca Raton, 1st edn, 2005 Search PubMed.
- P. Farris, J. Krutmann, Y.-H. Li, D. McDaniel and Y. Krol, J. Drugs Dermatol., 2013, 12, 1389–1394 CAS.
- B.-Y. Chen, C.-H. Kuo, Y.-C. Liu, L.-Y. Ye, J.-H. Chen and C.-J. Shieh, J. Nat. Prod., 2012, 75, 1810–1813 CrossRef CAS PubMed.
- V. Bertacche, N. Lorenzi, D. Nava, E. Pini and C. Sinico, J. Inclusion Phenom. Macrocyclic Chem., 2006, 55, 279–287 CrossRef CAS.
- J. M. López-Nicolás and F. García-Carmona, Food Chem., 2008, 109, 868–875 CrossRef PubMed.
- C. Lucas-Abellán, M. I. Fortea, J. A. Gabaldón and E. Núñez-Delicado, Food Chem., 2008, 111, 262–267 CrossRef.
- Z. Lu, B. Cheng, Y. Hu, Y. Zhang and G. Zou, Food Chem., 2009, 113, 17–20 CrossRef CAS.
- Z. Lu, R. Chen, H. Liu, Y. Hu, B. Cheng and G. Zou, J. Inclusion Phenom. Macrocyclic Chem., 2009, 63, 295–300 CrossRef CAS.
- S. Sapino, M. E. Carlotti, G. Caron, E. Ugazio and R. Cavalli, J. Inclusion Phenom. Macrocyclic Chem., 2009, 63, 171–180 CrossRef CAS.
- H. Li, X. Xu, M. Liu, D. Sun and L. Li, Thermochim. Acta, 2010, 510, 168–172 CrossRef CAS.
- J. M. López-Nicolás and F. García-Carmona, Food Chem., 2010, 118, 648–655 CrossRef.
- E. Troche-Pesqueira, I. Pérez-Juste, A. Navarro-Vázquez and M. M. Cid, RSC Adv., 2013, 3, 10242–10250 RSC.
- J. Chaudière and R. Ferrari-Iliou, Food Chem. Toxicol., 1999, 37, 949–962 CrossRef.
- J. E. Packer, T. F. Slater and R. L. Wilson, Nature, 1979, 278, 737–738 CrossRef CAS PubMed.
- C. Lucas-Abellán, M. T. Mercader-Ros, M. P. Zafrilla, M. I. Fortea, J. A. Gabaldón and E. Núñez-Delicado, J. Agric. Food Chem., 2008, 56, 2254–2259 CrossRef PubMed.
- C. Lucas-Abellán, M. T. Mercader-Ros, M. P. Zafrilla, J. A. Gabaldón and E. Núñez-Delicado, Food Chem. Toxicol., 2011, 49, 1255–1260 CrossRef PubMed.
- M. Ishikawa, Y. Sueishi, N. Endo, S. Oowada, M. Shimmei, H. Fujii and Y. Kotake, Int. J. Chem. Kinet., 2012, 44, 598–603 CrossRef CAS.
- E. Niki, J. Tsuchiya, R. Tanimura and Y. Kamiya, Chem. Lett., 1982, 789–792 CrossRef CAS.
- R. H. Bisby and A. W. Parker, FEBS Lett., 1991, 290, 205–208 CrossRef CAS PubMed.
- R. H. Bisby and A. W. Parker, Arch. Biochem. Biophys., 1995, 317, 170–178 CrossRef CAS PubMed.
- W. Gregor, G. Grabner, C. Adelwöhrer, T. Rosenau and L. Gille, J. Org. Chem., 2005, 70, 3472–3483 CrossRef CAS PubMed.
- K. Mukai, S. Mitani, K. Ohara and S. Nagaoka, Free Radical Biol. Med., 2005, 38, 1243–1256 CrossRef CAS PubMed.
- S. Nagaoka, T. Kakiuchi, K. Ohara and K. Mukai, Chem. Phys. Lipids, 2007, 146, 26–32 CrossRef CAS PubMed.
- J.-G. Fang, M. Lu, Z.-H. Chen, H.-H. Zhu, Y. Li, L. Yang, L.-M. Wu and Z.-L. Liu, Chem. – Eur. J., 2002, 8, 4191–4198 CrossRef CAS.
- C. Kerzig, S. Henkel and M. Goez, Phys. Chem. Chem. Phys., 2015, 17, 13915–13920 RSC.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703–2707 CrossRef CAS.
- M. Deak and H. Falk, Monatsh. Chem., 2003, 134, 883–888 CrossRef CAS.
- I. Džeba, T. Pedzinski and B. Mihaljević, J. Photochem. Photobiol., A, 2015, 299, 118–124 CrossRef.
- C. Bohne, E. B. Albuin and J. C. Scaiano, J. Am. Chem. Soc., 1990, 112, 4226–4231 CrossRef CAS.
- R. Simkovitch and D. Huppert, J. Phys. Chem. B, 2015, 119, 11684–11694 CrossRef CAS PubMed.
- S. J. Strickler and R. A. Berg, J. Chem. Phys., 1962, 37, 814–822 CrossRef CAS.
- J. Mohanty and W. M. Nau, Photochem. Photobiol. Sci., 2004, 3, 1026–1031 CAS.
- G. L. Duveneck, E. V. Sitzmann, K. B. Eisenthal and N. J. Turro, J. Phys. Chem., 1989, 93, 7166–7170 CrossRef CAS.
- J. W. T. Spinks and R. J. Woods, An Introduction to Radiation Chemistry, Wiley, New York, 2nd edn, 1976 Search PubMed.
- U. Lachish, A. Shafferman and G. Stein, J. Chem. Phys., 1976, 64, 4205–4211 CrossRef CAS.
- M. Goez and C. Kerzig, Angew. Chem., Int. Ed., 2012, 51, 12606–12608 CrossRef CAS PubMed.
- D. La Mendola, S. Sortino, G. Vecchio and E. Rizzarelli, Helv. Chim. Acta, 2002, 85, 1633–1643 CrossRef CAS.
- I. P. Pozdnyakov, L. Guo, E. M. Glebov, F. Wu, V. F. Plyusnin, V. P. Grivin and N. Deng, High Energy Chem., 2011, 45, 214–221 CrossRef CAS.
- V. A. Salomatova, I. P. Pozdnyakov, V. V. Yanshole, F. Wu, V. P. Grivin, N. M. Bazhin and V. F. Plyusnin, J. Photochem. Photobiol., A, 2014, 274, 27–32 CrossRef CAS.
- S. Monti and S. Sortino, Chem. Soc. Rev., 2002, 31, 287–300 RSC.
- S. Stojanović and O. Brede, Phys. Chem. Chem. Phys., 2002, 4, 757–764 RSC.
- Y. L. Loukas, V. Vraka and G. Gregoriadis, Pharm. Sci., 1996, 2, 523–527 CAS.
- J. W. Park, Fluorescence Methods for Studies of Cyclodextrin Inclusion Complexation and Excitation Transfer in Cyclodextrin Complexes, in Cyclodextrin Materials Photochemistry, Photophysics and Photobiology, ed. A. Douhal, Elsevier, Amsterdam, 2006, pp. 1–26 Search PubMed.
- B. D. Wagner, The Effects of Cyclodextrins on Guest Fluorescence, in Cyclodextrin Materials Photochemistry, Photophysics and Photobiology, ed. A. Douhal, Elsevier, Amsterdam, 2006, pp. 27–59 Search PubMed.
- E. Niki, Free Radical Biol. Med., 2010, 49, 503–515 CrossRef CAS PubMed.
- Y. Takahashi, K. Ichimori, M. Okano and H. Goto, J. Clin. Biochem. Nutr., 2015, 56, 105–110 CrossRef CAS PubMed.
- D. R. Weinberg, C. J. Gagliardi, J. F. Hull, C. F. Murphy, C. A. Kent, B. C. Westlake, A. Paul, D. H. Ess, D. G. McCafferty and T. J. Meyer, Chem. Rev., 2012, 112, 4016–4093 CrossRef CAS PubMed.
- J. J. Warren, T. A. Tronic and J. M. Mayer, Chem. Rev., 2010, 110, 6961–7001 CrossRef CAS PubMed.
- J. M. Mayer, D. A. Hrovat, J. L. Thomas and W. T. Borden, J. Am. Chem. Soc., 2002, 124, 11142–11147 CrossRef CAS PubMed.
- R. C. Guedes, K. Coutinho, B. J. Costa Cabral and S. Canuto, J. Phys. Chem. B, 2003, 107, 4304–4310 CrossRef.
- K. Suzuki, A. Kobayashi, S. Kaneko, K. Takehira, T. Yoshihara, H. Ishida, Y. Shiina, S. Oishi and S. Tobita, Phys. Chem. Chem. Phys., 2009, 11, 9850–9860 RSC.
- C. Kerzig and M. Goez, Phys. Chem. Chem. Phys., 2014, 16, 25342–25349 RSC.
- M. B. Davies, J. Austin and D. A. Partridge, Vitamin C: Its Chemistry and Biochemistry, The Royal Society of Chemistry, Cambridge, 1st edn, 1991 Search PubMed.
| This journal is © the Owner Societies 2016 |