Rationale for the implementation of reference electrodes in ionic liquids
Abstract
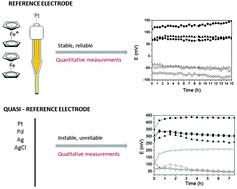
A thorough investigation of the reference electrodes for electrochemical measurements is presented in three ionic liquids (ILs), namely 1-butyl-3-butylimidazolium bis(trifluoromethanesulfonyl)imide [BMIM][Tf2N], 1-octyl-3-butylimidazolium bis(trifluoromethanesulfonyl)imide [OMIM][Tf2N] and 1-butyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide [BMPyrr][Tf2N]. The influence of technical aspects such as internal resistance, scan rate and concentration of the active species on cyclic voltammetry has been investigated using three quasi-reference electrodes (QREs) (AgCl, Pt, and Pd) and three reference electrodes (REs) (Fc+/Fc/Pt, Ag+/Ag, and Cl−/AgCl/Ag). The results show that the internal resistance has to be taken dynamically into account during any cyclic voltammetry experiment. Quasi-reference electrodes can be used only for qualitative electrochemical studies. Reference electrodes based on Ag+/Ag or Fc+/Fc dissolved in an IL have a stable potential over 15 h and thus can be used to perform reliable electrochemical experiments in ionic liquids and to get quantitative information such as potentials.

 Please wait while we load your content...
Please wait while we load your content...