Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Microwave-enhanced electrochemical cycling performance of the LiNi0.2Mn1.8O4 spinel cathode material at elevated temperature
Kumar
Raju
a,
Funeka P.
Nkosi
ab,
Elumalai
Viswanathan
c,
Mkhulu K.
Mathe
a,
Krishnan
Damodaran
c and
Kenneth I.
Ozoemena
*ab
aEnergy Materials, Materials Science and Manufacturing, Council for Scientific and Industrial Research (CSIR), Pretoria 0001, South Africa. E-mail: kozoemena@csir.co.za
bMolecular Sciences Institute, School of Chemistry, University of the Witwatersrand, Johannesburg PO Wits 2050, South Africa
cDepartment of Chemistry, University of Pittsburgh, Pittsburgh, PA 15260, USA
First published on 11th April 2016
Abstract
The well-established poor electrochemical cycling performance of the LiMn2O4 (LMO) spinel cathode material for lithium-ion batteries at elevated temperature stems from the instability of the Mn3+ concentration. In this work, a microwave-assisted solid-state reaction has been used to dope LMO with a very low amount of nickel (i.e., LiNi0.2Mn1.8O4, herein abbreviated as LMNO) for lithium-ion batteries from Mn3O4 which is prepared from electrolytic manganese oxide (EMD, γ-MnO2). To establish the impact of microwave irradiation on the electrochemical cycling performance at an elevated temperature (60 °C), the Mn3+ concentration in the pristine and microwave-treated LMNO samples was independently confirmed by XRD, XPS, 6LiMAS-NMR and electrochemical studies including electrochemical impedance spectroscopy (EIS). The microwave-treated sample (LMNOmic) allowed for the clear exposure of the {111} facets of the spinel, optimized the Mn3+ content, promoting structural and cycle stability at elevated temperature. At room temperature, both the pristine (LMNO) and microwave-treated (LMNOmic) samples gave comparable cycling performance (>96% capacity retention and ca. 100% coulombic efficiency after 100 consecutive cycling). However, at an elevated temperature (60 °C), the LMNOmic gave an improved cycling stability (>80% capacity retention and ca. 90% coulombic efficiency after 100 consecutive cycling) compared to the LMNO. For the first time, the impact of microwave irradiation on tuning the average manganese redox state of the spinel material to enhance the cycling performance of the LiNi0.2Mn1.8O4 at elevated temperature and lithium-ion diffusion kinetics has been clearly demonstrated.
Introduction
Rechargeable lithium-ion batteries (RLIBs) have emerged as the most dominant power sources for portable electronics and electric vehicles and will remain so for many years to come. Spinel, layered and olivine materials are the most important cathode materials for the RLIBs. Manganese-based spinel materials have become more attractive due to their inherent advantageous properties such as earth-abundance, low-cost, environmental benignity and satisfactory thermal stability.1–6 Lithium manganese oxide, LiMn2O4 (LMO), a spinel cathode material for RLIBs is a cathode material that drives some electric vehicles (e.g., Nissan Leaf and Chevrolet Volt). Two main challenges that this cathode material still confront are (i) the disproportionation reaction and (ii) Jahn–Teller effects, especially at elevated temperatures (55 °C). Indeed, the LMO combined with a robust spinel framework exhibits an astonishing cycle life associated with substantial capacity retention at room temperature, which has been intensively studied.7–11 However, the LMO suffers from rapid capacity-fading with cycling due to the manganese-dissolution at an elevated temperature which limits the practical use of this spinel material for large-scale applications. In particular, the manganese-dissolution is owed to the disproportionate reaction of Mn3+ with Mn2+ and Mn4+ ions; Mn2+ dissolves into the electrolyte, gets reduced to a metal and deposited as a film on the surface of the anode, whilst Mn4+ promotes the electro–inactive layer on the cathode side, creating defects in the lattice.12–17Disproportional reaction:
| 2Mn(solid)3+ → Mn(solid)4+ + Mn(solution)2+ | (1) |
The above named strategies for ameliorating capacity-fading in LMO at elevated temperature are either time-consuming or involve the extensive use of materials that could add to the cost of the final cathode material, and/or require the use of less environmentally-friendly chemicals including very harsh acids that must be handled with utmost caution. In this work, we have chosen 0.2 nickel (i.e., Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 9) since it has been shown that such a mole ratio is capable of stabilizing the M–O bonding in the [MnO6] octahedron than other mole ratios, leading to enhanced performance in electrochemical cycling.28 Therefore there is a need to explore the use of a simpler, fast, less expensive and ‘greener’ synthesis strategy. Motivated by this need, coupled with the realisation that the LMO chemistry can be improved by nickel-doping28,29 and microwave-irradiation,30,31 this new study demonstrates that by doping LMO with a very small amount of nickel (LiMn1.8Ni0.2O4) and subjecting it to microwave irradiation, one is able to manipulate the Mn3+/Mn4+ concentrations, expose the prominent {111} facets, stabilize the spinel structure, and enhance the cycling performance at an elevated temperature (60 °C). To our knowledge, this is the first report of the use of microwave-assisted preparation of doped-LMO for improving the performance of LMO under high temperature operating conditions. Interestingly, LiMn1.8Ni0.2O4 was obtained from an industrially-preferred solid-state method using Mn3O4 obtained from low-cost electrolytic manganese oxide (EMD, γ-MnO2).
Ni = 9) since it has been shown that such a mole ratio is capable of stabilizing the M–O bonding in the [MnO6] octahedron than other mole ratios, leading to enhanced performance in electrochemical cycling.28 Therefore there is a need to explore the use of a simpler, fast, less expensive and ‘greener’ synthesis strategy. Motivated by this need, coupled with the realisation that the LMO chemistry can be improved by nickel-doping28,29 and microwave-irradiation,30,31 this new study demonstrates that by doping LMO with a very small amount of nickel (LiMn1.8Ni0.2O4) and subjecting it to microwave irradiation, one is able to manipulate the Mn3+/Mn4+ concentrations, expose the prominent {111} facets, stabilize the spinel structure, and enhance the cycling performance at an elevated temperature (60 °C). To our knowledge, this is the first report of the use of microwave-assisted preparation of doped-LMO for improving the performance of LMO under high temperature operating conditions. Interestingly, LiMn1.8Ni0.2O4 was obtained from an industrially-preferred solid-state method using Mn3O4 obtained from low-cost electrolytic manganese oxide (EMD, γ-MnO2).
Experimental
Reagents and synthesis of LMNO and LMNOmic
First, the manganese precursor material (Mn3O4) was obtained from electrolytic manganese dioxide (MnO2 = 92.46% purity, Delta EMD (Pty) Ltd, South Africa) by using the established method of high-temperature annealing at 1050 °C in air for 74 h.32 The purity and morphology of Mn3O4 were established from SEM, TEM and XRD. NiO (>99% pure), Li2CO3 (>99% pure) and urea (>99% pure) were obtained from Sigma-Aldrich and used without further treatment. LiNi0.2Mn1.8O4 (LMNO) was synthesized using a similar method reported by Yang et al.33 In brief, stoichiometric amounts of reagents Li2CO3, NiO and the as-prepared Mn3O4 (molar ratio of Li![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn
Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 1.15
Ni = 1.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8
1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2) were ground using a mortar and pestle. A 10% excess of Li2CO3 was used to compensate for the easy loss of Li at high temperature heating. Urea (0.57 M per lithium) was added to the mixture and then ground to fine powder. The mixture was then preheated at 500 °C for about 7 min. Upon cooling down to room temperature in air, the preheated spinel precursor was ground into fine powder and then divided into two equal portions; the first portion was directly annealed at 900 °C for 6 h, while the second portion was subjected to microwave irradiation at 600 W for 20 min (using the Anton Paar Multiwave 3000 system, λ = 0.12236 m) before annealing at 900 °C for 6 h. The spinel materials without and with microwave irradiation are abbreviated herein as LMNO and LMNOmic, respectively.
0.2) were ground using a mortar and pestle. A 10% excess of Li2CO3 was used to compensate for the easy loss of Li at high temperature heating. Urea (0.57 M per lithium) was added to the mixture and then ground to fine powder. The mixture was then preheated at 500 °C for about 7 min. Upon cooling down to room temperature in air, the preheated spinel precursor was ground into fine powder and then divided into two equal portions; the first portion was directly annealed at 900 °C for 6 h, while the second portion was subjected to microwave irradiation at 600 W for 20 min (using the Anton Paar Multiwave 3000 system, λ = 0.12236 m) before annealing at 900 °C for 6 h. The spinel materials without and with microwave irradiation are abbreviated herein as LMNO and LMNOmic, respectively.
Characterization techniques
The XRD patterns of the as-prepared Mn3O4, LMNO and LMNOmic were obtained from a PANalytical X'Pert PRO diffractometer equipped with Ni-filtered Cu K-alpha radiation (λ = 1.541841 Å). X-ray photoelectron spectroscopy (XPS) was performed for LMNO and LMNOmic using a non-monochromatic aluminum (Al) Kα source (1486.6 eV) and an Al monochromatic Kα source (1486.6 eV), respectively. The XPS data analysis was performed with the XPS Peak 4.1 program and a Shirley function was used to subtract the background. The morphology of the as-synthesized powders was analysed using a JEOL-JSM 7500F scanning electron microscope operated at 2.0 kV. TEM and HRTEM images were obtained from a JEOL-Jem 2100 microscope operated at an acceleration voltage of 200 kV. All the NMR experiments were performed on a Bruker Avance 500 MHz (B0 = 11.7 Tesla) Wide bore spectrometer. 6Li and 7Li NMR measurements were done at corresponding Larmor frequencies of 73.59 and 194.36 MHz respectively using a 3.2 mm CPMAS probe. 6Li NMR was collected using a rotor synchronized Hahn echo sequence (90-tau-180-tau acquisition) at a 20 kHz spinning speed. 90° pulse lengths of 6 μs and a relaxation delay of 0.5 s were used. 7Li NMR was collected using a single pulse at MAS rates of 17, 20, 23 kHz for identifying the center bands. 2 μs pulse was used for excitation (90° pulse was 4.6 μs) and a relaxation delay of 0.5 s was used. All the spectra were referenced to standard 1 M LiCl solution at 0 ppm. All the electrochemical analyses were carried out in a coin cell (LIR-2032) fabricated with as-prepared LMNO and LMNOmic as the positive electrodes and lithium metal foil as the negative using a MACCOR series 4000 tester. The positive electrodes were prepared by coating the slurry mixture of the electrode material, acetylene black and polyvinylidene fluoride (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) onto a cleaned and polished aluminium foil, and dried in a vacuum oven at 80 °C overnight. The cells were assembled in an argon-filled MBraun® glovebox (O2, H2O < 0.5 ppm). The electrolyte was 1 M LiPF6 in a mixture of 1
10) onto a cleaned and polished aluminium foil, and dried in a vacuum oven at 80 °C overnight. The cells were assembled in an argon-filled MBraun® glovebox (O2, H2O < 0.5 ppm). The electrolyte was 1 M LiPF6 in a mixture of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) ethylene carbonate (EC)/dimethyl carbonate (DMC) while Cellgard 2300 was used as the separator. The cyclic voltammetry (CV) and electrochemical impedance (EIS) analysis were carried out on a Bio-Logic VMP3 potentiostat/galvanostat controlled by EC-Lab v10.40 software.
1 (v/v) ethylene carbonate (EC)/dimethyl carbonate (DMC) while Cellgard 2300 was used as the separator. The cyclic voltammetry (CV) and electrochemical impedance (EIS) analysis were carried out on a Bio-Logic VMP3 potentiostat/galvanostat controlled by EC-Lab v10.40 software.
Results and discussion
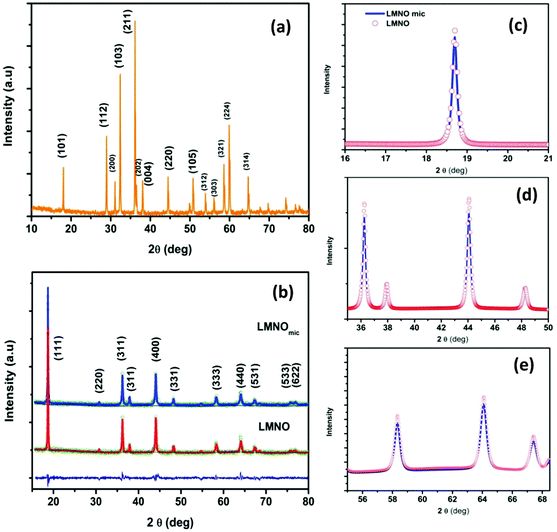
Fig. 1a shows the XRD pattern of the as-prepared Mn3O4 with peaks that can be indexed to the tetragonal Mn3O4 spinel (JCPDS no: 80-0382) with space group I41/amd. The obtained well-defined reflections with no impurity phases indicate that the as-prepared Mn3O4 is highly crystalline. Fig. 1b shows the XRD profiles of LMNO and LMNOmic with their Rietveld refinement. This confirms that the prepared materials have typical single phase spinel structures, which adopt the Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group with lithium and transition metals located in 8a and 16d sites respectively. The calculated lattice parameters of the pristine (a = 8.211 Å) and microwave treated (a = 8.213 Å) are essentially the same and are in agreement with the literature.34 All the three axes of cubic spinel are the same, thus a = b = c. We performed Rietveld refinement with Rp = 2.278 and Rwp = 2.922 for the LMNO, and Rp = 2.338, Rwp = 2.987 values for the LMNOmic powders. A closer look at the XRD profiles (Fig. 1c–e) indicates that the LMNOmic shows less intense peaks than the pristine LMNO, which suggests that the value of full width at half maximum (FWHM) for the LMNO-mic will be somewhat larger for the LMNOmic and it contains smaller sized particles compared to the LMNO.
m space group with lithium and transition metals located in 8a and 16d sites respectively. The calculated lattice parameters of the pristine (a = 8.211 Å) and microwave treated (a = 8.213 Å) are essentially the same and are in agreement with the literature.34 All the three axes of cubic spinel are the same, thus a = b = c. We performed Rietveld refinement with Rp = 2.278 and Rwp = 2.922 for the LMNO, and Rp = 2.338, Rwp = 2.987 values for the LMNOmic powders. A closer look at the XRD profiles (Fig. 1c–e) indicates that the LMNOmic shows less intense peaks than the pristine LMNO, which suggests that the value of full width at half maximum (FWHM) for the LMNO-mic will be somewhat larger for the LMNOmic and it contains smaller sized particles compared to the LMNO.
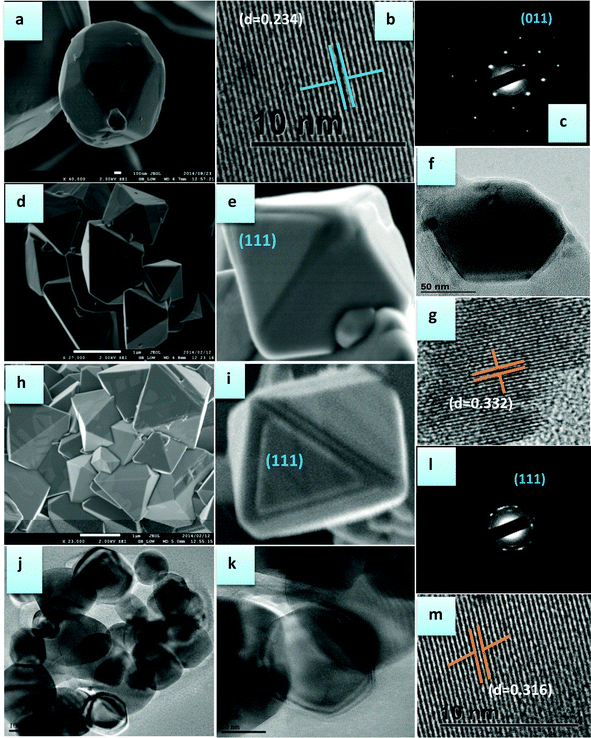
Fig. 2 depicts the morphological evolutions (SEM and TEM images) of the Mn3O4, LMNO and LMNO-mic samples. It is clear from the figures that the as-prepared Mn3O4 exhibits a tetragonally-distorted spinel morphology in micron size (Fig. 2a). From the HRTEM image of lattice fringes (Fig. 2b), it has an average neighbouring fringe with a distance of 0.234 nm, corresponding to the {011} facets. This is also confirmed by using SAED patterns (Fig. 2c).
Fig. 2d–g show the SEM images of LMNO, the magnified view of one well-defined octahedron particle display {111} facets (Fig. 2g) and the HRTEM image of the lattice fringe of LMNO of 0.332 nm. Fig. 2h–m show the SEM images of LMNOmic, the magnified view of one well-defined octahedron particle display {111} facets (Fig. 2m) and the HRTEM image of the lattice fringe of LMNO of 0.316 nm with corresponding SAED patterns of LMNOmic (Fig. 2l). Although the SEM images of LMNO and LMNO-mic exhibited almost the same octahedron morphology, there is a slight change in the particle sizes. Generally, the LMNOmic shows smaller sized particles (<1 μm) while those of the LMNO are in the 1 μm range. From HRTEM studies, it was difficult to observe smaller sized particles for the LMNO whereas the LMNOmic (Fig. 2j and k) easily showed particles with small sizes (100–200 nm range). EDX confirmed the stoichiometry of LMNO and LMNOmic as LiMn1.8Ni0.2O4.
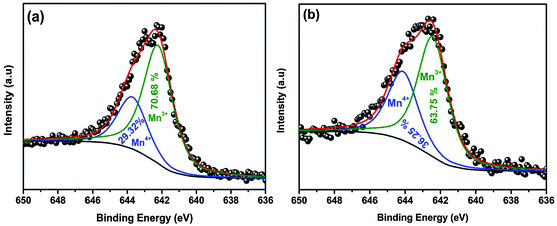
Fig. 3 compares the XPS of deconvoluted Mn 2p3/2 peaks of the LMNO and LMNOmic samples. Each of the deconvoluted spectra gives two binding energy values corresponding to the characteristic peaks of Mn3+/Mn4+ at 642.17/643.59 and 642.43/644.12 eV, and the values are very close in accordance with reported values. The Ni 2p peak could not be viewed owing to its low intensity as a result from the low Ni content in the samples. Table 1 summarizes the XPS data, including the ratio of Mn3+/Mn4+ and manganese valence of the LMNO and LMNOmic. From the XPS data in Table 1, the LMNOmic contains more of Mn4+ in its structure than in the LMNO. Evidently, microwave irradiation was able to tune the ratio of Mn3+/Mn4+ from 2.41 (for LMNO) to 1.75 (for LMNOmic), with the manganese average redox state (Mn valence) being 3.29 and 3.36 for LMNO and LMNOmic, respectively.
| Sample | Binding energy position (eV) | Cation distribution | Mn valence (average redox state) | |||
|---|---|---|---|---|---|---|
| Mn4+ | Mn3+ | Mn4+ (%) | Mn3+ (%) | Mn3+/Mn4+ | ||
| LMNO | 643.59 | 642.17 | 29.32 | 70.68 | 2.41 | 3.293 |
| LMNOmic | 644.12 | 642.43 | 36.25 | 63.75 | 1.75 | 3.363 |
Solid-state 6Li Magic Angle Spinning Nuclear Magnetic Resonance (6Li MAS-NMR) spectroscopy is highly sensitive to the local environment of the Li ion. 6Li MAS-NMR analyses were performed on LMNO and LMNOmic in order to investigate the effect of microwave irradiation on the local environment of Li ions. Fig. 4 illustrates the 6Li MAS-NMR spectra of LMNO and LMNOmic that were recorded at a spinning speed of 20 kHz. The dominant interactions between Li nuclear spins and Mn electron spins are either through-bond (Fermi-contact) or through-space (dipolar) interactions. Fermi-contact interactions shift Li NMR resonances to >500 ppm, whereas the electron-nuclear dipolar interactions give rise to large spinning sideband patterns during MAS-NMR. For instance, the spinel LiMn2O4 gives major resonance of Li nuclei around ∼520 ppm corresponding to the mixed-valence of Mn3+ and Mn4+ ions (the average oxidation state of Mn3.5+, i.e., cation distribution is 50% Mn3+ and 50% Mn4+). If the average oxidation state of Mn (i.e., Mn valence) around the local lithium site is increased, then the lithium chemical shift moves further downfield of 520 ppm.35–37 This increase in the oxidation state of manganese ions in the local environment of lithium results in a shift of the resonance to higher frequency. As shown in Fig. 4, two major 6Li resonances are observed at 528.4 and 482.0 ppm for LMNOmic and 528.0 and 478.2 ppm for LMNO, which can be assigned to the tetrahedrally coordinated Li+ surrounded by Ni2+ and Mn4+ ions.38 The slight shift of the peaks to the lower frequencies is attributed to the increased Mn3+ content in the LMNO. Importantly, the peaks in the high frequency region (750–850 ppm) are due to Mn4+, thus the broad high frequency resonance observed at 814.3 ppm for the LMNOmic clearly indicates that the microwave irradiation causes a further increase in Mn4+ concentration around Li+ which, in turn, results in the chemical shift of lithium resonance moving further downfield. Indeed, the 6Li MAS-NMR spectra clearly corroborate the XPS and XRD results that predict higher concentration of the Mn3+ in the LMNO than in the LMNOmic spinel structure.
Cyclic voltammetry (CV) studies were performed on LMNO and LMNOmic at room temperature at a scan rate of 0.1 mV s−1 in order to investigate the diffusion kinetics of lithium. Fig. 5a illustrates the CV profiles of LMNO and LMNOmic recorded in the potential window of 2 to 4.9 V vs. Li/Li+ that display very prominent anodic and cathodic peaks corresponding to the lithium extraction and insertion kinetics. The well-resolved pair of anodic peaks at 4.73 and 4.8 and their corresponding cathodic peaks at 4.6 and 4.7 V are related to the oxidation of Ni2+/Ni3+ and Ni3+/Ni4+ as well as Li extraction/insertion processes. Similarly, the shoulder peaks observed for both anodic (4.12 and 4.24 V) and cathodic (3.89 and 4.05 V) regions are characteristic of Mn3+/Mn4+ redox reactions despite having a small difference in cathodic peaks (3.87 and 4.06 V) for the microwave irradiated sample. It is noteworthy to mention that the anodic peak obtained at 3.85 V associated with the extraction of lithium resides at the octahedral sites. The peak intensity increases with an increase in cycling as a result of an incomplete extraction of lithium that occupies the octahedral sites at 3.12 V. In addition, the strong peaks resolved at 3.1 V for the anodic region and at 2.73 V for the cathodic region imply that the intercalation of lithium is more pronounced even below 3 V, the sharp peaks indicate that the as-prepared spinel has substantial stability even below 3 V.
Fig. 5b compares the galvanostatic charge–discharge of LMNO and LMNOmic samples at room temperature. The curves are typical signatures of LiMn1.8Ni0.2O4.29 The well-defined plateaus around 4.73, 4.12 and 4.05 V regions for both samples confirm the redox reactions of Ni and Mn. The pristine and microwave irradiated samples delivered comparable specific capacities of 121 and 108 mA h g−1 at room temperature, respectively. Both samples retained more than 95% of their initial capacity after 100 cycles and maintained excellent coulombic efficiency (Fig. 4c). Fig. 5d showed that both samples showed comparable rate capability, especially at 2C. At an elevated temperature of 60 °C (Fig. 5e & f), the LMNOmic outperformed the pristine LMNO-based cell, with a specific capacity of 108 mA h g−1 and good cycling stability, retaining ∼80% of its initial capacity with 90% coulombic efficiency after 100 repeated cycles. The LMNO delivered an initial capacity of 118 mA h g−1 at 60 °C but the cycling stability is very poor, retaining about 40% of its initial capacity after 100 cycles. At room temperature, both LMNO and LMNOmic exhibited almost similar electrochemical performances in terms of specific capacity and capacity retention. The microwave-treated spinel with a slightly higher average Mn redox state exhibited good cycle stability at 60 °C than the pristine LMNO. We may conclude that the high capacity retention of the LMNOmic at elevated temperature is due to the ability of the microwave irradiation to tune the Mn valence or the Mn3+/Mn4+ ratio to an appropriate level for enhanced electrochemical performance. The poor performance of the LMNO at 60 °C may be related to its higher Mn3+ content which may be taking part in the disproportionation reaction. Despite the fact that both spinel materials have the same surface {111} facets, the slightly higher content of Mn3+ in pristine may still allow the disproportionation reaction to occur.
To provide further insights into the effects of microwave-treatment on the spinel materials, EIS experiments were carried out on LMNO- and LMNOmic-based coin cells at different voltages after the freshly prepared cells were relaxed at OCV for 1 h. Fig. 6a–d illustrate the Nyquist plots of LMNO and LMNOmic obtained before and after 50 charge–discharge cycles at 60 °C. All the obtained EIS curves were satisfactorily fitted with an electrical equivalent circuit shown in Fig. 6e. The fitting parameters consist of a depressed semicircle at a high and intermediate frequency domain related to the solid–electrolyte interface (SEI) whose resistance is denoted as Rf, constant-phase element of surface film (Qf or CPEf), interfacial capacitance (CLi), the solution bulk resistance (Rs), the charge transfer resistance of Li ion insertion and extraction (RLi), the Warburg impedance (Zw) due to the Li ion diffusion in the solid state.39–42 As seen in Fig. 6a–d, the formation of a SEI layer becomes more stable for LMNOmic at all the voltage range at the elevated temperature after 50 consecutive charge–discharge cycles, which results in the appearance of a high frequency semicircle at all potentials investigated. The thin SEI layer formed may be because the octahedral shaped particles with {111} facets suppress the co-insertion of the electrolyte and deliver larger lithium diffusion.43Tables 2 and 3 summarise the values from the fitted Nyquist plots using the EEC for the LMNO and LMNOmic, respectively. From Fig. 6 and Tables 2 and 3, it is evident that the poor cycling performance of the LMNO at elevated temperature may be related to a systematic rise in impedance arising from Mn3+ dissolution.
| Applied potential (V) | EIS data for LMNO @ 60 °C | ||||||
|---|---|---|---|---|---|---|---|
| R s (Ω) | R f (Ω) | CPE (μF) | n | C Li (mF) | R Li (Ω) | Z w (Ω ω−1/2) | |
| Before cycling | |||||||
| 4.0 | 8.25 ± 0.43 | 49.51 ± 6.48 | 25.93 ± 2.02 | 0.72 ± 0.14 | 13.7 ± 1.8 | 120 ± 1.26 | 20.36 ± 0.8 |
| 4.1 | 7.78 ± 0.52 | 41.84 ± 6.86 | 17.44 ± 0.75 | 0.73 ± 0.05 | 14.53 ± 5.76 | 54.64 ± 1.8 | 8.10 ± 0.97 |
| 4.2 | 6.05 ± 0.45 | 54.53 ± 2.06 | 15.79 ± 6.59 | 0.72 ± 0.18 | 11.6 ± 0.76 | 49.77 ± 3.64 | 6.94 ± 0.86 |
| 4.8 | 5.54 ± 0.45 | 54.08 ± 2 | 17.08 ± 7.09 | 0.78 ± 0.21 | 9.51 ± 0.43 | 51.67 ± 2.49 | 5.22 ± 0.78 |
| After 50th cycle | |||||||
| 4.0 | 11.14 ± 0.57 | 762 ± 3 | 8.13 ± 0.42 | 0.72 ± 0.24 | 17.24 ± 1.42 | 236 ± 6 | 34.21 ± 1.24 |
| 4.1 | 6.35 ± 0.84 | 531 ± 7 | 6.42 ± 0.74 | 0.67 ± 0.17 | 11.9 ± 1.34 | 241 ± 3 | 28.87 ± 3.72 |
| 4.2 | 2.62 ± 0.65 | 515 ± 15 | 1.35 ± 0.28 | 0.68 ± 0.51 | 10.08 ± 1.04 | 383 ± 17 | 47.55 ± 0.78 |
| 4.8 | 5.43 ± 0.53 | 549 ± 20 | 5.92 ± 0.48 | 0.75 ± 0.57 | 1.41 ± 0.26 | 260 ± 4 | 36.98 ± 2.53 |
| Applied potential (V) | EIS data for LMNO-mic @ 60 °C | ||||||
|---|---|---|---|---|---|---|---|
| R s (Ω) | R f (Ω) | CPE (μF) | n | C Li (mF) | R Li (Ω) | Z w (Ω ω−1/2) | |
| Before cycling | |||||||
| 4.0 | 2.76 ± 0.34 | 11.15 ± 2.63 | 9.58 ± 0.45 | 0.72 ± 0.03 | 9.69 ± 0.9 | 46.51 ± 2.53 | 6.27 ± 2.62 |
| 4.1 | 2.58 ± 0.41 | 20.58 ± 3.57 | 9.37 ± 6.4 | 0.67 ± 0.13 | 25.5 ± 5.4 | 46.46 ± 3.91 | 10.07 ± 0.7 |
| 4.2 | 2.59 ± 0.39 | 17.13 ± 1.7 | 33.56 ± 18.05 | 0.52 ± 0.07 | 9.16 ± 6.6 | 42.46 ± 3.77 | 7.2 ± 2.4 |
| 4.8 | 5.15 ± 0.42 | 132.3 ± 20.6 | 12.41 ± 0.47 | 0.72 ± 0.15 | 11.44 ± 1.94 | 114 ± 1.68 | 12.97 ± 1.44 |
| After 50th cycle | |||||||
| 4.0 | 4.13 ± 0.48 | 409.3 ± 2.23 | 9.74 ± 0.68 | 0.63 ± 0.05 | 5.44 ± 0.26 | 272.3 ± 9.42 | 51.66 ± 13.19 |
| 4.1 | 3.86 ± 0.21 | 173.35 ± 2.54 | 10.23 ± 0.57 | 0.61 ± 0.18 | 3.63 ± 0.38 | 94.68 ± 2.81 | 32.24 ± 5.34 |
| 4.2 | 2.17 ± 0.34 | 164.71 ± 1.72 | 8.71 ± 1.73 | 0.64 ± 0.13 | 7.11 ± 1.53 | 105.41 ± 5.26 | 29.89 ± 2.71 |
| 4.8 | 1.86 ± 0.51 | 179.43 ± 2.78 | 9.18 ± 0.82 | 0.69 ± 0.57 | 4.69 ± 0.86 | 96.82 ± 2.37 | 36.22 ± 4.39 |
Lithium diffusion coefficients (DLi) of the LMNO- and LMNOmic-based coin cells were determined before and after 50 consecutive cycles at 25 °C and 60 °C respectively by using equation (eqn (2) and (3)) with Warburg impedance, σ, obtained from the slope of real impedance (Z′) vs. reciprocal square root of frequency (ω−1/2) in the low frequency region.44
 | (2) |
| Zω = σ(1 − j)ω−1/2 | (3) |
It is perhaps necessary to emphasize that while Mn3+ improves electronic conductivity; its content must be controlled to curb the negative effects of the disproportionation reaction (eqn (1)) and the accompanying severe capacity-fading at elevated temperatures. Indeed, there is a lot of evidence in the literature which prove that the higher content of surface Mn3+ leads to disproportionation of Mn3+ and severe capacity-fading despite the increased electronic conductivity.45 Our finding agrees with the need to have an “appropriate” amount of Mn3+ to get the best performance. For example, Zheng et al.46 clearly stated as follows: “the presence of an appropriate amount of oxygen deficiency and/or Mn3+ is critical to accelerate the Li+ ion transport within the crystalline structure”. In addition, it is to be noted that the enhancement of Li+ ion transport is not just a factor of the Mn3+ content, but other factors such as the morphology and particle size.31,47 Smaller sized particles are known to offer a short path length for Li+ to travel within the crystalline structure, and LMNOmic gave smaller sized particles than pristine LMNO. The well-defined octahedron morphology with {111} facets in the LMNOmic crystal structure, coupled with the local environment of the Li-ion in the spinel structure (evident from the 6Li MAS-NMR spectra, whereby Li+ is surrounded by Mn4+ and the highly conductive Ni2+) could have also enhanced the Li+ diffusivity.
Microwave chemistry occurs by two main mechanisms; dipolar polarization (alignment of dipoles in the microwave field) and ionic conduction (movement of ions in the microwave field) that ultimately led to the generation of heat energy.48 From our findings, microwave irradiation seems to have a significant impact on the redox chemistry of the spinel whereby the ‘excess’ Mn3+ is converted to Mn4+ for enhanced electrochemistry (notably, cycling durability and Li+ diffusion) at elevated temperature. However, further studies are necessary to fully explore this mechanism, and these will constitute the next steps of our future research activities on the topic.
Conclusion
It is well-documented that the poor cycling performance of LiMn2O4 (LMO) for lithium-ion batteries at elevated temperature is due to the instability of the concentration of Mn3+ in the spinel structure. This study describes, for the first time, the impact of microwave irradiation on LMO doped with a minute amount of nickel (LiNi0.2Mn1.8O4, LMNO) using Mn3O4 prepared from electrolytic manganese oxide (EMD, γ-MnO2) as the main precursor. From detailed spectroscopic, microscopic and electrochemical analyses, we have demonstrated that microwave irradiation could enhance the exposure of the {111} facets of the spinel and tune the Mn3+ concentration thereby promoting structural stability and cycling performance at elevated temperature (60 °C). Considering that the state-of-the-art methods of curbing the problem of capacity fading at elevated temperature are time-consuming and involve the use of harsh acidic treatment, the novelty of this finding gives promise for the potential adoption of a greener method of improving the electrochemical cycling performance at elevated temperatures.Acknowledgements
This work was supported by the CSIR (South Africa), the South Africa's Department of Science and Technology (DST) and National Research Foundation (NRF). Dr Kumar Raju thanks the CSIR for post-doctoral research fellowship.References
- M. Yohio, Lithium-Ion Batteries, Springer, New York, 2008 Search PubMed.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS PubMed.
- O. K. Park, Y. Cho, S. Lee, H. Yoo, H. K. Song and J. Cho, Energy Environ. Sci., 2011, 4, 1621 CAS.
- J. B. Goodenough and Y. Kim, Chem. Mater., 2010, 22, 587 CrossRef CAS.
- M. Jo, Y. K. Lee, K. M. Kim and J. Cho, J. Electrochem. Soc., 2010, 157, A481 CrossRef.
- H. Kawai, M. Nagata, H. Kageyama, H. Tukamoto and A. R. West, Electrochim. Acta, 1999, 45, 315 CrossRef CAS.
- E. Hosono, T. Kudo, I. Honma, H. Matsuda and H. S. Zhou, Nano Lett., 2009, 9, 1045 CrossRef CAS PubMed.
- J. W. Fergus, J. Power Sources, 2010, 195, 939 CrossRef CAS.
- B. Scrosati and J. Garche, J. Power Sources, 2010, 195, 2419 CrossRef CAS.
- F. Jiao, J. L. Bao, H. A. Hill and P. G. Bruce, Angew. Chem., Int. Ed., 2008, 47, 9711 CrossRef CAS PubMed.
- M. M. Thackeray, J. Am. Ceram. Soc., 1999, 82, 3347 CrossRef CAS.
- R. J. Gummow, A. D. Kock and M. M. Thackeray, Solid State Ionics, 1994, 69, 59 CrossRef CAS.
- Y. Shin and A. Manthiram, J. Electrochem. Soc., 2004, 151, A204 CrossRef CAS.
- S. Komaba, N. Kumagai and Y. Kataoka, Electrochim. Acta, 2002, 47, 1229 CrossRef CAS.
- D. Aurbach, J. Power Sources, 2000, 89, 206 CrossRef CAS.
- A. Blyr, C. Sigala, G. Amatucci, D. Guyomard, Y. Chabre and J.-M. Tarascon, J. Electrochem. Soc., 1998, 145, 194 CrossRef CAS.
- M. A. Andersson and K. Edström, J. Electrochem. Soc., 2001, 148, A1100 CrossRef.
- A. M. Kannan and A. Manthiram, Electrochem. Solid-State Lett., 2002, 5, A167 CrossRef CAS.
- M. M. Thackeray, C. S. Johnson, J. S. Kim, K. C. Lauzze, J. T. Vaughey, N. Dietz, D. Abraham, S. A. Hackney, W. Zeltner and M. A. Anderson, Electrochem. Commun., 2003, 5, 752 CrossRef CAS.
- M. Zhang, Y. Wang, J. N. Reimers and M. Gee, European Patent (EP) 1056143B1, 2007.
- G. Amatucci, A. Du Pasquier, A. Blyr, T. Zheng and J.-M. Tarascon, Electrochim. Acta, 1999, 45, 255 CrossRef CAS.
- N. M. Hagh, F. Cosandey, S. Rangan, R. Bartynski and G. Amatucci, J. Electrochem. Soc., 2010, 157, A305 CrossRef CAS.
- S. T. Myung, K. S. Lee, D. K. Kim, B. Scrosati and Y. K. Sun, Energy Environ. Sci., 2011, 4, 935 CAS.
- G. P. Zhong, Y. Y. Wang, X. J. Zhao, Q. S. Wang, Y. Yu and C. H. Chen, J. Power Sources, 2012, 216, 368 CrossRef CAS.
- L. S. Yong and F. Li, US Patent Application, 2012/0237837 A1, 2012.
- K. R. Chemelewski, E. S. Lee, W. Li and A. Manthiram, Chem. Mater., 2013, 25, 2890 CrossRef CAS.
- B. Hai, A. K. Shukla, H. Duncan and G. Chen, J. Mater. Chem. A, 2013, 1, 759 CAS.
- Y. J. Wei, L. Y. Yan, C. Z. Wang, X. G. Xu, F. Wu and G. Chen, J. Phys. Chem. B, 2004, 108, 18547 CrossRef CAS.
- M. A. Kebede, N. Kunjuzwa, C. J. Jafta, M. K. Mathe and K. I. Ozoemena, Electrochim. Acta, 2014, 128, 172 CrossRef CAS.
- C. J. Jafta, M. K. Mathe, N. Manyala, W. D. Roos and K. I. Ozoemena, ACS Appl. Mater. Interfaces, 2013, 5, 7592 CAS.
- N. P. Funeka, C. J. Jafta, M. Kebede, L. le Roux, M. K. Mathe and K. I. Ozoemena, RSC Adv., 2015, 5, 32256 RSC.
- S. Komaba, T. Tsuchikawa and A. Ogata, ECS Trans., 2008, 16, 201 CrossRef CAS.
- K. Yang, J. Su, L. Zhang, Y. Long, X. Lv and Y. Wen, Particuology, 2012, 10, 765 CrossRef CAS.
- A. Manthiram, K. R. Chemelewski and E. S. Lee, Energy Environ. Sci., 2014, 7, 1339 CAS.
- P. Mustarelli, V. Massarotti, M. Bini and D. Capsoni, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 55, 12018 CrossRef CAS.
- Y. J. Lee, C. Eng and C. P. Grey, J. Electrochem. Soc., 2001, 148, A249 CrossRef CAS.
- Y. J. Lee, F. Wang, S. Mukerjee, J. McBreen and C. P. Grey, J. Electrochem. Soc., 2000, 147, 803 CrossRef CAS.
- Y. J. Lee, F. Wang and C. P. Grey, J. Am. Chem. Soc., 1998, 120, 12601 CrossRef CAS.
- D. Aurbach, K. Gamolsky, B. Markovsky, G. Salitra, Y. Gofer, U. Heider, R. Oesten and M. Schmidt, J. Electrochem. Soc., 2000, 147, 1322 CrossRef CAS.
- D. Lu, W. Li, X. Zuo, Z. Yuan and Q. Huang, J. Phys. Chem. C, 2007, 111, 12067 CAS.
- Q. C. Zhuang, T. Wei, L. L. Du, Y. L. Cui, L. Fang and S. G. Sun, J. Phys. Chem. C, 2010, 114, 8614 CAS.
- M. Mohamedi, M. Makino, K. Dokko, T. Itoh and I. Uchida, Electrochim. Acta, 2002, 48, 79 CrossRef CAS.
- M. Hirayana, H. Ido, K. Kim, W. Cho, K. Tamura, J. Mizuki and R. Kanno, J. Am. Chem. Soc., 2010, 132, 15268 CrossRef PubMed.
- C. J. Jafta, K. I. Ozoemena, M. K. Mathe and W. D. Roos, Electrochim. Acta, 2012, 85, 411 CrossRef CAS.
- J. Xiao, X. Chen, P. V. Sushko, M. L. Sushko, L. Kovarik, J. Feng, Z. Deng, J. Zheng, G. L. Graff, Z. Nie, D. Choi, J. Liu, J. G. Zhang and M. S. Whittingham, Adv. Mater., 2012, 24, 2109 CrossRef CAS PubMed.
- J. Zheng, J. Xiao, X. Yu, L. Kovarik, M. Gu, F. Omenya, X. Chen, X. Q. Yang, J. Liu, G. L. Graff, M. S. Whittingham and J. G. Zhang, Phys. Chem. Chem. Phys., 2012, 14, 13515 RSC.
- H. M. Cho and Y. S. Meng, J. Electrochem. Soc., 2013, 160, A1482 CrossRef CAS.
- M. Gasgnier, L. Albert, J. Derouet and L. Beaury, J. Alloys Compd., 1993, 198, 73 CrossRef CAS.
| This journal is © the Owner Societies 2016 |