Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reactivity of atomically dispersed Pt2+ species towards H2: model Pt–CeO2 fuel cell catalyst†
Yaroslava
Lykhach
*a,
Alberto
Figueroba
b,
Matteo Farnesi
Camellone
c,
Armin
Neitzel
a,
Tomáš
Skála
 d,
Fabio R.
Negreiros
c,
Mykhailo
Vorokhta
d,
Nataliya
Tsud
d,
Kevin C.
Prince
ef,
Stefano
Fabris
c,
Konstantin M.
Neyman
d,
Fabio R.
Negreiros
c,
Mykhailo
Vorokhta
d,
Nataliya
Tsud
d,
Kevin C.
Prince
ef,
Stefano
Fabris
c,
Konstantin M.
Neyman
 bg,
Vladimír
Matolín
d and
Jörg
Libuda
ah
bg,
Vladimír
Matolín
d and
Jörg
Libuda
ah
aLehrstuhl für Physikalische Chemie II, Friedrich-Alexander-Universität Erlangen-Nürnberg, Egerlandstrasse 3, 91058 Erlangen, Germany. E-mail: yaroslava.lykhach@fau.de
bDepartament de Química Física and Institut de Quimica Teòrica i Computacional (IQTCUB), Universitat de Barcelona, c/Martí Franquès 1, 08028 Barcelona, Spain
cCNR-IOM DEMOCRITOS, Istituto Officina dei Materiali, Consiglio Nazionale delle Ricerche and SISSA, Via Bonomea 265, I-34136, Trieste, Italy
dCharles University, Faculty of Mathematics and Physics, Department of Surface and Plasma Science, V Holešovičkách 2, 18000 Prague, Czech Republic
eElettra-Sincrotrone Trieste SCpA, Strada Statale 14, km 163.5, 34149 Basovizza-Trieste, Italy
fIOM, Strada Statale 14, km 163.5, 34149 Basovizza-Trieste, Italy
gInstitució Catalana de Recerca i Estudis Avançats (ICREA), 08010 Barcelona, Spain
hErlangen Catalysis Resource Center, Friedrich-Alexander-Universität Erlangen-Nürnberg, Egerlandstrasse 3, 91058 Erlangen, Germany
First published on 19th February 2016
Abstract
The reactivity of atomically dispersed Pt2+ species on the surface of nanostructured CeO2 films and the mechanism of H2 activation on these sites have been investigated by means of synchrotron radiation photoelectron spectroscopy and resonant photoemission spectroscopy in combination with density functional calculations. Isolated Pt2+ sites are found to be inactive towards H2 dissociation due to high activation energy required for H–H bond scission. Trace amounts of metallic Pt are necessary to initiate H2 dissociation on Pt–CeO2 films. H2 dissociation triggers the reduction of Ce4+ cations which, in turn, is coupled with the reduction of Pt2+ species. The mechanism of Pt2+ reduction involves reverse oxygen spillover and formation of oxygen vacancies on Pt–CeO2 films. Our calculations suggest the existence of a threshold concentration of oxygen vacancies associated with the onset of Pt2+ reduction.
1. Introduction
Hydrogen fuel cells (FCs) have attracted significant attention during the last few years as environmentally friendly power sources for a variety of small to medium-scale applications, including automotive vehicle propulsion or even chip-integrated micro-devices.1–3 The high demand for noble metals is among the major challenges associated with low-temperature FC technology. Recently, it was shown that novel catalytic materials containing atomically dispersed Pt2+ species4–8 on the surface of nanostructured CeO2 are highly efficient anode catalysts for hydrogen FCs.8–10The mechanism of H2 activation on nanostructured CeO2 containing ionic Pt2+, however, remains controversial. One of the reasons is that the role of atomically dispersed Pt2+ on CeO2 has not been studied individually, i.e. in the absence of other active species such as Pt4+, Pt0, and oxygen vacancies.11–13 For instance, most of the studies employed Pt-doped CeO2 materials in the form of solid solutions, Ce1−xPtxO2−δ, prepared by the solution combustion method.11,12 This preparation method yields oxygen vacancies as the result of charge compensation upon substitution of Ce4+ ions by Pt2+ in the Ce1−xPtxO2−δ solid solution.12,14 In the most stable configuration, the Pt2+ is displaced from the Ce4+ site to adopt a square planar arrangement with four lattice oxygen ions and one oxygen vacancy is formed per Pt2+ site.15 In Ce1−xPtxO2−δ catalysts, the charge compensating oxygen vacancy is located within the square planar arrangement of Pt2+ ions forming a Pt–O3 moiety.5,12 In the corresponding configuration, Pt2+ has three Pt–O bonds with a length of about 200 pm.5,12
In contrast to the combustion solution method, physical vapor deposition (PVD) and radio frequency magnetron sputtering yield a Pt–O4 moiety without the oxygen vacancies.8 It was found that Pt2+ is anchored on the CeO2 support at {100} nanofacets, which form so-called O4 nanopockets, abundant at the surface of nanostructured cerium oxide.8 Here, the oxide support serves as a polydentate ligand for Pt2+ species and their binding can be described similarly to that in classical coordination compounds.16 The formation of the Pt–O4 moiety is not accompanied by creation of the charge compensating oxygen vacancy. Instead, two Ce4+ cations are reduced to Ce3+ per one Pt2+ site. The corresponding Ce3+ ions can be re-oxidized to Ce4+ in an oxygen atmosphere during the catalyst preparation. The exceptionally high stability of the Pt–O4 moiety8,17 is likely to be responsible for the excellent durability of the Pt–CeO2 based anode catalyst even under dynamic operation conditions.9,10
In the present study we provide new insights into the mechanism of hydrogen activation on Pt2+–CeO2 in the absence of oxygen vacancies and identify the active sites for hydrogen dissociation. Our model catalyst approach allows us to isolate Pt2+ sites as well as to control the amount of metallic Pt0 in the Pt–CeO2 films. We employed high-resolution synchrotron photoelectron spectroscopy (SRPES) and resonant photoemission spectroscopy (RPES) to monitor the oxidation states of Pt2+ and Ce4+ ions with ultimate surface sensitivity.
2. Materials and methods
2.1. Experimental details: SRPES and RPES
High-resolution SRPES experiments were performed at the Materials Science Beamline (MSB), Elettra synchrotron light facility in Trieste, Italy. The MSB, with a bending magnet source provides synchrotron light in the energy range of 21–1000 eV. The ultrahigh vacuum (UHV) end-station (base pressure 1 × 10−10 mbar) is equipped with a multichannel electron energy analyzer (Specs Phoibos 150), a rear view low energy electron diffraction (LEED) optics, a sputter gun (Ar), and a gas inlet system. The basic setup of the chamber includes a dual Mg/Al X-ray source.The Pt–CeO2 oxide films were prepared on a well-ordered CeO2(111) buffer layer grown on a single crystal Cu(111) substrate. Cu(111) (MaTecK GmbH, 99.999%) was cleaned by several cycles of Ar+ sputtering (300 K, 60 min) and annealing (723 K, 5 min) until no traces of carbon or any other contaminant were found in the photoelectron spectra. Then, an epitaxial CeO2(111) layer was deposited onto the clean Cu(111) substrate by PVD of Ce metal (Goodfellow, 99.99%) in an oxygen atmosphere (pO2 = 5 × 10−5 Pa, Linde, 99.999%) at 523 K. This preparation method18 yielded a continuous, stoichiometric CeO2(111) film with a thickness of 1.5 nm as determined from the attenuation of the Cu 2p3/2 intensity. In the next step, the mixed Pt–CeO2 films were prepared by means of simultaneous PVD of Ce and Pt (Goodfellow, 99.99%) metals in an oxygen atmosphere (pO2 = 5 × 10−5 Pa) onto the CeO2(111)/Cu(111) at 110 K. The low deposition temperature results in a growth of Pt–CeO2 nanoparticles on the CeO2(111) film.8
The Pt concentration of 5%, 15% and 18% per volume of the mixed layers was achieved by using different Pt/Ce deposition rate ratios. The deposition rate ratios were determined from the ratios of nominal thicknesses of Pt to CeO2 deposited during the same period of time. All Pt–CeO2/CeO2(111) were deposited onto a 1.5 nm thick CeO2(111) buffer layer at 110 K and maintained a nominal thickness of Pt–CeO2 film of 0.3 nm.
During the course of experiments, all Pt–CeO2/CeO2(111) samples were briefly annealed at 700 K followed by cooling to 110 K. This treatment eliminated the less stable Pt4+ species yielding exclusively Pt2+ for 5% Pt–CeO2 film. For 15% and 18% Pt–CeO2, Pt2+ is formed together with metallic Pt0 particles of different size (see ref. 8 for details). Then, the Pt–CeO2/CeO2(111) samples were exposed to 50 L of H2 (2.67 × 10−5 Pa, 250 s) at different temperatures between 110 and 700 K. Hydrogen (Linde 99.999%) was dosed by backfilling the UHV chamber.
The Pt 4f spectra were acquired with photon energies of 180 eV. The binding energies in the spectra acquired with synchrotron radiation were calibrated with respect to the Fermi level. Additionally, Al Kα radiation (1486.6 eV) was used to measure O 1s, Ce 3d, Pt 4f, and Cu 2p3/2 core levels. All spectra were acquired at constant pass energy and at an emission angle for the photoelectrons of 20° or 0° with respect to the sample normal, while using the X-ray source or synchrotron radiation, respectively. All spectral components were fitted with a Voigt profile. Pt 4f spectra were fitted by doublet peaks with a spin–orbit splitting of 3.30 eV and a fixed branching ratio of 1.33. Cu 3p spectra were fitted by a doublet with a fixed spin–orbit splitting of 2.42 eV and a fixed branching ratio of 2. A combination of a linear and Shirley background was subtracted from Pt 4f spectra.
Valence band spectra were acquired at three different photon energies, 121.4, 124.8, and 115.0 eV, that correspond to the resonant enhancements in Ce3+, Ce4+ ions, and to off-resonance conditions, respectively. Analysis of the spectra obtained with these photon energies forms the basis of RPES.19,20 The ratio between the corresponding resonant intensities, D(Ce3+)/D(Ce4+), denoted as a Resonant Enhancement Ratio (RER) is a direct measure of the degree of reduction of cerium oxide and can be used to quantify the concentration of Ce3+ ions in the films.21 All SRPES data were processed using KolXPD fitting software.22 The values of total spectral resolution were 1 eV (Al Kα), 200 meV (hν = 115–180 eV), 400 meV (hν = 410 eV) and 650 meV (hν = 650 eV). During the experiment, the sample temperature was controlled by a DC power supply passing a current through Ta wires holding the sample. Temperatures were monitored by a K-type thermocouple attached to the back of the sample.
2.2. Computational details: model Pt2+ sites on extended ceria surfaces
The theoretical study of the adsorption and dissociation of H2 on model Pt sites at extended CeO2 surfaces was based on the density functional theory (DFT) calculations employing the Perdew–Burke–Ernzerhof (PBE)23 generalized gradient approximation (GGA) for the exchange and correlation (XC) functional. In line with previous studies,24–26 the ions were described with Vanderbilt's ultrasoft pseudopotentials,27 while the crystal wavefunction and density were expanded with a plane-wave basis set limited by energy cutoffs of 40 and 320 Ry, respectively. It is well established that the usual local and gradient-corrected approximations of the XC functional cannot describe the correct insulating electronic structure of ceria-based materials.24,28,29 This is because the electron self-interaction effects arising from the high localization of the Ce 4f electrons are not properly cancelled out by these XC approximations. The addition of an on-site Hubbard U term to the GGA functional (GGA+U) has been proven to be a practical and efficient method to simulate ceria-based systems.24,28,29 For the periodic surface-slab calculations we used the GGA+U method as implemented by Cococcioni and de Gironcoli,30 employing a value of the parameter U = 4.5 eV in line with previous works.24,25,31 Integrals in the Brillouin zone were calculated at the Γ point. These calculations were performed using the Quantum Espresso package.32The CeO2(111) surface was modeled with 3 O–Ce–O tri-layers separated by more than 12 Å of vacuum normal to the surface. The coordinates of the lowermost tri-layer were constrained to their bulk-like optimized positions, while all other atoms were allowed to relax during the structural optimizations. The Pt6 cluster supported on the CeO2(111) surface was modeled as described in ref. 26. The CeO2 surface step was modeled with a vicinal surface exposing (110) facets between (111) terraces, and was described with monoclinic periodic slabs separated by more than 10 Å of vacuum in the direction perpendicular to the (111) terrace and labeled as step I in the ref. 33. The dimensions of the cell were 17.97 × 11.67 Å along the [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 2] and [1
2] and [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] directions, where the [1
0] directions, where the [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] direction is parallel to the step edge. The vicinal surface slabs were 3 CeO2 tri-layers thick. It has been shown that this thickness is sufficient to converge the structural and thermodynamic step properties.33 The Pt2+ ion at this step edge was obtained by adsorbing a Pt atom at the hollow site of the (110) facet of the vicinal surface. During the geometry optimization, the atomic positions of the lowermost CeO2 tri-layer were constrained as well as those of the Ce atoms in the central tri-layer, except for the Ce atoms below the step edge. All structures were relaxed by minimizing the atomic forces, until the maximum component of the residual forces on each ion was less than 0.02 eV Å−1.
0] direction is parallel to the step edge. The vicinal surface slabs were 3 CeO2 tri-layers thick. It has been shown that this thickness is sufficient to converge the structural and thermodynamic step properties.33 The Pt2+ ion at this step edge was obtained by adsorbing a Pt atom at the hollow site of the (110) facet of the vicinal surface. During the geometry optimization, the atomic positions of the lowermost CeO2 tri-layer were constrained as well as those of the Ce atoms in the central tri-layer, except for the Ce atoms below the step edge. All structures were relaxed by minimizing the atomic forces, until the maximum component of the residual forces on each ion was less than 0.02 eV Å−1.
To compute the minimum energy path for H2 dissociation we employed the Climbing-Image Nudge-Elastic-Band method34 using the Broyden scheme. These calculations were performed in the supercells described above including up to 17 replica images.
2.3. Computational details: model Pt2+ sites on ceria nanoparticles
The adsorption of atomic Pt on the {100} sites resulting in the formation of the Pt–O4 moiety on the ceria nanoparticles (NPs) has been investigated by means of periodic spin-polarized density functional calculations using the VASP code.35–37 In line with previous studies,19,38–40 present calculations of ceria NP systems have been performed using the PW9141 XC potential corrected with a value of U = 4 eV. The valence states were represented using plane-wave basis set with a cut-off of 415 eV for the kinetic energy. The core-valence electrons interaction has been described through the projector augmented wave method.42 Only the Γ-point has been used to sample the reciprocal space. The electron density was self-consistently converged with a 10−4 eV total energy threshold and structures were optimized until forces acting on all atoms were smaller than 0.02 eV Å−1. Formation energies of n oxygen vacancies in mPt–Ce40O80 models were calculated with respect to the energy of O2 molecule, E(O2), as follows: Evac = {E(mPt–Ce40O80−n) + n/2[E(O2)] − E(mPt–Ce40O80)}/n. Whereas ceria NPs of many different shapes and sizes coexist in experiments, simulated models have to be restricted to a model selected from a variety of low-energy nanoparticulate systems.39,40,43 We have chosen the NP Ce40O80 to represent in a qualitative fashion properties of the experimentally treated nanostructures. Our benchmark calculations have shown, for instance, that increasing the NP to Ce80O160 insignificantly affects the adsorption of metal atoms.8 Note also that the increase of the U-value to 4.5 eV (the value used in the calculations of the extended ceria surfaces, see Section 2.2) will strengthen the binding of Pt atom to the O4 site on the NP Ce40O80 by only 4%.83. Results and discussion
3.1. Reactivity of Pt–CeO2 films towards molecular hydrogen
Pt 4f spectra obtained from 5%, 15%, and 18% Pt–CeO2 films prepared by physical vapor co-deposition of Pt and Ce metals in an oxygen atmosphere onto CeO2/Cu(111) buffer layer followed by a brief annealing to 700 K in UHV are shown in Fig. 1 (top traces).The Pt 4f spectrum obtained from 5% Pt–CeO2 film contains a single spin–orbit doublet at 73.0 eV (Pt 4f7/2) associated with Pt2+ species in a square planar coordination, i.e. Pt–O4 moiety.8 A second broad spin–orbit doublet arises from the underlying Cu(111) substrate at 75.1 eV (Cu 3p3/2). Earlier we reported that Pt4+ ions formed in small amounts on Pt–CeO2 films prepared by PVD at 110 K are largely eliminated by annealing above 300 K in UHV.8,44 Besides the major contribution from Pt2+ species, two additional spin–orbit doublets emerge in the Pt 4f spectra obtained from 15% and 18% Pt–CeO2 films at 71.1–71.2 (Pt 4f7/2) and 72.0 eV (Pt 4f7/2). We associate the corresponding peaks with the presence of metallic (Pt0) and oxidized Pt particles (Pt*). We assume that the Pt0 particles on the samples with 15% and 18% Pt are partially covered by atomic oxygen due to the reverse oxygen spillover triggered by annealing.19,26 The same phenomenon likely leads to oxidation of smaller Pt particles resulting in the Pt* component in the spectra of both 15% and 18% Pt–CeO2 films.45 Earlier a similar component was detected by Hatanaka et al.46 and Wu et al.47 at 72.1 eV in the spectra of small oxidized Pt clusters supported on CeO2.
Previous calculations based on DFT predicted that the dissociation of molecular H2 on pristine CeO2(111) surfaces leads to surface hydroxylation and reduction of cerium cations.48–51 The dissociation of H2 is associated with a high activation barrier (∼1.0 eV)48–50 and therefore requires high pressures (∼105 Pa)52–56 and/or elevated temperatures. In our previous studies, however, we did not observe any activity of noble-metal-free CeO2(111) towards H2 dissociation under the conditions of a UHV model experiment (<10−4 Pa).57 This observation suggests that the reaction rate of hydrogen dissociation on pure CeO2(111) under the UHV conditions is too low to yield measurable changes in the oxidation state of cerium cations.
In order to detect whether H2 dissociation is possible on the Pt–CeO2 films, we monitored the oxidation states of both Pt and Ce cations. In particular, we employed resonant photoemission spectroscopy20 to quantify the oxidation state of Ce cations with high surface sensitivity (see Methods). The Pt 4f spectra obtained from 5%, 15%, and 18% Pt–CeO2 films exposed to hydrogen at different temperatures are shown in Fig. 1. The corresponding development of the relative concentrations of Pt2+, Pt0, and Pt* along with Ce3+ are plotted in Fig. 2. We found that interaction of H2 with the 5% Pt–CeO2 film does not cause reduction of Pt2+ or Ce4+ (Fig. 2a and d). This observation strongly suggests that the Pt2+ sites do not facilitate dissociation of molecular hydrogen under the experimental conditions employed.
To clarify this experimental finding we performed DFT calculations to compare the reaction pathways and the energetics for H2 dissociation on both the Pt2+ (Fig. 3, red line) and the CeO2(111) surface sites (Fig. 3, black line). The Pt–CeO2 system was modeled as a low-energy vicinal surface exposing CeO2(111) terraces and step edges along the [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0] direction.33 We found that Pt atoms bind at step facets forming planar Pt–O4 moieties that have structural and electronic properties very similar to Pt–O4 species identified on ceria nanoparticles.8 Our present calculations show that the activation energy for H2 dissociation at the Pt2+ sites is even larger than the barrier on the pristine CeO2(111) surface.
0] direction.33 We found that Pt atoms bind at step facets forming planar Pt–O4 moieties that have structural and electronic properties very similar to Pt–O4 species identified on ceria nanoparticles.8 Our present calculations show that the activation energy for H2 dissociation at the Pt2+ sites is even larger than the barrier on the pristine CeO2(111) surface.
 | ||
| Fig. 3 Minimum energy paths for H2 dissociation on the pristine CeO2(111) surface (black, adapted with permission from ref. 51. Copyright 2015 American Chemical Society), the ionic Pt2+ sites (red), and the supported Pt6 cluster (green) – middle panel. The top and bottom panels display the initial (IS), transition (TS), and final (FS) states of H2 dissociation on the Pt2+–CeO2 and Pt6/CeO2(111) systems. The activation energies in TS states (open symbols) are given in parentheses. Ce4+, Ce3+, and O2− ions are displayed as yellow, brown, and red spheres, respectively. | ||
Compared to the value of 1.0 eV calculated for the pristine CeO2(111) surface,51 the barrier increases to 1.2 eV in the presence of the Pt2+ sites at the steps. The lower propensity towards H2 adsorption on these Pt2+ sites can be explained by electrostatic arguments as proposed by García-Melchor and López.48 The higher activation energy calculated for the Pt2+ sites implies that H2 desorption is strongly favored against dissociation, in accordance with the experiment showing no activity for the 5% Pt–CeO2 system.
In sharp contrast to the 5% Pt–CeO2, we detected facile reduction of Pt2+ species coupled with the reduction of Ce4+ on both 15% and 18% Pt–CeO2 samples upon reaction with H2 at elevated temperatures (see Fig. 1b, c, 2b, c, e and f). Eventually, all Pt2+ species are reduced quantitatively to metallic Pt0. Remarkably, very small amounts of Pt0 turned out to be sufficient to initiate the reduction process (see Fig. 1b, 2b and e). Note that such small amounts of metallic Pt0 as are present on the 15% Pt–CeO2 film (corresponding to a nominal coverage of less than 0.05 Å Pt) could be hardly detected by conventional X-ray photoelectron spectroscopy. Quite consistently, our DFT simulations show that sub-nm sized Pt clusters supported on ceria are, indeed, highly active for H2 dissociation. For instance, we calculated an activation energy of ∼0.3 eV (Fig. 3, green line) for Pt6 particle supported on CeO2(111), which can be easily overcome even at low temperature.
3.2. The mechanism of Pt2+ reduction in the presence of metallic Pt particles
In view of the high stability of the Pt–O4 moiety,8 the observed reduction of Pt2+ sites upon reaction with hydrogen may appear surprising. However, we reported previously that the adsorption of atomic hydrogen, leading to hydroxylation of the oxygen ions of the Pt–O4 moiety and reduction of Ce4+ results in destabilization and, possibly, in reduction of Pt2+ species.8 In the presence of metallic Pt0 on Pt–CeO2, atomic hydrogen could spillover from Pt particles to the support leading to hydroxylation of the Pt–O4 moiety. In fact, the calculated reaction pathway on Pt6/CeO2 (Fig. 3, final state bottom panels) suggests the tendency for H to migrate to the cluster periphery and accumulate at the boundary in contact with the oxide support.On Pt/CeO2(111) model catalysts, reversible hydrogen spillover is typically indicated by an increase of the RER between 190 and 260 K by about ΔRER = 0.3.57 On both the 15% and the 18% Pt–CeO2 samples (Fig. 2e and f) the corresponding effect is much weaker. Therefore, we assume that there is only little tendency for hydrogen spillover from the metallic Pt particles to the oxide for the Pt–CeO2 catalysts employed in the present work. This suggests that hydrogen spillover and hydroxylation of Pt–O4 are not the key steps in Pt2+ reduction during the reaction with H2.
Thus, the mechanism of Pt2+ reduction is likely to be associated with the formation of oxygen vacancies upon reverse oxygen spillover19 from Pt–CeO2 to the Pt particles. Upon exposure to H2 the spilt-over oxygen is continuously removed from the Pt particles by reaction with hydrogen and formation of water which desorbs immediately. This reaction channel leads to formation of oxygen vacancies accompanied by the reduction of Ce4+. The corresponding scenario is consistent with the absence of hydroxyl groups in the O 1s spectra on 15% and 18% Pt–CeO2 films exposed to H2 above 350 K (data not shown). The developments of the Pt2+ (Fig. 2b and c) and the Ce3+ (Fig. 2e and f) concentrations show that reduction of Ce4+ slightly precedes the reduction of Pt2+. This suggests that the Pt2+ ions are destabilized in the presence of oxygen vacancies and at sufficiently high vacancy concentration are eventually reduced to Pt0.
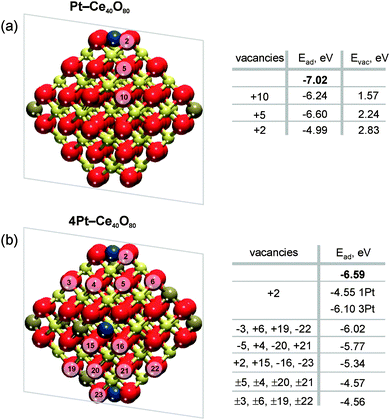
Indeed, our DFT calculations show that the formation of oxygen vacancies notably lowers the adsorption energy of Pt atom in Pt–Ce40O80 and 4Pt–Ce40O80 NPs (see Fig. 4). The stability of Pt2+ depends on various parameters such as the proximity of the oxygen vacancies, their number, the Pt2+ concentration, and the distribution of Ce3+ ions (see ESI† for details).
The criterion for Pt2+ being stable against formation of metallic particles is that the magnitude of adsorption energy of atomic Pt, Ead, is larger than the bulk cohesive energy value of Pt (−5.85 eV).58 For isolated Pt2+ in the {100} O4 nanopocket of stoichiometric CeO2 this is clearly the case (Ead = −7.02 eV). The formation of a single oxygen vacancy in a position outside the {100} nanopocket (Pt–Ce40O79, vacancy positions +5 or +10, see Fig. 4) is not energetically sufficient to trigger the reduction of Pt2+ cations. The same holds in the case where four vacancies have been created outside the pockets on NP with four Pt2+ ions (4Pt–Ce40O76, vacancy positions −3, 6, 19, −22 or +4, −5, −20, 21, see Fig. 4).
The adsorption energy of atomic Pt in the 2+ state falls dramatically below the cohesive energy (to −4.55 or −4.99 eV) if a single vacancy is created inside the square pocket (position +2). Such decrease is a direct consequence of the loss of the square-planar coordination for the Pt2+ cations. However, the vacancy formation energy is strongly enhanced at the pocket sites themselves (see Tables S1 and S2, ESI†). This suggests that, at least initially, the oxygen vacancies will be formed preferentially outside the Pt–O4 moiety. If two of such oxygen vacancies are created per Pt2+ ion, we indeed find configurations in which the Pt2+ adsorption energy falls well below the cohesive energy. This is the case, for instance, upon creation of eight oxygen vacancies in the 4Pt–Ce40O80 system (4Pt–Ce40O72, vacancy position ±4, ±5, ±20, ±21 or ±3, ±6, ±22, ±19, see Fig. 4). Thus, DFT calculations indicate that the onset of Pt2+ reduction occurs when approximately two oxygen vacancies are created per each Pt2+ site.
Interestingly, a rough estimation of the ratio between the concentrations of oxygen vacancies and the Pt2+ cations at the onset of Pt2+ reduction can be obtained from the experimental data (see ESI,† Table S3). The concentration of oxygen vacancies produced by oxygen spillover can be calculated from the temperature dependent change in the Ce3+ concentration, taking into account that two Ce3+ ions are produced per oxygen vacancy (note that the Ce3+ ions present at low temperature originate from the charge transfer from metallic particles without the generation of oxygen vacancies).19,59 As well, a certain number of Ce3+ ions emerge upon the redox reaction between Pt and CeO2 during the growth of Pt2+–CeO2 films.8 In addition we have to take into account the consumption of Pt2+ ions because the reduction of one Pt2+ ion leads to formation of two additional Ce4+ centers, which subsequently may be reduced to Ce3+ by formation of one oxygen vacancy. Taking both contributions into account, we obtain a ratio of 1.5 between the concentration of the oxygen vacancies and the concentration of Pt2+ ions in the temperature region between 450 and 500 K, where the onset of Pt2+ reduction is observed (see ESI† for details of the evaluation procedure). It is noteworthy that this value is in fairly good agreement with the results of our DFT calculations.
4. Conclusions
We have studied H2 activation on Pt–CeO2 model catalysts under UHV conditions by means of SRPES, RPES, and DFT calculations. The isolated Pt2+ sites on the catalyst surface are found to be inactive towards H2 dissociation. The activation of H2 on Pt–CeO2 requires trace amounts of metallic Pt0 to be present on the surface. The low reactivity of the Pt2+ sites is manifested by a high activation barrier for H2 dissociation which exceeds even the barrier on pristine CeO2(111). In the presence of small amounts of metallic Pt0, the surface becomes very active towards hydrogen dissociation and Pt2+ is rapidly reduced. The proposed reduction mechanism involves reverse spillover of oxygen from the Pt–CeO2 support to the Pt0 particles followed by the clean-off reaction with hydrogen. Reduction of the ceria support subsequently leads to substantial destabilization of the ionic Pt2+ species. Once a threshold concentration of oxygen vacancies is reached, Pt2+ is reduced and metallic Pt particles are formed.Author contributions
Y. L., A. N., T. S., M. V., and N. T. performed the SRPES and RPES experiments. K. C. P. contributed to the SRPES and RPES experiments and operation of the experimental facilities. J. L. and V. M. supervised the experimental work. Y. L., A. N., and J. L. were involved in the analysis of the experimental data. A. F., M. F. C. and F. R. N. performed the computational modelling supervised by K. M. N. and S. F. All co-authors were involved in the preparation of the manuscript.Acknowledgements
This work was funded by the European Community (FP7-NMP.2012.1.1-1 project ChipCAT, Reference No. 310191), by the Deutsche Forschungsgemeinschaft (DFG) within the Excellence Cluster “Engineering of Advanced Materials” in the framework of the excellence initiative, by Spanish MINECO (grants CTQ2012-34969 and CTQ2015-64618-R), by the Generalitat de Catalunya (grants 2014SGR97 and XRQTC), and by the Czech Science Foundation (grant 15-06759S). The authors acknowledge a support by the COST Action CM1104 “Reducible oxide chemistry, structure and functions”. Computer resources, technical expertise and assistance were provided by the Red Española de Supercomputación and by the ISCRA initiative of the CINECA consortium. Y. L., A. N., M. V., N. T., and K. C. P. thank Elettra for excellent working conditions and support.References
- R. F. Service, Science, 2002, 296, 1222–1224 CrossRef CAS PubMed.
- L. Carrette, K. A. Friedrich and U. Stimming, ChemPhysChem, 2000, 1, 162–193 CrossRef CAS PubMed.
- M. Z. Jacobson, W. G. Colella and D. M. Golden, Science, 2005, 308, 1901–1905 CrossRef CAS PubMed.
- M. Flytzani-Stephanopoulos and B. C. Gates, Annu. Rev. Chem. Biomol. Eng., 2012, 3, 545–574 CrossRef CAS PubMed.
- M. S. Hegde, G. Madras and K. C. Patil, Acc. Chem. Res., 2009, 42, 704–712 CrossRef CAS PubMed.
- E. W. McFarland and H. Metiu, Chem. Rev., 2013, 113, 4391–4427 CrossRef CAS PubMed.
- M. S. Hegde and P. Bera, Catal. Today, 2015, 253, 40–50 CrossRef CAS.
- A. Bruix, Y. Lykhach, I. Matolínová, A. Neitzel, T. Skála, N. Tsud, M. Vorokhta, V. Stetsovych, K. Ševčiková, J. Mysliveček, R. Fiala, M. Václavů, K. C. Prince, S. Bruyere, V. Potin, F. Illas, V. Matolín, J. Libuda and K. M. Neyman, Angew. Chem., Int. Ed., 2014, 53, 10525–10530 CrossRef CAS PubMed.
- R. Fiala, M. Vaclavu, M. Vorokhta, I. Khalakhan, J. Lavková, V. Potin, I. Matolínová and V. Matolín, J. Power Sources, 2015, 273, 105–109 CrossRef CAS.
- R. Fiala, M. Vaclavu, A. Rednyk, I. Khalakhan, M. Vorokhta, J. Lavkova, V. Potin, I. Matolinova and V. Matolin, Catal. Today, 2015, 240(part B), 236–241 CrossRef CAS.
- G. Dutta, U. V. Waghmare, T. Baidya and M. S. Hegde, Chem. Mater., 2007, 19, 6430–6436 CrossRef CAS.
- P. Bera, K. R. Priolkar, A. Gayen, P. R. Sarode, M. S. Hegde, S. Emura, R. Kumashiro, V. Jayaram and G. N. Subbanna, Chem. Mater., 2003, 15, 2049–2060 CrossRef CAS.
- B. D. Mukri, U. V. Waghmare and M. S. Hegde, Chem. Mater., 2013, 25, 3822–3833 CrossRef CAS.
- M. Nolan, J. Mater. Chem., 2011, 21, 9160–9168 RSC.
- D. O. Scanlon, B. J. Morgan and G. W. Watson, Phys. Chem. Chem. Phys., 2011, 13, 4279–4284 RSC.
- A. G. Hu, K. M. Neyman, M. Staufer, T. Belling, B. C. Gates and N. Rosch, J. Am. Chem. Soc., 1999, 121, 4522–4523 CrossRef CAS.
- H. A. Aleksandrov, K. M. Neyman and G. N. Vayssilov, Phys. Chem. Chem. Phys., 2015, 17, 14551–14560 RSC.
- F. Šutara, M. Cabala, L. Sedláček, T. Skála, M. Škoda, V. Matolín, K. C. Prince and V. Cháb, Thin Solid Films, 2008, 516, 6120–6124 CrossRef.
- G. N. Vayssilov, Y. Lykhach, A. Migani, T. Staudt, G. P. Petrova, N. Tsud, T. Skála, A. Bruix, F. Illas, K. C. Prince, V. Matolín, K. M. Neyman and J. Libuda, Nat. Mater., 2011, 10, 310–315 CrossRef CAS PubMed.
- V. Matolin, I. Matolinova, L. Sedlacek, K. C. Prince and T. Skala, Nanotechnology, 2009, 20, 215706 CrossRef CAS PubMed.
- T. Staudt, Y. Lykhach, N. Tsud, T. Skála, K. C. Prince, V. Matolín and J. Libuda, J. Catal., 2010, 275, 181–185 CrossRef CAS.
- J. Libra, KolXPD: Spectroscopy Data Measurement and Processing, http://www.kolibrik.net/science/kolxpd/, Accessed 15.03.2015, 2015.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- S. Fabris, G. Vicario, G. Balducci, S. de Girancoli and S. Baroni, J. Phys. Chem. B, 2005, 109, 22860–22867 CrossRef CAS PubMed.
- L. Szabová, O. Stetsovych, F. Dvořák, M. Farnesi Camellone, S. Fabris, J. Mysliveček and V. Matolín, J. Phys. Chem. C, 2012, 116, 6677–6684 Search PubMed.
- F. R. Negreiros and S. Fabris, J. Phys. Chem. C, 2014, 118, 21014–21020 CAS.
- D. Vanderbilt, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 7892–7895 CrossRef.
- J. Paier, C. Penschke and J. Sauer, Chem. Rev., 2013, 113, 3949–3985 CrossRef CAS PubMed.
- M. V. Ganduglia-Pirovano, J. L. F. Da Silva and J. Sauer, Phys. Rev. Lett., 2009, 102, 026101 CrossRef PubMed.
- M. Cococcioni and S. de Gironcoli, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 035105 CrossRef.
- L. Szabova, M. F. Camellone, M. Huang, V. Matolin and S. Fabris, J. Chem. Phys., 2010, 133, 234705 CrossRef PubMed.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni, I. Dabo, A. D. Corso, S. de Gironcoli, S. Fabris, G. Fratesi, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, M. Lazzeri, L. Martin-Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A. P. Seitsonen, A. Smogunov, P. Umari and R. M. Wentzcovitch, J. Phys.: Condens. Matter, 2009, 21, 395502 CrossRef PubMed.
- S. M. Kozlov, F. Viñes, N. Nilius, S. Shaikhutdinov and K. M. Neyman, J. Phys. Chem. Lett., 2012, 3, 1956–1961 CrossRef CAS.
- G. Henkelman, B. P. Uberuaga and H. Jónsson, J. Chem. Phys., 2000, 113, 9901–9904 CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. N. Vayssilov, A. Migani and K. Neyman, J. Phys. Chem. C, 2011, 115, 16081–16086 CAS.
- A. Migani, G. N. Vayssilov, S. T. Bromley, F. Illas and K. M. Neyman, Chem. Commun., 2010, 46, 5936–5938 RSC.
- A. Migani, G. N. Vayssilov, S. T. Bromley, F. Illas and K. M. Neyman, J. Mater. Chem., 2010, 20, 10535–10546 RSC.
- J. P. Perdew and Y. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 45, 13244–13249 CrossRef.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef.
- M. A. Sk, S. M. Kozlov, K. H. Lim, A. Migani and K. M. Neyman, J. Mater. Chem. A, 2014, 2, 18329–18338 CAS.
- A. Neitzel, Y. Lykhach, T. Skála, N. Tsud, M. Vorokhta, D. Mazur, K. C. Prince, V. Matolín and J. Libuda, Phys. Chem. Chem. Phys., 2014, 16, 24747–24754 RSC.
- D. J. Miller, H. Öberg, S. Kaya, H. Sanchez Casalongue, D. Friebel, T. Anniyev, H. Ogasawara, H. Bluhm, L. G. M. Pettersson and A. Nilsson, Phys. Rev. Lett., 2011, 107, 195502 CrossRef CAS PubMed.
- M. Hatanaka, N. Takahashi, N. Takahashi, T. Tanabe, Y. Nagai, A. Suda and H. Shinjoh, J. Catal., 2009, 266, 182–190 CrossRef CAS.
- T. Wu, X. Pan, Y. Zhang, Z. Miao, B. Zhang, J. Li and X. Yang, J. Phys. Chem. Lett., 2014, 5, 2479–2483 CrossRef CAS PubMed.
- M. García-Melchor and N. López, J. Phys. Chem. C, 2014, 118, 10921–10926 Search PubMed.
- D. Fernández-Torre, J. Carrasco, M. V. Ganduglia-Pirovano and R. Pérez, J. Chem. Phys., 2014, 141, 014703 CrossRef PubMed.
- G. Vicario, G. Balducci, S. Fabris, S. de Gironcoli and S. Baroni, J. Phys. Chem. B, 2006, 110, 19380–19385 CrossRef CAS PubMed.
- F. R. Negreiros, M. F. Camellone and S. Fabris, J. Phys. Chem. C, 2015, 119, 21567–21573 CAS.
- S. Bernal, J. J. Calvino, G. A. Gifredo, J. M. Rodriguez-Izquiero, V. Perrichon and A. Laachir, J. Catal., 1992, 137, 1–11 CrossRef CAS.
- A. Laachir, V. Perrichon, A. Badri, J. Lamotte, E. Catherine, J. C. Lavalley, J. El Fallah, L. Hilaire, F. Le Normand, E. Quéméré, G. N. Sauvion and O. Touret, J. Chem. Soc., Faraday Trans., 1991, 87, 1601–1609 RSC.
- G. Vilé, B. Bridier, J. Wichert and J. Pérez-Ramírez, Angew. Chem., Int. Ed., 2012, 51, 8620–8623 CrossRef PubMed.
- M. Boaro, M. Vicario, C. de Leitenburg, G. Dolcetti and A. Trovarelli, Catal. Today, 2003, 77, 407–417 CrossRef CAS.
- J. Carrasco, G. Vilé, D. Fernández-Torre, R. Pérez, J. Pérez-Ramírez and M. V. Ganduglia-Pirovano, J. Phys. Chem. C, 2014, 118, 5352–5360 CAS.
- Y. Lykhach, T. Staudt, M. Vorokhta, T. Skála, V. Johánek, K. C. Prince, V. Matolín and J. Libuda, J. Catal., 2012, 285, 6–9 CrossRef CAS.
- C. Kittel, Introduction to Solid State Physics, Wiley, 8th edn, 2004 Search PubMed.
- Y. Lykhach, M. Kozlov, T. Skála, A. Tovt, V. Stetsovych, N. Tsud, F. Dvořák, V. Johánek, A. Neitzel, J. Mysliveček, S. Fabris, V. Matolín, K. M. Neyman and J. Libuda, Nat. Mater., 2016, 15 DOI:10.1038/nmat4500.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cp00627b |
| This journal is © the Owner Societies 2016 |