High-performance lithium storage in an ultrafine manganese fluoride nanorod anode with enhanced electrochemical activation based on conversion reaction†
Abstract
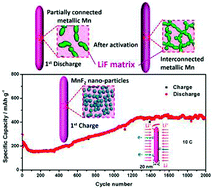
A facile, one-step solvothermal reaction route for the preparation of manganese fluoride nanorods is successfully developed using manganese(II) chloride tetrahydrate (MnCl2·4H2O) as the manganese source and the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate (BmimBF4) as the fluorine source. X-ray diffraction, field-emission scanning electron microscopy and high-resolution transmission electron microscopy (HRTEM) are conducted to characterize the structural and microstructural properties of the synthesized MnF2. The pure-phase tetragonal MnF2 displays nanorod-like morphology with a diameter of about 20 nm and a length of several hundreds of nanometers. The electrochemical performance of the MnF2 nanorod anode for rechargeable lithium batteries is investigated. A reversible discharge capacity as high as 443 mA h g−1 at 0.1 C is obtained for the lithium uptake reaction with an initial discharge plateau around 0.7 V. The striking enhancement in electrochemical Li storage performance in ultrafine MnF2 nanorods can be attributed to the small diameters of the nanorods and efficient one-dimensional electron transport pathways. Long cycle performance for 2000 cycles at 10 C with a stabilized capacity of about 430 mA h g−1 after activation is also achieved. Furthermore, lithiated and delithiated MnF2 anodes are analyzed with HRTEM to elucidate the conversion mechanism for the electrochemical reaction of MnF2 nanorods with Li at a microscopic level.

 Please wait while we load your content...
Please wait while we load your content...