Roles of the scalar and vector components of the solvation effects on the vibrational properties of hydrogen- or halogen-bond accepting stretching modes†
Abstract
Solvation-induced vibrational frequency shifts and infrared (IR) intensity changes of the hydrogen- or halogen-bond accepting stretching modes, especially their dependence on the angular position of the hydrogen- or halogen-bond donating molecule, are examined theoretically. Calculations are carried out for some modes of hydrogen- or halogen-bonding molecular complexes, including the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of dimethyl sulfoxide-13C2⋯H2O, the C
O stretch of dimethyl sulfoxide-13C2⋯H2O, the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretch of acetonitrile⋯H2O, and the amide I′ mode of the N-methylacetamide-d1⋯BrNC 1 : 1 complex. It is shown that, in all the example cases dealt with in this study, the frequency shift depends rather strongly on the hydrogen- or halogen-bond angle (e.g., S
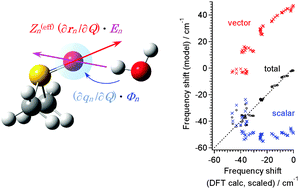
N stretch of acetonitrile⋯H2O, and the amide I′ mode of the N-methylacetamide-d1⋯BrNC 1 : 1 complex. It is shown that, in all the example cases dealt with in this study, the frequency shift depends rather strongly on the hydrogen- or halogen-bond angle (e.g., S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H angle), with a larger low-frequency shift as the hydrogen or halogen bond becomes more bent, indicating the generality of the results obtained for the amide I′ mode of the N-methylacetamide-d1⋯2H2O 1 : 1 complex in a previous study. Contrary to our vague expectation, the frequency shift is not well correlated to the hydrogen- or halogen-bond distance or strength, but nevertheless, it is well reproduced by an electrostatic interaction model if it is carefully constructed by considering the scalar and vector components separately in a reasonable way. On the basis of this electrostatic interaction model, the reason why our vague expectation is not realized is clarified, and a unified understanding is achieved on the hydration-induced high-frequency shift of the C
O⋯H angle), with a larger low-frequency shift as the hydrogen or halogen bond becomes more bent, indicating the generality of the results obtained for the amide I′ mode of the N-methylacetamide-d1⋯2H2O 1 : 1 complex in a previous study. Contrary to our vague expectation, the frequency shift is not well correlated to the hydrogen- or halogen-bond distance or strength, but nevertheless, it is well reproduced by an electrostatic interaction model if it is carefully constructed by considering the scalar and vector components separately in a reasonable way. On the basis of this electrostatic interaction model, the reason why our vague expectation is not realized is clarified, and a unified understanding is achieved on the hydration-induced high-frequency shift of the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretch and the low-frequency shifts of the S
N stretch and the low-frequency shifts of the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch and amide I′. With regard to the IR intensity, it is shown that, in some of the example cases, it also has rather strong angular position dependence. The mechanism of the IR intensity changes is estimated by analyzing the dipole derivative vector, especially its angular relation with the hydrogen or halogen bond.
O stretch and amide I′. With regard to the IR intensity, it is shown that, in some of the example cases, it also has rather strong angular position dependence. The mechanism of the IR intensity changes is estimated by analyzing the dipole derivative vector, especially its angular relation with the hydrogen or halogen bond.

 Please wait while we load your content...
Please wait while we load your content...