 Open Access Article
Open Access ArticleFormation of simple nitrogen hydrides NH and NH2 at cryogenic temperatures through N + NH3 → NH + NH2 reaction: dark cloud chemistry of nitrogen
Sendres
Nourry
ab and
Lahouari
Krim
*ab
aSorbonne Universités, UPMC Univ Paris 06, UMR 8233, Monaris, F 75005, Paris, France. E-mail: Lahouari.krim@upmc.fr
bCNRS, UMR 8233, Monaris, F 75005, Paris, France
First published on 10th June 2016
Abstract
Although NH3 molecules interacting with ground state nitrogen atoms N(4S) seem not to be a very reactive system without providing additional energy to initiate the chemical process, we show through this study that, in the solid phase, at very low temperature, NH3 + N(4S) reaction leads to the formation of the amidogen radical NH2. Such a dissociation reaction previously thought to occur exclusively through UV photon or energetic particle irradiation is in this work readily occurring just by stimulating the mobility of N(4S)-atoms in the 3–10 K temperature range in the solid sample. The N(4S)–N(4S) recombination may be the source of metastable molecular nitrogen N2(A), a reactive species which might trigger the NH3 dissociation or react with ground state nitrogen atoms N(4S) to form excited nitrogen atoms N(4P/2D) through energy transfer processes. Based on our obtained results, it is possible to propose reaction pathways to explain the NH2 radical formation which is the first step in the activation of stable species such as NH3, a chemical induction process that, in addition to playing an important role in the origin of molecular complexity in interstellar space, is known to require external energy supplies to occur in the gas phase.
I. Introduction
Ammonia is one of the most important simple molecules that may be the source of the N–H and N–N bonds that lead to the formation of pre-biotic molecules in interstellar space and in some planetary and satellite atmospheres. As a consequence of its high microwave absorbance, several studies consider ammonia molecules to be the primary source of opacity in many extraterrestrial atmospheres.1 As a result, numerous space observations and laboratory simulations have been carried out to provide a clear description of the role of NH3 molecules in the chemical activities in space. Hofstadter et al.1 underlined the importance of latitudinal variations of ammonia in the atmosphere of Uranus, through an analysis of a radio map of the planet made with the very large array, while Loeffler et al. carried out laboratory simulation by heating water-ammonia ices2 previously irradiated with protons to simulate Saturn's energetic ion environment. They illustrated that the eruption of high-pressure bubbles of hydrogen and nitrogen molecules might be due to the radiolytic decomposition of water-ammonia ices, one of the phenomena responsible for the variations of NH3 abundances. Regarding the variations of NH3 abundances, studies of the ammonia abundances vs. temperatures and photo-dissociation processes in some nearby galaxies, undertaken very recently by Takano et al., have already shown that galaxies with low temperatures tend to have low abundances of ammonia.3 However, those studies have only proved that the low abundances of ammonia cannot be explained only by the photo-dissociation effects and that other chemical processes might be involved in the evolution of their abundances. On the other hand, interstellar ammonia, considered to be originating from molecular cloud materials,4 might be one of the formation sources of N–H-bearing reactive species such as NH, NH2, and N2H3. However since the detection of the first interstellar neutral N-bearing molecules,5 the ratios of the three simple nitrogen hydrides NH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH2
NH2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3 were found to be strongly dependent on dark cloud conditions. In 1991 Millar et al. predicted6 a dark cloud value NH3/NH2 < 3 abundances, incompatible with the observations of van Dishoeck et al.7 or Hily-Blant et al.8. In fact, Hily-Blant et al. reported the ratio of NH
NH3 were found to be strongly dependent on dark cloud conditions. In 1991 Millar et al. predicted6 a dark cloud value NH3/NH2 < 3 abundances, incompatible with the observations of van Dishoeck et al.7 or Hily-Blant et al.8. In fact, Hily-Blant et al. reported the ratio of NH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH2
NH2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3 equal to 5
NH3 equal to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 in the cold envelope of IRAS16293-2422, while, using the Caltech Submillimeter Observatory to probe the NH2 radical in interstellar clouds, van Dishoeck et al. found a value of 0.5 for the NH2/NH3 ratio. Those disparities in NH2 and NH3 abundance measurements have caused many groups to state that the NH2 radical may be formed through different reaction mechanisms depending on the dark cloud conditions.
300 in the cold envelope of IRAS16293-2422, while, using the Caltech Submillimeter Observatory to probe the NH2 radical in interstellar clouds, van Dishoeck et al. found a value of 0.5 for the NH2/NH3 ratio. Those disparities in NH2 and NH3 abundance measurements have caused many groups to state that the NH2 radical may be formed through different reaction mechanisms depending on the dark cloud conditions.
It is well established that the photo-dissociation of NH3 leads mainly to the formation of NH and NH2 radicals as primary photoproducts. Consequently, many laboratory investigations have focused their NH2 formation research on ultraviolet processing of interstellar or satellite containing NH3 ice analogs9,10 in order to mimic the photo-chemistry processes of icy objects exposed to UV radiation in different regions of space, such as interstellar clouds, Saturn's rings, comets and planetary atmospheres. In the same context, earlier studies carried out by Miligan et al. and Schnepp et al. have already characterized the formation of NH2 radicals11,12 through studies of photolysis of solid ammonia formed at 14 K, where the NH2 infrared fundamental vibration has been measured at 1499 cm−1. In those studies related to ammonia decomposition, the NH2 radical has been characterized only by the bending mode signal around 1500 cm−1 as the NH stretching is unfortunately partially obscured by the large IR-absorption bands of ammonia aggregates. Similarly, in 1984, Nishi et al. underlined13 the formation of a hydrogen bound NH2–NH3 complex on the surface of irradiated icy solids containing NH3. More recently, Loeffler et al. studied, using IR spectroscopy and mass spectrometry, the UV photolysis of solid ammonia and ammonia-dihydrated samples formed at 40 K.14 They showed that photolysis of NH3 leads to the formation of NH2 as a first primary photoproduct. In this context we have also investigated the UV photolysis of ammonia15 ice at 3 K, confirming the results obtained by Loeffler et al. and showing that the only detectable IR-signal due to the photo-dissociation of solid NH3, a wide absorption band due to the NH2 radical, is located around 1500 cm−1 (Fig. 1).
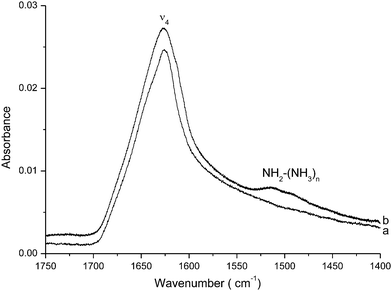 | ||
| Fig. 1 UV-irradiation of ammonia ice formed at 3 K. (a) Before irradiation and (b) after 30 min irradiation using a UV-hydrogen lamp. | ||
This band has been attributed to the NH2 radical interacting with ammonia aggregates by carrying out the same experiments using neon matrix isolation.15Fig. 2 shows the formation of different complexes between NH2 and NH3 while the ammonia concentration increases in the neon matrix. The formation of (NH2)(NH3), (NH2)(NH3)2 and (NH2)(NH3)n was clearly established thanks to the comparison between the theoretical and experimental vibrational frequencies.
Although from the previous studies NH3 photo-dissociation might be considered an important source of NH and NH2 radicals, the aim of the present study is to characterize the formation of reactive nitrogen hydride radicals from non-energetic processes involving non-reactive but very abundant species in space, namely ground state nitrogen atoms N(4S) and ammonia molecules NH3. We show through this experimental investigation that while the absence of additional energy sources associated with an extremely low temperature may present conditions less favorable for the N(4S) + NH3 reaction to occur, NH and NH2 radicals may be easily formed in the solid phase just by stimulating a mobility of N-atoms around NH3 molecules in the 3–10 K temperature range. These results show that complex nitrogen chemistry, involving nitrogen atoms and initiated by thermal processes, may take place in dense molecular clouds. Indeed, nitrogen is considered as one of the most abundant elements in various very cold regions of space.16 Knauth et al.17 detected N2 molecules in interstellar space, with a column density of 4.6 × 1013 cm−2, several orders of magnitude lower than that of atomic nitrogen 2.0 × 1017 cm−2, demonstrating that nitrogen is mainly atomic. On the other hand, several interstellar gas-grain chemistry models predicted that N2 should be formed on ice mantles through N–N addition reactions and that much of the missing nitrogen is present in icy grains. In such a context as for N + N recombination on ice mantles, NH3 + N reaction might be also very frequent. Paradoxically, to date, no theoretical investigations have even been carried out to describe the reaction between ground or excited state nitrogen atoms and NH3 molecules. Based on previous theoretical models describing reactions with N atoms, many studies have already shown that all reactions involving excited N-atoms such as N(2D) and N(4P) are highly exothermic, with almost no activation energy, while reactions involving ground state nitrogen atoms N(4S) are found to be endothermic and they need a high additional energy supply to the proceed. Even with the lack of N + NH3 theoretical models, we show, in the present article, that the hydrogen atom abstraction reaction from the NH3 molecule is due to the mobility of nitrogen atoms in the solid phase. This is the first study of the NH3 + N(4S) reaction which was carried out in the solid phase at very low temperature. We have explored such a reaction to characterize the three simple nitrogen hydrides NH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH2
NH2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3 through the N + NH3 → NH + NH2 reaction pathway. We show, in the course of this study, that the NH2 radical is formed from ammonia under low temperatures, low pressures, and non-energetic conditions, circumstances different from those involving high energy photons or particles. As we are particularly interested in solid-phase reactivity, we conducted a detailed investigation of the NH3 + N(4S) reaction by co-condensing NH3 molecules and N atoms at 3 and 10 K. The obtained solid samples were further analyzed spectroscopically using a Fourier Transform Infrared (FT-IR) spectrometer.
NH3 through the N + NH3 → NH + NH2 reaction pathway. We show, in the course of this study, that the NH2 radical is formed from ammonia under low temperatures, low pressures, and non-energetic conditions, circumstances different from those involving high energy photons or particles. As we are particularly interested in solid-phase reactivity, we conducted a detailed investigation of the NH3 + N(4S) reaction by co-condensing NH3 molecules and N atoms at 3 and 10 K. The obtained solid samples were further analyzed spectroscopically using a Fourier Transform Infrared (FT-IR) spectrometer.
II. Technical background
The experimental methods used for the present study have been previously described.18,19 Nevertheless, it is necessary to remind the reader of the basic scheme. The setup is maintained at 10−8 mbar, and samples were prepared by condensing gaseous mixtures on the surface of a substrate mirror maintained at 3 K. The co-deposition lasts for 30 min. To this end, a pulsed tube, closed cyclic helium cryogenerator is used (Cryomech PT405, USA). Prior to condensation onto the cold mirror, nitrogen atoms are obtained by passing pure nitrogen gas through a microwave-driven radical atomic source (SPECS PCR-ECS). Nitrogen atoms are generated with a flux of about 1015 atoms cm−2 s−1, corresponding to a 4% N2-dissociation rate. The dissociation rate has been evaluated by monitoring the IR signals corresponding to solid N2 formed at 3 K under the same experimental conditions with the microwave discharge on and off. The obtained samples are analyzed using a Bruker 120 HR Fourier Transform InfraRed (FT-IR) spectrometer in the transmission-reflection mode between 4500 and 500 cm−1. The angle of the IR beam is 8° with respect to the normal of the deposition mirror. A resolution of 0.5 cm−1 is used and 300 scans are recorded for each spectrum. Pure NH3 molecules are concomitantly injected, at 3 or 10 K, with the N/N2 discharged mixture and for the sake of comparison all the IR spectra are registered at 3 K. The formed solid sample is kept in a chamber under vacuum (10−8 mbar) where the only pollutions detected as traces are CO, CO2 and H2O, during the sample deposition when the pressure increased to 10−5 mbar. Molecular N2 and NH3 were purchased from “Air Liquide” and Messer, respectively, with a purity of 99.9995%. The purity of samples was confirmed spectroscopically. Experiments at 3 and 10 K were carried out, however, the 3 K lowest temperature was preferable for the present study in order to control the mobility of nitrogen atoms trapped in the solid samples. In fact, at temperatures as low as 3 K, the resulting samples may be warmed up from 3 to 10 K to study the diffusion of nitrogen atoms and then the evolution of the reaction, thermally stimulated.III. Results
Fig. 3a shows the spectrum of a sample obtained by co-injection of ammonia and N/N2 reactants at 3 K, corresponding to NH3 + N + N2 reaction. As a reference, a spectrum of the sample containing only NH3 and N2 as reactants, at the same temperature, is shown in Fig. 3b. Comparison between these two spectra reveals that at 3 K, there are no new reaction products due to the NH3 + N reaction. | ||
| Fig. 3 NH3 and NH2 spectral regions. (a) NH3 + N + N2 reaction and co-injection of the NH3 and N/N2 mixture at 3 K. (b) NH3 + N2 reaction and co-injection of NH3 and N2 gas at 3 K. | ||
In fact by zooming in the NH2 characteristic spectral region around 1500 cm−1, Fig. 4 shows no signal due to NH2 species which, as mentioned above, has been measured in the solid phase by Loeffler et al.14 at 1508 cm−1. As NH3 + N + N2 and NH3 + N2 systems give the same IR response, we attest that no reaction may be possible between ammonia and nitrogen atoms at 3 K. These first results also prove that the N-atoms reaching the solid sample are mainly ground state nitrogen atoms N(4S), atomic species less reactive than excited nitrogen atoms N(2D) and N(4P). In order to monitor the NH3 + N reaction, the former solid sample obtained at 3 K has been kept in the dark, far away from any excitation source such as room light or IR radiation from our spectrometer, for a period ranging from 1 min to several hours. This was performed to ensure that all the reactants are in their ground states and that the N + NH3 reaction is not triggered by any other external source of energy. After several hours in the dark, the recorded IR spectrum of the sample is similar to that obtained just after N + NH3 co-deposition, showing that our sample was not altered in any way while kept in the dark at 3 K. In fact, at 3 K, the mobility of the reactants, particularly nitrogen atoms, is very limited and thus reduces the encounter probability and hence the interaction between the reactants. In order to induce some motion, the sample was gradually heated from 3 to 10 K and an infrared spectrum was subsequently acquired at 3 K.
 | ||
| Fig. 4 NH2 spectral region. (a) NH3 + N + N2 reaction and co-injection of the NH3 and N/N2 mixture at 3 K. (b) NH3 + N2 reaction and co-injection of NH3 and N2 gas at 3 K. | ||
Fig. 5a shows the results of the sample heating between 3 and 10 K. Detectable signals due to the NH2 radical around 1500 cm−1 are observed, but only in samples containing nitrogen atoms and NH3 molecules. Fig. 5b shows the results of the heating of our reference sample containing just NH3 and N2 as reactants and no new signals are identified.
Similar experiments have been carried out by varying the amount of injected NH3 during the NH3 + N/N2 co-injection. Fig. 6, corresponding to the NH3 spectral region, and Fig. 7, to that of the NH2 radical, show the influence of ammonia concentration on NH3 + N reaction. The spectra presented in these two figures result from a reactant co-deposition at 3 K, followed by a sample heating at 10 K to thermally induce the NH3 + N reaction. In order to have an order of magnitude of the ammonia concentrations in each studied sample, we have calculated the column density n (molecules per cm2) of ammonia, using the band area of the absorption ν2 vibrational mode, associated with its appropriate20 band strength (A = 1.2 × 10−17 cm per molecules). The column densities are defined as:21
The association of Fig. 6 and 7 allows for the assignment of the detected signal at 1508 cm−1 to the NH2 radical. The attribution of this signal, observed even at very low NH3 concentration (Fig. 6a and 7b), is in good agreement with Loeffler's measurements.14 The signals detected at 1480 and 1520 cm−1, observed at relatively high NH3 concentrations, might be due to the NH2 radical interacting with NH3 molecules (Fig. 6b, c and 7b, c). Our assignments are also consistent with our previous recent study characterizing complexes between NH2 and NH3 in a neon matrix through the UV photolysis of ammonia.15 We showed, by combining neon matrix isolation studies with theoretical calculations, that trapped in the neon matrix the NH2 radical and (NH2)(NH3)n aggregates show characteristic signals at 1501.1 and 1487.1 cm−1, respectively. Table 1 gathers the calculated and measured values of the spectral positions of NH2 and complexes between NH2 and NH3.
In our previous study, the formation and isolation in the neon matrix of NH2, (NH2)(NH3), (NH2)(NH3)2 and (NH2)(NH3)n species were clearly established by irradiating samples with different NH3/Ne amounts. We show in the present study that the NH2 radical is formed by stimulating thermally the mobility of N(4S)-atoms around ammonia molecules. However from Fig. 6 and 7, we note that while in diluted samples ([NH3] = 0.2 × 1017 molecules per cm2) the NH2 signal is observed as the main reaction product, an increase in the NH3 concentration stimulates directly the formation of larger (NH2)(NH3)n aggregates. The direct formation of (NH2)(NH3)n under our experimental conditions may be due to the fact that the NH3 + N reaction takes place only by heating the sample from 3 to 10 K which may also favor the formation of ammonia aggregates in the nitrogen matrix.
One of the advantages of carrying out this experiment study at 3 K, in addition to proving that all chemical processes start from reactants in their ground states and that the reactions occur without providing any external energy to the NH3 + H system, is the possibility to deduce the amount of reacting ammonia by calculating the integrated band intensities of NH3 at 3 and 10 K before and after the reaction take place, respectively. The ratio between the two calculated band intensities allows us to deduce the amount of reacting ammonia. Table 2 gathers the integrated band intensities at 3 and 10 K of the ν2 band of ammonia in a reactive sample containing N, N2 and NH3 species and in a reference sample containing only N2 and NH3 molecules as reactants.
| S 3K (cm−1) | S 10K (cm−1) | S 10K/S3K | |
|---|---|---|---|
| NH3 + N + N2 | 0.85 | 0.76 | 0.89 |
| NH3 + N2 | 0.79 | 0.81 | 1 |
The calculations of the integrated band intensities of ammonia at 3 and 10 K in the reference sample show almost the same value around 0.8 cm−1, which confirms the fact that there is no NH3 consumption and thus no reaction between molecular nitrogen and ammonia. We note that in the reactive sample containing nitrogen atoms the integrated band intensities of NH3 are 0.85 cm−1 and 0.76 cm−1 at 3 and 10 K, respectively. This shows that NH3 concentration is decreasing at 10 K; thus a reaction is occurring between NH3 and nitrogen atoms and the NH2 radical formation is directly linked to the presence of N-atoms in our solid samples. The ratio between the integrated band intensities at 3 and 10 K easily allows the estimation of the NH3 amount consumed during the N + NH3 reaction, which is found to be 0.89, showing that almost 11% of NH3 has reacted with nitrogen atoms to produce NH2.
IV. Discussion
We have characterized the NH3 + N reaction in the solid phase, at very low temperature, under non-energetic conditions and where the NH3 and N reactants are in the ground state. We have shown, by carrying out our experiments at temperatures as low as 3 K, that the first step of the NH3 + N reaction may be blocked due to the reduced mobility of the trapped species in the solid phase. Leaving the formed solid sample in the dark for a while, to allow all trapped reactants to relax down to the ground state, is the only possible way to prove that the studied N + NH3 reaction starts from reactants in their ground states and without providing any additional energy to the system. The appearance of the NH2 radical as a reaction product by inducing the mobility of N(4S)-atoms between 3 and 10 K translates to the fact that a hydrogen abstraction reaction from ammonia under non-energetic conditions may occur at very low temperature.From a theoretical point of view, we did not find any calculation in the literature to characterize reactions involving ground or excited state nitrogen atoms with ammonia, while many theoretical investigations have been carried out to study similar reactions such as N + H2O, N + CH4 and N + CH3OH.22 Based on those previous theoretical models describing reactions with N-atoms, all reactions involving excited nitrogen atoms N(2D/4P) are highly exothermic, with almost no activation energy, and reactions involving ground state nitrogen atoms N(4S) are found to be endothermic with very important activation energies. We suppose that NH3 + N(4S) reaction is not an exception and that it may need a high additional energy supply to occur. However, in the present experimental study, we show that the N + NH3 reaction may occur only by inducing a mobility of ground state nitrogen atoms at very low temperature, between 3 and 10 K. In fact, the heating of the sample from 3 to 10 K stimulates the mobility of ground state nitrogen atoms which may recombine through reaction 1 to the form metastable molecular nitrogen N2(A).23 This metastable nitrogen species, which is highly reactive,24 can be a source of high energy, 6.2 eV.
| N(4S) + N(4S) → N2(A) | (1) |
| N2(A) → N2(X) + 6.2 eV | (2) |
| N2(A) + N2(X) → N2(X) + N2(A) | (3) |
| N2(A) + N2(X) → N2(X,v′) + N2(X,v) | (4) |
| N2(A) + N(4S) → N(4P/2D) + N2(X) | (5) |
| N(4P/2D) + NH3 → NH + NH2 | (6) |
| N2(A) + NH3 → N2(X) + NH2 + H | (7) |
V. Conclusions
We show through this experimental study that, in the solid phase and at cryogenic temperatures, the NH3 + N(4S) reaction might be one of the sources of the amidogen radical NH2 in some cold regions of the interstellar space, in addition to those usually discussed which involve high energy photons and particles. Based on our obtained results, we propose reaction pathways to explain the NH and NH2 radical formation which may be the first step in the activation of stable species such as NH3, a significant process in the molecular complexity of the Universe. For the present study, the reactants have been frozen at a very low temperature and therefore their interactions have been controlled by stimulating the mobility of nitrogen atoms through a gradual heating of the solid sample in order to show that a hydrogen abstraction reaction from NH3 may occur without supplying any external energy to the NH3 + N system.References
- M. D. Hofstadter and D. O. Muhleman, Icarus, 1989, 81, 396 CrossRef CAS.
- M. J. Loeffler, U. Raut and R. A. Baragiol, Astrophys. J., Lett., 2006, 649, 133 CrossRef.
- S. Takano, T. Takano, N. Nakai, K. Kawaguchi and P. Schilke, Astron. Astrophys., 2013, 552, 34 CrossRef.
- J. Keene, G. A. Blake and T. G. Phillips, Astrophys. J., Lett., 1983, 271, 27 CrossRef.
- A. C. Cheung, D. M. Rank, C. H. Townes, D. D. Thornton and W. J. Welch, Phys. Rev. Lett., 1968, 21(25), 1701 CrossRef CAS.
- T. J. Millar, A. Bennett, J. M. C. Rawlings, P. D. Brown and S. B. Charnley, Astron. Astrophys., Suppl. Ser., 1991, 87, 585 Search PubMed.
- E. F. van Dishoeck, D. J. Jansen, P. Schilke and T. G. Phillips, Astrophys. J., Lett., 1993, 416, L83 CrossRef CAS.
- P. Hily-Blant, S. Maret, A. Bacmann, S. Bottinelli, B. Parise, E. Caux, A. Faure, E. A. Bergin, G. A. Blake, A. Castets, C. Ceccarelli, J. Cernicharo, A. Coutens, N. Crimier, K. Demyk, C. Dominik, M. Gerin, P. Hennebelle, T. Henning, C. Kahane, A. Klotz, G. Melnick, L. Pagani, P. Schilke, C. Vastel, V. Wakelam, A. Walters, A. Baudry, T. Bell, M. Benedettini, A. Boogert, S. Cabrit, P. Caselli, C. Codella, C. Comito, P. Encrenaz, E. Falgarone, A. Fuente, P. F. Goldsmith, F. Helmich, E. Herbst, T. Jacq, M. Kama, W. Langer, B. Lefloch, D. Lis, S. Lord, A. Lorenzani, D. Neufeld, B. Nisini, S. Pacheco, T. Phillips, M. Salez, P. Saraceno, K. Schuster, X. Tielens, F. van der Tak, M. H. D. van der Wiel, S. Viti, F. Wyrowski and H. Yorke, Astron. Astrophys., 2010, 521, 52 CrossRef.
- P. A. Gerakines, W. A. Schutte and P. Ehrenfreund, Astron. Astrophys., 1996, 312, 289 CAS.
- W. Zheng, D. Jewitt, Y. Osamura and R. I. Kaiser, Astrophys. J., Lett., 2008, 674, 1242 CrossRef CAS.
- D. E. Miligan and M. E. Jacox, J. Chem. Phys., 1965, 43, 4487 CrossRef.
- O. Schnepp and K. Dressler, J. Chem. Phys., 1960, 32, 1682 CrossRef CAS.
- N. Nishi, H. Shinohara and T. Okuyama, J. Chem. Phys., 1984, 80, 3898 CrossRef CAS.
- M. J. Loeffler and R. A. Baragiola, J. Chem. Phys., 2010, 133, 214506 CrossRef CAS PubMed.
- E. L. Zins and L. Krim, RSC Adv., 2013, 3, 10285 RSC.
- A. Waszczak, 2013. Hydrogen and nitrogen cosmochemistry: a review. Ge 232 term paper.
- D. C. Knauth, B.-G. Andersson, S. R. McCandliss and H. W. Moos, Nature, 2004, 429–636 Search PubMed.
- E. L. Zins, P. R. Joshi and L. Krim, Mon. Not. R. Astron. Soc., 2011, 415, 3107 CrossRef CAS.
- C. Pirim, L. Krim, C. Laffon, P. Parent, F. Pauzat, J. Pilmé and Y. Ellinger, J. Phys. Chem. A, 2010, 114, 3320 CrossRef CAS PubMed.
- C. R. Richey and P. A. Gerakines, Astrophys. J., 2012, 759, 74 CrossRef.
- C. J. Bennett, C. Jamieson, A. M. Mebel and R. I. Kaiser, Phys. Chem. Chem. Phys., 2004, 6, 735 RSC.
- C.-M. Ouk, N. Zvereva-Loete, Y. Scribano and B. Bussery-Honvault, J. Comput. Chem., 2012, 33, 2211 CrossRef CAS PubMed.
- A. M. Pravilov, L. G. Smirnova and A. F. Vilesov, Chem. Phys. Lett., 1984, 109, 4 CrossRef.
- V. Guerra, P. A. Sa and J. Loureiro, J. Phys. D: Appl. Phys., 2001, 34, 1745 CrossRef CAS.
- D. Kuszner and N. Schwentner, J. Chem. Phys., 1993, 98, 9 CrossRef.
- H. Kajihara, F. Okada and S. Koda, Chem. Phys., 1994, 186, 395 CrossRef CAS.
| This journal is © the Owner Societies 2016 |





