Fundamental insights into the electronic structure of zigzag MoS2 nanoribbons†
Abstract
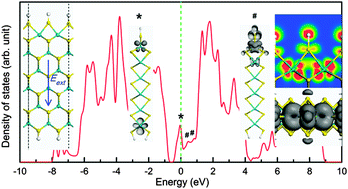
The structural and electronic properties of zigzag MoS2 nanoribbons are investigated using first-principles density functional theory. Our models are motivated by the experimental observations, in which both Mo edges are terminated by S atoms. Our calculations show that the edge can introduce some extra states into the energy gap, which lead nanoribbons to exhibit a metallic characteristic. Such extra states around the Fermi level are flat or dispersed. Through detailed analyses, we identify and discriminate them based on the major contributors. By applying an external transverse electric field, Eext the extra states around the Fermi level can shift apparently, especially for those attributed to Mo-edge atoms. It can be explained by the charge redistribution in the MoS2 nanoribbons due to Eext. In addition, the nanoribbon can be changed from metal to an n/p-type semiconductor according to different edge hydrogenation. After full edge hydrogenation, we observe a characteristic of anti-bonding orbitals between H and S atoms at the Mo-edge. Interestingly, the energy of anti-bonding orbitals and electric conductivity of nanoribbons can be tailored by Eext. The results suggest a strategy controlling the performance of MoS2 for hydrogen evolution.


 Please wait while we load your content...
Please wait while we load your content...