Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fission of multiply charged alkali clusters in helium droplets – approaching the Rayleigh limit†
Michael
Renzler
a,
Martina
Harnisch
a,
Matthias
Daxner
a,
Lorenz
Kranabetter
a,
Martin
Kuhn
a,
Paul
Scheier
*a and
Olof
Echt
*ab
aInstitut für Ionenphysik und Angewandte Physik, University of Innsbruck, Technikerstrasse 25, A-6020 Innsbruck, Austria. E-mail: paul.scheier@uibk.ac.at; Michael.Renzler@uibk.ac.at; Martina.Harnisch@uibk.ac.at; Matthias.Daxner@uibk.ac.at; Lorenz.Kranabetter@student.uibk.ac.at; Martin.Kuhn@uibk.ac.at; Fax: +43 512 507 2922; Tel: +43 512 507 52660
bDepartment of Physics, University of New Hampshire, Durham, New Hampshire 03824, USA. E-mail: olof.echt@unh.edu; Fax: +1 603 862 2998; Tel: +1 603 862 3548
First published on 16th March 2016
Abstract
Electron ionization of helium droplets doped with sodium, potassium or cesium results in doubly and, for cesium, triply charged cluster ions. The smallest observable doubly charged clusters are Na92+, K112+, and Cs92+; they are a factor two to three smaller than reported previously. The size of sodium and potassium dications approaches the Rayleigh limit nRay for which the fission barrier is calculated to vanish, i.e. their fissilities are close to 1. Cesium dications are even smaller than nRay, implying that their fissilities have been significantly overestimated. Triply charged cesium clusters as small as Cs193+ are observed; they are a factor 2.6 smaller than previously reported. Mechanisms that may be responsible for enhanced formation of clusters with high fissilities are discussed.
1. Introduction
The instability of highly charged droplets is a phenomenon of broad relevance that occurs on a wide range of length scales. Lord Rayleigh applied a continuum model to determine the maximum charge of macroscopic water droplets beyond which spherical droplets become unstable with respect to spontaneous deformation. This so-called Rayleigh limit occurs when the Coulomb energy exceeds twice the surface energy.1 Charge-separation of droplets near the Rayleigh-limit is of practical relevance to electrospray sources and inkjet printing2,3 and it may prove to be useful to separate carbon nanotubes.4 The disintegration of micron-sized liquid glycol droplets at the Rayleigh limit has been imaged by Leisner and coworkers.5,6 Imaging by Mason et al. revealed that the explosive behavior of millimeter-sized alkali droplets injected into water is due to the Rayleigh instability.7At the other end of the length scale one encounters fission of atomic nuclei.8 In between, on the mesoscopic scale, charge-driven instabilities manifest themselves in the absence of multiply charged atomic clusters Anz+ in mass spectra below an experimental appearance size nexp,9 first documented by Sattler et al.10 Numerous reports have explored the energetics and dynamics of cluster ions in the vicinity of nexp (for reviews, see ref. 11–13). Early work was directed at determining values of nexp, fission barriers, and size distributions of fission fragments with a focus on van der Waals systems.14–21 Later work was focused on metal clusters and the role of the electronic shell structure.22–26
Initially it was assumed that clusters of size nexp were near the Rayleigh limit nRay at which the fission barrier vanishes. In other words, it was believed that their fissility was near X = 1 where X is the ratio between the Coulomb energy and twice the surface energy.1 A more useful relation, when dealing with atomic or molecular clusters of size n, is X = nRay/n.12,27 However, the observation of metastable fission, on the timescale of microseconds, revealed that fission near nexp is thermally activated and competes with evaporation of monomers.15,18,21,28–30 In most experiments cluster ions are “boiling hot;” their vibrational temperatures are set by the heat of evaporation and are difficult to vary.31 Thus, in spite of the expected temperature dependence, experimental values of nexp have been highly reproducible.32
Given the competition between fission and evaporation, a meaningful definition of a theoretical appearance size9na is the size at which the rate of thermally activated fission equals the rate of monomer evaporation.27,28 For multiply charged alkali clusters computed na values agree, indeed, closely with experimental appearance sizes nexp24,25,34 but they are much larger than values computed for the Rayleigh limit nRay. The fissility of the smallest multiply charged alkali cluster ions produced by photoionization ranges from nRay/nexp = 0.28 to 0.38.12,35 Huber and coworkers demonstrated that colder cluster ions can be produced in collisions with highly charged atomic ions.40 They attained fissilities as large as X = 0.87 for ten-fold charged sodium clusters but for low charge states the increase in fissility over photoionization experiments was rather modest. The authors conjectured that fissilities reached in their experiment were limited by the initial cluster temperature.
Here we show that alkali clusters with fissilities approaching 1 can be identified in mass spectra if the neutral precursors are embedded in superfluid helium nanodroplets. The smallest detected doubly charged sodium clusters contain 9 atoms, a factor three less than in previous experiments;41 and for cesium dications nexp is even lower than values computed for nRay.24,34 Furthermore, we observe triply charged cesium ions that are a factor 2.6 smaller than those reported previously by Martin and coworkers.42
2. Experimental
Helium nanodroplets were produced by expanding helium (Messer, purity 99.9999%) at a stagnation pressure of 20 bar through a 5 μm nozzle, cooled to 9.5 K (sodium and potassium) or 9.1 ± 0.1 K (cesium) in a vacuum. Droplets that form in the expansion contain an average number of 105 to 106 atoms; at a temperature of 0.37 K they are superfluid.43 The resulting supersonic beam traversed a pick-up cell into which sodium, potassium or cesium (Sigma Aldrich, purity 99.95% based on a trace metal analysis) was vaporized. The doped helium droplets were ionized by electrons at or below 70 eV; cations were accelerated and extracted by a pulsed voltage into a high-resolution reflectron-type time-of-flight mass spectrometer. The mass resolution (measured at full-width-at-half maximum) was 2900. Additional experimental details have been provided elsewhere.44Mass spectra were evaluated by means of custom-designed software.45 The routine includes automatic fitting of mass peaks and subtraction of background; it explicitly considers isotopic patterns of all ions. The abundance of specific ions (specific value of n) is derived by a matrix method. Critical regions were also inspected visually and fitted manually in order to ensure that impurity peaks and background were properly taken into account.
3. Results
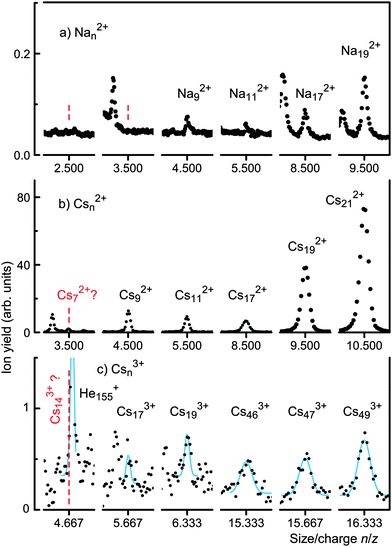
Fig. 1 displays sections of mass spectra recorded for sodium- and cesium-doped helium droplets. The abscissa has been labeled in terms of the size-to-charge ratio n/z of Anz+ (A = Na, Cs) or, in other words, the mass/charge ratio m/z divided by the atomic mass of sodium or cesium, respectively (these elements are monoisotopic).Fig. 1a shows sections of a spectrum of sodium-doped droplets in the vicinity of n/z values that are half-integer, i.e. sections where doubly charged ions containing an odd number of atoms would appear (even-numbered Nan2+ cannot be identified because they coincide with singly charged cluster ions half as large). Each section covers a range of Δn/z = ±0.01, equivalent to Δm/z = ±0.23 u. The spectrum clearly establishes the occurrence of odd-numbered Nan2+ for n ≥ 9. The abundance of Na52+ and Na72+ (marked by vertical dashed lines) is an order of magnitude weaker than that of Na92+, and statistically not significant. The smallest dication observed by Martin and coworkers in a multi-step photoionization study using an F2 laser (photon energy 7.9 eV) was Na272+.41,42
Fig. 1b shows sections of a spectrum that reveal the appearance of Csn2+ for n ≥ 9. Each section covers a range of Δn/z = ±0.01, equivalent to Δm/z = ±1.3 u. The ion yield at the position of Cs72+ (marked by a vertical dashed line) is not statistically significant. The smallest dication observed by Martin and coworkers was Cs192+.42
Fig. 1c shows sections where Csn3+ ions would appear (3n/z = integer). Solid lines represent gaussians fitted to the data. Their positions were fixed at integer values of 3n/z and their fixed widths were computed from the mass resolution of the instrument. The right-most trace shows Cs493+, the smallest trication identified by Martin and coworkers.42 Our spectra reveal trications as small as Cs193+. However, their abundance is two orders of magnitude less than that of small Csn2+ shown in Fig. 1b. Establishing the existence or absence of even smaller trications is challenging though. Cs173+ appears as a small peak whose statistical significance is doubtful. Cs163+ is masked by a strong impurity peak. Cs143+ would occur at Δm/z = 0.178 u to the left of He155+ as indicated by a vertical dashed line in Fig. 1c. A fit of two gaussians in this region (solid line) shows no significant contribution from Cs143+.46
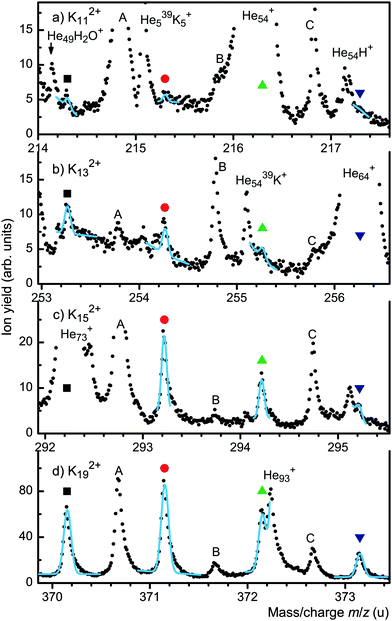
We now turn to potassium which has two isotopes of significant abundance, 39K (mass 38.964 u, natural abundance 93.26%) and 41K (40.962 u, 6.73%). Combined with the presence of ions that result from water impurities this leads to congested spectra. Fig. 2 shows sections of a mass spectrum that reveal the presence of odd-numbered Kn2+ for n ≥ 11. Each panel covers the four most abundant isotopologues containing zero to three 41K; the expected positions of these isotopologues are marked by symbols.
In Fig. 2d all four K192+ isotopologues are resolved. A set of four gaussians with individually chosen backgrounds was fitted to the four peaks. Positions and relative peak heights were fixed at the theoretical values; a common value was chosen for the width. The fit (solid cyan line) matches the experimental data very well. All other significant mass peaks in Fig. 2d are identified in the figure or its caption. Spectra covering smaller odd-numbered Kn2+ were analyzed similarly. Isotopologues overwhelmed by other peaks (Hem+, HemK+ or reaction products of H2O with Kn+) were excluded from the fit. For K132+ and K152+ three isotopologues are clearly seen in Fig. 2. The yield of K112+ is weak but the amplitudes of the first two isotopologues, containing zero or one 41K, are non-zero with a statistical significance greater than 4σ. K92+ and K72+ could not be identified.
Even-numbered doubly charged potassium clusters can be identified for n ≥ 20 thanks to the presence of isotopologues. Other doubly charged cluster ions that could be identified are H2OKn2+ (n ≥ 16), OHKn2+ and HKn2+.
Fig. 3 summarizes our results for doubly charged cluster ions. Solid dots represent their abundances, deduced from mass spectra after correction for background, other interfering mass peaks, and, for potassium, the presence of isotopologues. Odd-numbered Nan2+ and Csn2+ are observed with high statistical significance for n ≥ 9; the yields of Na52+, Na72+ and Cs72+ are zero within the error bars. Diamonds represent the results of previous experiments by Martin and coworkers who identified Nan2+ for n ≥ 27 and Csn2+ for n ≥ 19.41,42 Their data and error bars were estimated from published mass spectra and scaled to match our values at n = 27 (Na) and 21 (Cs).
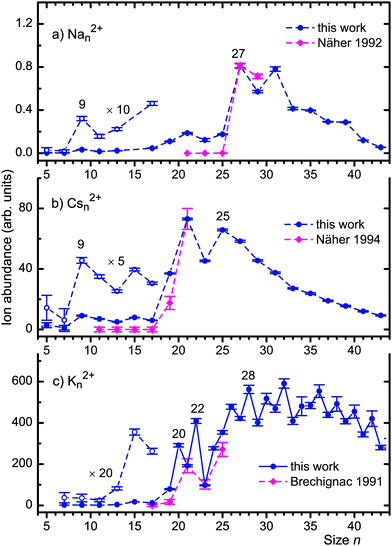 | ||
| Fig. 3 Full dots: ion abundance of doubly charged Nan2+, Csn2+ and Kn2+versus size n. Weak signals for small n are re-plotted with enhanced amplitude. Solid lines connect data available for even and odd n; dashed lines connect data available for odd n only. Diamonds represent the results of photoionization studies by Näher et al.41,42 and Brechignac et al.23 Their values were deduced from published mass spectra and scaled to match our values at n = 27 (Na) or 21 (Cs, K). | ||
The results for potassium-doped helium droplets are summarized in Fig. 3c. Odd-numbered Kn2+ ions appear for n ≥ 11, and even-numbered dications can be identified for n ≥ 20. The smallest potassium dication reported previously, in a multiphoton ionization study by Brechignac and coworkers using a nitrogen laser (photon energy 3.67 eV), was K192+.23 Their data (estimated from a mass spectrum) are shown as diamonds. Their mass resolution was not sufficient for the identification of even-numbered Kn2+.
Our results for Csn3+ are compiled in Fig. 4. The experimental appearance size is nexp = 19 or, perhaps, 17. The abundances of the next smaller trications (C143+, C133+, and C113+) that do not strongly interfere with other ions are all zero within the statistical error. Cs203+ forms a remarkably strong peak. All larger trications with non-integer values of n/3 are statistically significant except perhaps Cs263+. Diamonds represent the results of previous experiments by Martin and coworkers who identified trications for n ≥ 49.42 Their data were estimated from a mass spectrum and scaled. The difference in the size dependence of the two datasets beyond n ≈ 55 is likely caused by differences in the size distributions of neutral precursors and the way ions are formed; the difference is not relevant for the present discussion.
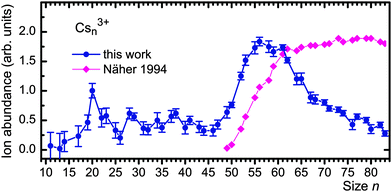 | ||
| Fig. 4 Full dots: ion abundance of Csn3+versus size n. Diamonds represent the results of photoionization studies by Näher et al.42 | ||
Mass spectra of helium droplets doped with sodium and other alkalis produce copious amounts of alkali cations (monomers as well as dimers) complexed with helium.47–49 These ions are often referred to as snowballs because electrostrictive forces solidify the helium matrix that surrounds the ion. One may wonder if HemAn2+ (A = sodium, potassium, cesium) dications can be detected for n > 1. The answer is no, but that is hardly surprising. Consider, for example, sodium. The most abundant dication is Na272+. Among all possible HemNa272+ ions the detection threshold would be best for He2Na272+; the ion could be identified if its abundance were at least 5% that of Na272+. To put this in perspective: the abundance of Nan+ with one or two helium attached is about 10% for Na+, 4% for Na2+, but only 0.5% for Na3+ (relative to the corresponding bare alkali ions). We cannot safely identify any singly charged HemNan+ ions containing more than 3 sodium atoms. Furthermore, although we observe atomic Na2+ its abundance is low; no HemNa2+ ions are detected. However, the abundance of Cs2+ is much higher and HemCs2+ ions appear at the one-percent level. The only other complexes of dications with helium reported so far (by the Meiwes-Broer group using femtosecond laser pulses50,51) are Mg2+, Ag2+ and Pb2+.
4. Discussion
The main results of our experiments are(1) Detection of Nan2+, Kn2+, Csn2+, and Csn3+ that are much smaller (by a factor 3 for Nan2+) than previously observed,
(2) Close agreement of the experimental appearance size nexp, the size of the smallest observed multiply charged clusters, with the Rayleigh limit nRay, the size for which the calculated fission barrier vanishes (i.e. cluster ions with fissility X = 1).
We emphasize that observation (1) is not merely the result of enhanced detection efficiency or resolution. These factors may lower nexp but hardly by more than a few percent.32 Instead, in the present work the dramatic decrease of nexp results from enhanced production of small multiply charged ions. This is apparent from the distributions displayed in Fig. 3 and 4. For example, in our work the abundance ratio of Cs192+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cs212+ is 2.4 times larger than that reported by Martin and coworkers.42 For K192+
Cs212+ is 2.4 times larger than that reported by Martin and coworkers.42 For K192+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K212+ our value is five times larger than the value reported by Brechignac et al.23 For Na252+
K212+ our value is five times larger than the value reported by Brechignac et al.23 For Na252+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na272+ we obtain about 1
Na272+ we obtain about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; Martin and coworkers52 could not observe Na252+ even though the mass resolution of their instrument (20
5; Martin and coworkers52 could not observe Na252+ even though the mass resolution of their instrument (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was seven times better than that of our instrument. From their spectrum41 we estimate that their ratio was below 1
000) was seven times better than that of our instrument. From their spectrum41 we estimate that their ratio was below 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500.
500.
Before discussing possible mechanisms for the enhanced production of small multiply charged cluster ions we turn to observation (2). Values of nRay and appearance sizes na calculated for doubly charged sodium, potassium and cesium clusters and for Cs trications are listed in Table 1 together with experimental appearance sizes nexp observed in past experiments and in the present work. One notes that (i) previously reported nexp values are close to na (about 20% lower for K and Cs, 10% higher for Na), (ii) our current nexp values are close to nRay. In other words, the fissility of the smallest observed multiply charged clusters is close to 1.
| n Ray (theory) | n a (theory) | n exp (publ) | n exp (this work) | |
|---|---|---|---|---|
| a Ref. 53. b Ref. 54. c Ref. 25. d Ref. 24. e Ref. 34. f Ref. 55. g Ref. 42. h Ref. 36. i Ref. 23. | ||||
| Nan2+ | 6a,b, 7c, 11d, 12e | 24e, 27c,d | 27f,g | 9 |
| Kn2+ | 7c,h, 11e, 12d | 22c, 24e | 19i | 11 |
| Csn2+ | 11e, 12d | 23d,e | 19g | 9 |
| Csn3+ | 19d, 27e | 51d, 57e | 49g | 19 (or 17) |
As nRay marks the size where the fission barrier vanishes one should always find nRay < nexp. The values nRay = 6 or 7 calculated for Nan2+ by Barnett et al.,53 Blaise et al.54 and Vieira et al.25 are consistent with this inequality, but the values 11 or 12 computed by Li et al.24 and Garcias et al.34 are not. Similarly, nRay values calculated for Kn2+ by Brechignac et al.36 and Vieira et al.25 are compatible with our experimental value for nexp while other calculated values are too high. In general, nRay values obtained by ab initio molecular dynamics simulations of Kn2+36 and Nan2+53,54 tend to be smaller, more compatible with our experimental results. For Csn2+ all calculated nRay values exceed our value nexp = 9. For Csn3+ we observe nexp = 19 (or perhaps 17) which is in reasonable agreement with the Rayleigh limit nRay = 19 deduced using a many-body potential based on local density calculations.24 Another value, nRay = 27 calculated by applying a semi-empirical model to compute fission barriers,34 is clearly too large.
Why is our experimental approach so efficient in the production of long-lived multiply charged ions near X = 1? There are two major differences between the present and previous studies, (i) the mechanism by which ions are formed, and (ii) the presence of the helium droplet that cools the neutral precursor to 0.37 K and may help to cool and/or cage the cluster after ionization.56
Electron ionization of doped helium droplets involves one or more intermediate steps. If the energy of the incident electrons exceeds the ionization threshold of helium (24.59 eV), ionization proceeds primarily via formation of He+ which will hop, on the timescale of femtoseconds, by resonant charge transfer.57 He+ may ionize the dopant, or localize by forming a vibrationally excited He2+. We have measured the yield of Csnz+ (z = 1, 2) versus electron energy and found a stepwise onset near 21 eV. This implies that ionization proceeds by Penning ionization, involving metastable, electronically excited He*, He2*, or anions.58,59 Moreover, neutral cesium clusters will reside on the surface of the droplet.60 Neutral sodium and potassium clusters will reside on the surface if they contain fewer than ≈20 or ≈80 atoms, respectively.44,60,61 Location on the surface favors Penning ionization over ionization by He+.61
According to Huber and coworkers, the ability to form multiply charged clusters near X = 1 is mainly limited by the initial cluster temperature.40 Our experiments involve ultracold (0.37 K) precursors but it is questionable if this is sufficient to suppress fission of nascent multiply charged clusters given that our mass spectra indicate strong fragmentation: in Fig. 3c K222+ stands out particularly strong. According to the spherical jellium model this ion, containing 20 valence electrons (in the configuration 1s21p61d102s2), is particularly stable.62 As growth of neutral clusters in helium droplets is a statistical process, the enhanced K222+ abundance must be a result of ionization induced fragmentation.63
Thus, we face a conundrum: evidence for fragmentation (appearance of magic numbers) and, at the same time, evidence for suppression of fission (X approaching 1). We will sketch two factors that may be relevant but more work is needed to pin down the exact mechanism. First, it is conceivable that multiply charged cluster ions form by successive ionization, involving two or more incident electrons as observed in some of our past work.59,64 Penning ionization at the surface will result in an alkali cluster ion An+ which will dive into the droplet and be cooled to 0.37 K. At some later time (within microseconds) another incident electron will form He* or He*− which will form Am2+ (m ≤ n) by Penning ionization. Although successive ionization of bare clusters by photons does not result in multiply charged clusters of high fissility,42 effective cooling and caging of singly charged cluster ions submersed in helium may be sufficient to suppress fission. Unfortunately the low ion yield of the newly observed small multiply charged cluster ions makes a systematic study of the dependence of their yield on the electron emission current all but impossible.
Second, fission proceeds on a slower timescale than monomer evaporation because the frequency of collective modes is relatively low; viscosity will further slow down the reaction.14,27,65 The extension of the timescale may be particularly strong for solid precursors.12 Möller and coworkers have reported a dramatic difference in the fission pattern of solid xenon clusters as compared to liquid neon clusters for fissilities near X = 1.37 The slower the rate of fission the larger the chance that the helium matrix can remove the excess energy and quench the reaction, provided the fissility is below 1.
The search for long-lived multiply charged clusters with high fissilities is motivated by the expectation of new physics.12,37,40,55 For metal clusters one expects that fission will change from highly asymmetric (emission of trimer ions) to symmetric as X approaches 1.12,26,66 Moreover, the strong effects of the electronic shell structure that govern fission of warm, liquid clusters with X well below 1 may be greatly reduced or even absent.12 Fission of doubly charged alkali clusters is often likened to alpha decay of radioactive nuclei because of the preference for emission of trimer ions which are favored by electronic shell effects.27 However, with increasing fissility and decreasing temperature the balance shifts toward more symmetric fission;26,66 this shift will also contribute to a slowing of the fission reaction.
5. Conclusions
Electron ionization of alkali clusters embedded in helium droplets results in doubly and triply charged clusters that are much smaller than observed previously; their fissility is approaching X = 1. We have discussed some factors that might enhance the yield of these small multiply charged cluster ions by suppressing rapid fission. Preparing clusters of high fissility is a first step; future experiments will have to characterize their fission reactions, for example by gentle heating with low-energy photons. Molecular dynamics simulations of multiply charged clusters embedded in helium would be helpful to unravel details.Acknowledgements
This work was supported by the Austrian Science Fund, Wien (FWF Projects I978 and P26635).References
- L. Rayleigh, Philos. Mag., 1882, 14, 184–186 CrossRef.
- W. Gu, P. E. Heil, H. Choi and K. Kimb, Appl. Phys. Lett., 2007, 91, 064104 CrossRef.
- S. Consta and A. Malevanets, Phys. Rev. Lett., 2012, 109, 148301 CrossRef PubMed.
- G. T. Liu, Y. C. Zhao, K. H. Zheng, Z. Liu, W. J. Ma, Y. Ren, S. S. Xie and L. F. Sun, Nano Lett., 2009, 9, 239–244 CrossRef PubMed.
- D. Duft, T. Achtzehn, R. Müller, B. A. Huber and T. Leisner, Nature, 2003, 421, 128 CrossRef PubMed.
- E. Giglio, B. Gervais, J. Rangama, B. Manil, B. A. Huber, D. Duft, R. Müller, T. Leisner and C. Guet, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2008, 77, 036319 CrossRef PubMed.
- P. E. Mason, F. Uhlig, V. Vanek, T. Buttersack, S. Bauerecker and P. Jungwirth, Nat. Chem., 2015, 7, 250–254 CrossRef PubMed.
- N. Bohr and J. A. Wheeler, Phys. Rev. A: At., Mol., Opt. Phys., 1939, 56, 426–450 Search PubMed.
- In the literature appearance sizes are often called critical sizes. The terms may pertain to experimental as well as theoretical values; we refer to experimental values as nexp and theoretical values as na, respectively. The Rayleigh limit nRay is the size at which the fissility X equals 1, i.e. the calculated fission barrier vanishes.
- K. Sattler, J. Mühlbach, O. Echt, P. Pfau and E. Recknagel, Phys. Rev. Lett., 1981, 47, 160–163 CrossRef.
- O. Echt and T. D. Märk, in Clusters of Atoms and Molecules II, ed. H. Haberland, Springer-Verlag, Berlin, 1st edn, 1994, vol. 56, pp. 183–220 Search PubMed.
- U. Näher, S. Bjørnholm, S. Frauendorf, F. Garcias and C. Guet, Phys. Rep., 1997, 285, 245–320 CrossRef.
- O. Echt, P. Scheier and T. D. Märk, C. R. Phys., 2002, 3, 353–364 CrossRef.
- J. G. Gay and B. J. Berne, Phys. Rev. Lett., 1982, 49, 194–198 CrossRef.
- D. Kreisle, O. Echt, M. Knapp, E. Recknagel, K. Leiter, T. D. Märk, J. J. Saenz and J. M. Soler, Phys. Rev. Lett., 1986, 56, 1551–1554 CrossRef PubMed.
- D. Kreisle, K. Leiter, O. Echt and T. D. Märk, Z. Phys. D, 1986, 3, 319–322 CrossRef.
- K. Leiter, D. Kreisle, O. Echt and T. D. Märk, J. Phys. Chem., 1987, 91, 2583–2586 CrossRef.
- O. Echt, D. Kreisle, E. Recknagel, J. J. Saenz, R. Casero and J. M. Soler, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3236–3248 CrossRef.
- N. G. Gotts, P. G. Lethbridge and A. J. Stace, J. Chem. Phys., 1992, 96, 408–421 CrossRef.
- I. Mähr, F. Zappa, S. Denifl, D. Kubala, O. Echt, T. D. Märk and P. Scheier, Phys. Rev. Lett., 2007, 98, 023401 CrossRef PubMed.
- G. H. Wu, X. J. Chen, A. J. Stace and P. Linse, J. Chem. Phys., 2011, 134, 031103 CrossRef PubMed.
- M. Nakamura, Y. Ishii, A. Tamura and S. Sugano, Phys. Rev. A: At., Mol., Opt. Phys., 1990, 42, 2267–2278 CrossRef.
- C. Brechignac, P. Cahuzac, F. Carlier, J. Leygnier and A. Sarfati, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 44, 11386–11393 CrossRef.
- Y. B. Li, E. Blaisten-Barojas and D. A. Papaconstantopoulos, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 15519–15532 CrossRef.
- A. Vieira and C. Fiolhais, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 7352–7359 CrossRef.
- C. Yannouleas, U. Landman, C. Brechignac, P. Cahuzac, B. Concina and J. Leygnier, Phys. Rev. Lett., 2002, 89, 173403 CrossRef PubMed.
- P. Fröbrich, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, 6450–6453 CrossRef.
- W. A. Saunders, Phys. Rev. Lett., 1990, 64, 3046–3049 CrossRef CAS PubMed.
- C. Brechignac, P. Cahuzac, F. Carlier and M. De Frutos, Phys. Rev. Lett., 1990, 64, 2893–2896 CrossRef CAS PubMed.
- S. Krückeberg, G. Dietrich, K. Lutzenkirchen, L. Schweikhard, C. Walther and J. Ziegler, Z. Phys. D, 1997, 40, 341–344 CrossRef.
- C. E. Klots, Nature, 1987, 327, 222–223 CrossRef.
- Experiments performed with high sensitivity may identify slightly smaller values of nexp, see e.g.ref. 33 and references therein.
- M. Daxner, S. Denifl, P. Scheier and O. Echt, Int. J. Mass Spectrom., 2014, 365–366, 200–205 CrossRef CAS PubMed.
- F. Garcias, R. J. Lombard, M. Barranco, J. A. Alonso and J. M. Lopez, Z. Phys. D, 1995, 33, 301–305 CrossRef CAS.
- It is possible to exceed X = 1, for example by photoionizing small singly charged clusters,36 photoexciting core electrons in neutral cluster,37 or exposing clusters to intense laser fields38,39 but only the fragments of those clusters are observable.
- C. Brechignac, P. Cahuzac, F. Carlier, M. De Frutos, R. N. Barnett and U. Landman, Phys. Rev. Lett., 1994, 72, 1636–1639 CrossRef CAS PubMed.
- M. Hoener, C. Bostedt, S. Schorb, H. Thomas, L. Foucar, O. Jagutzki, H. Schmidt-Böcking, R. Dörner and T. Möller, Phys. Rev. A: At., Mol., Opt. Phys., 2008, 78, 021201 CrossRef.
- A. E. Kaplan, B. Y. Dubetsky and P. L. Shkolnikov, Phys. Rev. Lett., 2003, 91, 143401 CrossRef CAS PubMed.
- A. Heidenreich, J. Jortner and I. Last, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10589–10593 CrossRef CAS PubMed.
- F. Chandezon, S. Tomita, D. Cormier, P. Grübling, C. Guet, H. Lebius, A. Pesnelle and B. A. Huber, Phys. Rev. Lett., 2001, 87, 153402 CrossRef CAS PubMed.
- U. Näher, H. Göhlich, T. Lange and T. P. Martin, Phys. Rev. Lett., 1992, 68, 3416 CrossRef PubMed.
- U. Näher, S. Frank, N. Malinowski, U. Zimmermann and T. P. Martin, Z. Phys. D, 1994, 31, 191–197 CrossRef.
- J. Tiggesbäumker and F. Stienkemeier, Phys. Chem. Chem. Phys., 2007, 9, 4748–4770 RSC.
- L. An der Lan, P. Bartl, C. Leidlmair, H. Schöbel, R. Jochum, S. Denifl, T. D. Märk, A. M. Ellis and P. Scheier, J. Chem. Phys., 2011, 135, 044309 CrossRef PubMed.
- S. Ralser, J. Postler, M. Harnisch, A. M. Ellis and P. Scheier, Int. J. Mass Spectrom., 2015, 379, 194–199 CrossRef CAS PubMed.
- The next close encounter between Csn3+ and helium cluster ions occurs at Cs293+ which would be separated from He321+ by Δm/z = 0.083 u. This coincidence causes no problem because Hem+ ions are not discernible beyond m ≈ 100.
- S. Müller, M. Mudrich and F. Stienkemeier, J. Chem. Phys., 2009, 131, 044319 CrossRef PubMed.
- M. Theisen, F. Lackner and W. E. Ernst, J. Chem. Phys., 2011, 135, 074306 CrossRef PubMed.
- L. An der Lan, P. Bartl, C. Leidlmair, R. Jochum, S. Denifl, O. Echt and P. Scheier, Chem. – Eur. J., 2012, 18, 4411–4418 CrossRef CAS PubMed.
- T. Döppner, T. Diederich, S. Gode, A. Przystawik, J. Tiggesbäumker and K. H. Meiwes-Broer, J. Chem. Phys., 2007, 126, 244513 CrossRef PubMed.
- T. Döppner, T. Diederich, A. Przystawik, N. X. Truong, T. Fennel, J. Tiggesbäumker and K. H. Meiwes-Broer, Phys. Chem. Chem. Phys., 2007, 9, 4639–4652 RSC.
- T. P. Martin, T. Bergmann, H. Göhlich and T. Lange, J. Phys. Chem., 1991, 95, 6421–6429 CrossRef CAS.
- R. N. Barnett, U. Landman and G. Rajagopal, Phys. Rev. Lett., 1991, 67, 3058–3061 CrossRef CAS PubMed.
- P. Blaise, S. A. Blundell, C. Guet and R. R. Zope, Phys. Rev. Lett., 2001, 87, 063401 CrossRef CAS PubMed.
- F. Chandezon, C. Guet, B. A. Huber, D. Jalabert, M. Maurel, E. Monnand, C. Ristori and J. C. Rocco, Phys. Rev. Lett., 1995, 74, 3784–3787 CrossRef CAS PubMed.
- A. Kautsch, M. Koch and W. E. Ernst, Phys. Chem. Chem. Phys., 2015, 17, 12310–12316 RSC.
- A. M. Ellis and S. F. Yang, Phys. Rev. A: At., Mol., Opt. Phys., 2007, 76, 032714 CrossRef.
- A. Mauracher, M. Daxner, J. Postler, S. E. Huber, S. Denifl, P. Scheier and J. P. Toennies, J. Phys. Chem. Lett., 2014, 5, 2444–2449 CrossRef CAS PubMed.
- M. Renzler, M. Daxner, N. Weinberger, S. Denifl, P. Scheier and O. Echt, Phys. Chem. Chem. Phys., 2014, 16, 22466–22470 RSC.
- C. Stark and V. V. Kresin, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 085401 CrossRef.
- L. An der Lan, P. Bartl, C. Leidlmair, H. Schöbel, S. Denifl, T. D. Märk, A. M. Ellis and P. Scheier, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 115414 CrossRef.
- W. A. De Heer, Rev. Mod. Phys., 1993, 65, 611–676 CrossRef CAS.
- Even-numbered Nan2+ and Csn2+ cannot be identified because the elements are monisotopic. Triply charged cesium cluster ions would have closed shells if n = 23, 43, and 61. Those ions are identified in Fig. 4 but they do not stand out as particularly strong. Csn+ would have closed electronic shells at n = 9 and 21, but only the first feature is reflected in the mass spectrum.
- H. Schöbel, P. Bartl, C. Leidlmair, M. Daxner, S. Zöttl, S. Denifl, T. D. Märk, P. Scheier, D. Spångberg, A. Mauracher and D. K. Bohme, Phys. Rev. Lett., 2010, 105, 243402 CrossRef PubMed.
- F. Calvo, Phys. Rev. A: At., Mol., Opt. Phys., 2006, 74, 043202 CrossRef.
- C. Brechignac, P. Cahuzac, B. Concina and J. Leygnier, Phys. Rev. Lett., 2004, 92, 083401 CrossRef CAS PubMed.
Footnote |
| † PACS numbers: 36.40.Qv, 79.77.+g, 36.40.Wa, 36.40.Qv, 34.50.Gb. |
| This journal is © the Owner Societies 2016 |