Aromatic stabilization of functionalized corannulene cations†
Abstract
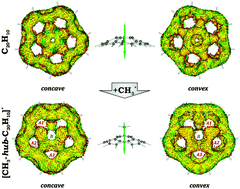
The first comprehensive theoretical investigation of aromaticity in functionalized corannulene cations of general formula [CH3–C20H10]+ was accomplished. The experimentally known system [CH3-hub-C20H10]+ was augmented by two other possible isomers, namely, rim- and spoke-ones. Changes in aromaticity, when going from neutral corannulene to its functionalized cations, were monitored with the help of descriptors of different nature such as structure-based HOMA, topological PDI and FLU, and magnetic NICS. A highly efficient tool for analysis and visualization of delocalization and conjugation named ACID was also utilized. In the final step, a complete set of 1H and 13C chemical shifts was calculated and compared with the available experimental data. Conservation of aromaticity of 6-membered rings along with vanishing anti-aromatic character of central 5-membered rings was found to be the main reason for the exceptional stability of the hub-isomer. At the same time, functionalization of the corannulene moiety at the rim- or spoke-site resulted in dramatic elimination of aromaticity of 6-membered rings, whereas anti-aromatic character of the central ring remained. Altogether, it led to much lower stability of these isomers in comparison with that of the hub-one.
- This article is part of the themed collection: Electron delocalization and aromaticity: 150 years of the Kekulé benzene structure

 Please wait while we load your content...
Please wait while we load your content...