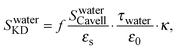Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Quantifying transient interactions between amide groups and the guanidinium cation
V.
Balos
,
M.
Bonn
and
J.
Hunger
*
Max Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany. E-mail: hunger@mpip-mainz.mpg.de
First published on 14th December 2015
Abstract
Correction for ‘Quantifying transient interactions between amide groups and the guanidinium cation’ by V. Balos et al., Phys. Chem. Chem. Phys., 2015, 17, 28539–28543.
We correct an error to our previous paper,1 which does not affect any of the conclusions in that paper, but changes some of the inferred values. For the extraction of the concentration of free NMA in solution from the measured dielectric spectra, as shown in Fig. 3, the contribution of the hydration, Shyd of NaCl, LiCl and MgCl2, was calculated incorrectly. The erroneous calculation originates from the kinetic depolarization correction (eqn (S5) in the ESI of the original article):
The number of NMA molecules interacting with the different cations obtained from fitting eqn (3) to the data shown in Fig. 3 are nLiCl = (2.8 ± 0.4) and nMgCl2 = (2.6 ± 0.7). The featureless, nearly linear decrease in cNMA,free upon addition of NaCl does not allow for determination of nNaCl independent of K (nNaCl = (16 ± 15)).
The association constants assuming n = 2 are reduced accordingly to K = 0.13 L2 mol−2 (Mg2+), 0.13 L2 mol−2 (Li+), and 0.04 L2 mol−2 (Na+).
As the erroneous calculation only affected Shyd and for solutions of GdmCl Shyd = 0, our results for GdmCl are not altered. Thus, our main conclusions and the reported association constants between GdmCl and amide are not affected, except that the interaction of GdmCl with amides is not similar to NaCl, but intermediate between NaCl and LiCl.
References
1 V. Balos, M. Bonn and J. Hunger, Phys. Chem. Chem. Phys., 2015, 17, 28539–28543.2 R. Buchner, G. T. Hefter and P. M. May, J. Phys. Chem. A, 1999, 103, 1–9.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © the Owner Societies 2016 |