Open Access Article
Open Access ArticleTip enhanced Raman spectroscopy imaging of opaque samples in organic liquid†
T.
Touzalin
,
A. L.
Dauphin
,
S.
Joiret
,
I. T.
Lucas
* and
E.
Maisonhaute
*
Sorbonne Universités, UPMC Univ Paris 06, UMR 8235, Laboratoire Interfaces et Systèmes Electrochimiques, F-75005 Paris, France. E-mail: ivan.lucas@upmc.fr; emmanuel.maisonhaute@upmc.fr
First published on 17th May 2016
Abstract
Implementation of Tip Enhanced Raman Spectroscopy in liquid is still a challenge. We demonstrate herein its feasibility in an upright illumination/collection configuration. Through a thin layer of organic solvent covering the sample, laser focussing on the tip is possible, enabling TERS imaging in liquid.
Introduction
Beyond topography resolved at a sub-molecular scale, methodologies derived from Scanning Probe Microscopy (SPM) provide a range of useful information about the electrical, optical, mechanical or magnetic properties of the sample under scrutiny.1 SPM can be implemented under various environments ranging from low temperature and ultra-high vacuum conditions to liquid media. This versatility accounts for the very broad scientific area covered by SPM. In research domains like molecular electronics or biology, the stringent need for additional chemical identification at the molecular scale has been so far addressed by UHV Inelastic Scanning Tunneling Spectroscopy derived from Scanning Tunneling Microscopy (STM) or methods derived from Atomic Force Microscopy, for example force spectroscopy.2Near-field scanning optical microscopy (NSOM) associating vibrational spectroscopy (Raman, infrared: IR) and SPM stands as a powerful alternative by providing unambiguous and more precise chemical identification with high sensibility and high spatial resolution. IR-NSOM has been a fast emerging technology, due to the strong IR signature of most compounds and the availability of suitable probes, but its implementation in liquids is really tedious due to the strong IR absorption of usual solvents.3–6 In parallel, the development of Raman-SNOM, i.e. Tip Enhanced Raman Spectroscopy (TERS),7,8 has necessitated tailoring specific probes which support localized surface plasmon resonance close to the apex of the tip, at the so-called “hot spot”.9 When a visible laser light of suitable wavelength is shined on a gold or silver tip, the electric field at the hot spot is considerably amplified and translates into a strong near field Raman scattering signal enhancement at the tip/sample interface.10–14 Low laser excitation power can be therefore applied, minimizing considerably the far-field contribution to the scattered signal. The strong signal enhancement compensates for the low efficiency of the inelastic scattering process of many compounds, enabling detection and imaging of single molecules.15,16 While silver provides the largest enhancement, gold is less prone to oxidation and therefore more adapted to liquids.11,17,18
However, implementation of TERS in liquid also brings out other difficulties. The main one is the delicate optical alignment to achieve accurate focusing of the laser beam on the tip apex and also to ensure an efficient collection of the scattered signal especially through a layer of liquid. Most literature reports describe transparent samples set on an inverted optical microscope; focusing of the laser beam on the TERS probe is therefore easily achieved directly through the sample.19–23 With opaque samples that necessitate top or side illumination/collection, the optical signal is inevitably deviated and considerably attenuated by refraction at each interface. Ren et al. recently elegantly circumvented this problem by mounting the sample in a liquid cell equipped with a side thin glass window and obtained an efficient side illumination of a STM probe enabling single point TERS spectroscopy of a pyridinyl derivative in water.24 The window was positioned about 1 mm apart from the probe. This solution unfortunately freezes the tip vs. sample/window positioning, obliterating TERS sample imaging capabilities in liquid. Moreover for further development of TERS in organic solvent, even single point spectroscopy through 1 mm of liquid is not conceivable. We therefore propose in this work an upright illumination/collection configuration that enables TERS imaging in organic liquid (hexadecane).
Results and discussion
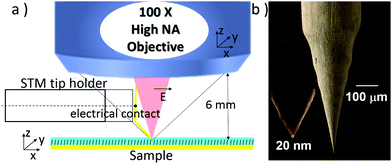
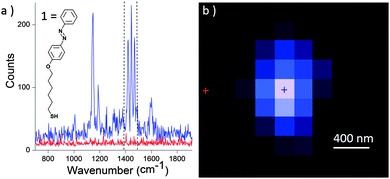
In our setup described in Fig. 1, a polarized excitation laser (λexc = 632.8 nm) is focused on the STM probe apex through a long distance 100× objective (working distance: 6 mm) with high numerical aperture (N.A. = 0.7). Therefore, the STM tip holder can fit between the objective and the sample. An electronic micrograph of a typical tip is presented in Fig. 1b. A gold tip is bent with an angle of 30 to 45° to the normal of the sample as previously depicted by Zenobi et al. in air.25,26 This angle allows the apex of the tip to reach the focal point of the objective for an optimized signal collection and enables a better alignment of the electric field along the shaft of the tip. This also maintains the imaging capability of the STM probe. The objective is mounted onto a piezo positioning system which allows an accurate screening of the optical hotspot on the gold tip, i.e. the position of the laser on the tip giving the maximum enhancement of the Raman signal intensity.The TERS signal of the thioazobenzene derivative 1 (see Fig. 2) self-assembled on a gold surface was first collected in air. The sample was prepared by soaking a gold substrate in a 0.2 mM ethanolic solution of 1, followed by immersion in pure ethanol for 1 h in order to remove any physisorbed molecule (see ESI† for more details). Screening of the tip hotspot is achieved by performing an objective mapping, i.e. a raster scanning of the objective in the directions parallel (XY) and normal (XZ, YZ) to the sample surface synchronized with the registering of the overall Raman signal intensity, while maintaining the tip at the tunnel contact with the sample. Fig. 2a displays two Raman spectra of 1 collected at the exact hot spot location and also slightly away, highlighting respectively the strong near field signal (TERS) and the extremely weak far-field contribution (microRaman) using only 14.1 μW of laser power. Particularly, the bands at 1143, 1415, 1443 and 1465 cm−1 appear with a large signal/noise ratio as already reported in the literature.27,28 The far field signal collected with a higher laser power and longer exposure time is presented in Fig. S1 of ESI.† A typical Raman mapping of the integrated spectrum intensity between 1394 and 1495 cm−1 during a XY objective map is presented in Fig. 2b and confirms the strong enhancement of the signal around the hotspot location and the abrupt drop of the intensity in its close proximity (600 nm).
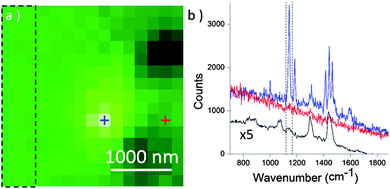
Then, a thin layer of liquid hexadecane was added onto the sample. This sample/solvent combination represents an ideal system to evaluate the possible screening of the sample signal by the surrounding solvent since the Raman spectrum of pure hexadecane presented in Fig. S2 of ESI,† displays several bands that overlap with those of 1. Using this specific solvent that has a low dielectric constant, no electrical insulation of the STM tip is required to avoid current leakage due to electrochemical reactions during tip polarization. The main limitation of our configuration lies in the tip/liquid meniscus that interferes with the optical path. The height and radius of curvature of this meniscus strongly depends on the diameter and angle of the tip. We observed that a liquid thickness of a few micrometers (2–20 μm) however still allowed an accurate focusing of the laser on the tip. Under such conditions, search of the hot spot in liquid became possible as revealed by the XY objective map depicted in Fig. 3a. The spectra at the exact hotspot (blue) and slightly off (red) appear in Fig. 3b. A weak contribution from the solvent is apparent on all collected spectra as revealed on the average spectrum collected for a large area of the Raman map outside the hot spot (black spectra). At the hot spot however, the contribution from 1 largely dominated, demonstrating for the first time that TERS is effective in hexadecane.
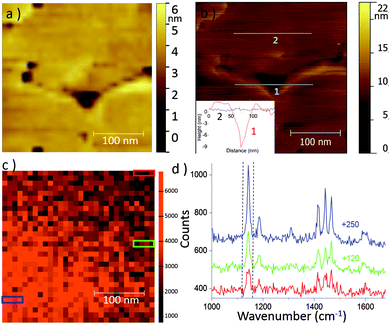
To show that our setup is also suitable for TERS imaging in liquid, a 300 × 300 nm2 area of the sample was scanned in STM mode in hexadecane while recording TERS spectra. With a 30 × 30 pixels2 image and 0.107 s acquisition time per spectrum (laser power = 98 μW), recording a full TERS image took only 97 s. A higher resolution STM image (256 × 256 pixels) was also recorded immediately after. In Fig. 4, the comparison between low and high resolution STM images shows that a 30 × 30 pixels2 image is sufficient to evidence topography details, like pin holes present over the gold surface. Furthermore, in the high resolution image, the topography is well-described meaning that the STM-TERS tip was sharp enough to minimize convolution effects.
A TERS map obtained by integrating the band intensity of 1 between 1125 and 1162 cm−1 is presented in Fig. 4c. It reveals that the signal intensity on the lower left part of the image is more intense. Such local intensity fluctuations would never be detected in microRaman. Fig. 4d displays three spectra obtained by averaging the 4 consecutive spectra inside the 3 three different colored rectangles indicated in Fig. 4c. The lower intensity corresponds to 40% of the highest one. Note that the scan proceeded from left to right and bottom to top. To explain the drop in intensity along the vertical direction of the raster scan, a change in the laser focusing on the tip may be invoked. This could originate from thermal effect induced by laser irradiation, altering either the refraction at the air/liquid interface or the meniscus height. Two XY objective maps recorded before and after TERS mapping of the sample (Fig. S3, ESI†), within a 17 min interval, show however a signal loss of at most 20% but also a slight shift of the hot spot position (at most 200 nm). This slight progressive defocusing cannot therefore account for all the signal fluctuation on the TERS sample map. Furthermore, defocusing of the laser cannot be responsible for variations along the horizontal direction. We therefore rather incriminate a local variation in the surface coverage of 1 due to non-homogenous SAM formation on gold, possibly combined with a modification of the molecular orientation of 1 on the surface. This is probably due to the SAM preparation.27,28 As a consequence of this surface concentration change, no relation between the topography and the Raman intensity could be clearly established.29,30 This experiment illustrates that TERS imaging is operational in organic solvent using an upright configuration. The next stage will be to precisely determine the spatial resolution of TERS in liquid by imaging small nanoobjects in a similar way to what was recently performed in air with carbon nanotubes.31
Conclusions
This work demonstrates that it is possible, using an upright optical configuration, to implement TERS imaging of an opaque sample in contact with an organic liquid, with fast acquisition time and minimal laser power. With this set-up, the efficient excitation/collection of the scattered Raman signal enables extraction of the chemical signature of a molecular monolayer from the one of the surrounding solvent. To achieve such performances, the liquid thickness has to be optimized (around 20 μm at most here), in order to reduce the distortion of the optical path and the loss of signal at the tip/liquid interface (meniscus). These promising results pave the road to TERS imaging in other solvents or electrolytes.22,24,32–34Acknowledgements
This work was funded by UPMC, CNRS, the FP7-People program (CIC) of the European union, the Labex “MiChem” (ANR-11-IDEX-0004-02) and the Region Ile de France through the framework DIM Nano-K. We also thank Dr. Marc Chaigneau (Horiba) for helpful discussion.References
-
C. J. Chen, Introduction to Scanning Tunneling Microscopy, Oxford University Press, Oxford, 2nd edn, 2007 Search PubMed
.
- J. Landoulsi and V. Dupres, Phys. Chem. Chem. Phys., 2013, 15, 8429–8440 RSC
.
- A. Deniset-Besseau, C. B. Prater, M. J. Virolle and A. Dazzi, J. Phys. Chem. Lett., 2014, 5, 654–658 CrossRef CAS PubMed
.
- S. Heedy, M. Lo, E. Dillon, Q. C. Hu, C. Prater, R. Shetty, K. Kjoller, C. Marcott, A. Dazzi and C. Yip, Biophys. J., 2014, 106, 205A CrossRef
.
- I. T. Lucas, A. S. McLeod, J. S. Syzdek, D. S. Middlemiss, C. P. Grey, D. N. Basov and R. Kostecki, Nano Lett., 2015, 15, 1–7 CrossRef CAS PubMed
.
- O. Khatib, J. D. Wood, A. S. McLeod, M. D. Goldflam, M. Wagner, G. L. Damhorst, J. C. Koepke, G. P. Doidge, A. Rangarajan, R. Bashir, E. Pop, J. W. Lyding, M. H. Thiemens, F. Keilmann and D. N. Basov, ACS Nano, 2015, 9, 7968–7975 CrossRef CAS PubMed
.
- B. Pettinger, G. Picardi, R. Schuster and G. Ertl, Electrochemistry, 2000, 68, 942–949 CAS
.
- R. M. Stockle, Y. D. Suh, V. Deckert and R. Zenobi, Chem. Phys. Lett., 2000, 318, 131–136 CrossRef CAS
.
- X. Wang, Z. Liu, M. D. Zhuang, H. M. Zhang, Z. X. Xie, D. Y. Wu, B. Ren and Z. Q. Tian, Appl. Phys. Lett., 2007, 91, 101105 CrossRef
.
- Z. Yang, J. Aizpurua and H. Xu, J. Raman Spectrosc., 2009, 40, 1343–1348 CrossRef CAS
.
-
E. C. Le Ru and P. G. Etchegoin, Principles of Surface Enhanced Raman Spectroscopy and related phenomena, Elsevier Science Bv, Amsterdam, 2009 Search PubMed
.
- T. Schmid, L. Opilik, C. Blum and R. Zenobi, Angew. Chem., Int. Ed., 2013, 52, 5940–5954 CrossRef CAS PubMed
.
- L. Langeluddecke, P. Singh and V. Deckert, Appl. Spectrosc., 2015, 69, 1357–1371 CrossRef PubMed
.
- C. Blum, L. Opilik, J. M. Atkin, K. Braun, S. B. Kammer, V. Kravtsov, N. Kumar, S. Lemeshko, J. F. Li, K. Luszcz, T. Maleki, A. J. Meixner, S. Minne, M. B. Raschke, B. Ren, J. Rogalski, D. Roy, B. Stephanidis, X. Wang, D. Zhang, J. H. Zhong and R. Zenobi, J. Raman Spectrosc., 2014, 45, 22–31 CrossRef CAS
.
- W. H. Zhang, B. S. Yeo, T. Schmid and R. Zenobi, J. Phys. Chem. C, 2007, 111, 1733–1738 CAS
.
- R. Zhang, Y. Zhang, Z. C. Dong, S. Jiang, C. Zhang, L. G. Chen, L. Zhang, Y. Liao, J. Aizpurua, Y. Luo, J. L. Yang and J. G. Hou, Nature, 2013, 498, 82–86 CrossRef CAS PubMed
.
- P. Aubertin, M. Aissa, N. Raouafi, S. Joiret, A. Courty and E. Maisonhaute, Nano Res., 2015, 8, 1615–1626 CrossRef CAS
.
- E. Sutter, K. Jungjohann, S. Bliznakov, A. Courty, E. Maisonhaute, S. Tenney and P. Sutter, Nat. Commun., 2014, 5, 4946 CrossRef CAS PubMed
.
- C. Hoppener and L. Novotny, Nano Lett., 2008, 8, 642–646 CrossRef PubMed
.
- C. Hoppener and L. Novotny, Nanotechnology, 2008, 19, 384012 CrossRef PubMed
.
- A. Nakata, T. Nomoto, T. Toyota and M. Fujinami, Anal. Sci., 2013, 29, 865–869 CrossRef CAS PubMed
.
- D. Kurouski, M. Mattei and R. P. Van Duyne, Nano Lett., 2015, 15, 7956–7962 CrossRef CAS PubMed
.
- T. Schmid, B. S. Yeo, G. Leong, J. Stadler and R. Zenobi, J. Raman Spectrosc., 2009, 40, 1392–1399 CrossRef CAS
.
- Z.-C. Zeng, S.-C. Huang, D.-Y. Wu, L.-Y. Meng, M.-H. Li, T.-X. Huang, J.-H. Zhong, X. Wang, Z.-L. Yang and B. Ren, J. Am. Chem. Soc., 2015, 137, 11928–11931 CrossRef CAS PubMed
.
- J. Stadler, T. Schmid and R. Zenobi, Nano Lett., 2010, 10, 4514–4520 CrossRef CAS PubMed
.
- J. Stadler, B. Oswald, T. Schmid and R. Zenobi, J. Raman Spectrosc., 2013, 44, 227–233 CrossRef CAS
.
- G. Picardi, M. Chaigneau, R. Ossikovski, C. Licitra and G. Delapierre, J. Raman Spectrosc., 2009, 40, 1407–1412 CrossRef CAS
.
- H. K. Wickramasinghe, M. Chaigneau, R. Yasukuni, G. Picardi and R. Ossikovski, ACS Nano, 2014, 8, 3421–3426 CrossRef CAS PubMed
.
- P. Z. El-Khoury, Y. Gong, P. Abellan, B. W. Arey, A. G. Joly, D. H. Hu, J. E. Evans, N. D. Browning and W. P. Hess, Nano Lett., 2015, 15, 2385–2390 CrossRef CAS PubMed
.
- W. H. Zhang, X. D. Cui, B. S. Yeo, T. Schmid, C. Hafner and R. Zenobi, Nano Lett., 2007, 7, 1401–1405 CrossRef CAS PubMed
.
- C. Chen, N. Hayazawa and S. Kawata, Nat. Commun., 2014, 5, 3312 Search PubMed
.
- V. Climent, J. D. Zhang, E. P. Friis, L. H. Ostergaard and J. Ulstrup, J. Phys. Chem. C, 2012, 116, 1232–1243 CAS
.
- X. S. Zhou, B. W. Mao, C. Amatore, R. G. Compton, J. L. Marignier, M. Mostafavi, J. F. Nierengarten and E. Maisonhaute, Chem. Commun., 2016, 52, 251–263 RSC
.
- P. Fortgang, E. Maisonhaute, C. Amatore, B. Delavaux-Nicot, J. Iehl and J. F. Nierengarten, Angew. Chem., Int. Ed., 2011, 50, 2364–2367 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and methods, supplementary Raman spectra and XY objective scan maps. See DOI: 10.1039/c6cp02596j |
| This journal is © the Owner Societies 2016 |