Crystallographic origin of cycle decay of the high-voltage LiNi0.5Mn1.5O4 spinel lithium-ion battery electrode†
Abstract
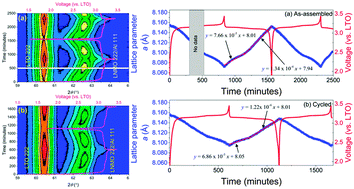
High-voltage spinel LiNi0.5Mn1.5O4 (LNMO) is considered a potential high-power-density positive electrode for lithium-ion batteries, however, it suffers from capacity decay after extended charge–discharge cycling, severely hindering commercial application. Capacity fade is thought to occur through the significant volume change of the LNMO electrode occurring on cycling, and in this work we use operando neutron powder diffraction to compare the structural evolution of the LNMO electrode in an as-assembled 18650-type battery containing a Li4Ti5O12 negative electrode with that in an identical battery following 1000 cycles at high-current. We reveal that the capacity reduction in the battery post cycling is directly proportional to the reduction in the maximum change of the LNMO lattice parameter during its evolution. This is correlated to a corresponding reduction in the MnO6 octahedral distortion in the spinel structure in the cycled battery. Further, we find that the rate of lattice evolution, which reflects the rate of lithium insertion and removal, is ∼9 and ∼10% slower in the cycled than in the as-assembled battery during the Ni2+/Ni3+ and Ni3+/Ni4+ transitions, respectively.
- This article is part of the themed collection: Neutron Scattering in Catalysis and Energy Materials

 Please wait while we load your content...
Please wait while we load your content...