Calorimetric and spectroscopic studies on solvation energetics for H2 storage in the CO2/HCOOH system†
Abstract
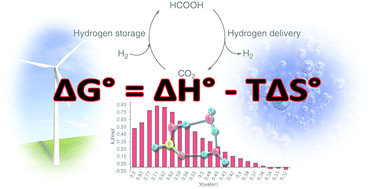
Solvents playing a crucial role in many chemical reactions and additives can be used to shift the reaction equilibrium. Herein we study the enthalpy of mixing for selected solvents (aqueous, organic) and basic additives (amines, aqueous KOH) when mixed with formic acid with the aim to optimize hydrogen storage/delivery in the CO2/HCOOH system. Formic acid, resulting from carbon dioxide hydrogenation, reaches highest yields when effectively “removed” from the reaction equilibrium. In terms of energy efficiency, any heat released during CO2 hydrogenation has to be reused in the reverse reaction, during the production of hydrogen. In any scenario, the usage of basic chemicals, non-innocent solvents, causes higher energy release in CO2 hydrogenation, which has to be reused in the hydrogen delivery process. Therefore, the enthalpy of mixing is a valuable parameter for designing hydrogen storage devices since it allows the estimation of energy balance for the CO2 hydrogenation/H2 liberation cycle. The highest formic acid concentrations in direct catalytic CO2 hydrogenation under acidic conditions were reached in DMSO. DMSO exhibits considerably stronger interactions with formic acid compared to water as was observed in calorimetric measurements. This difference can be ascribed, at least partly, to stronger hydrogen bonding of FA to DMSO than to water in the corresponding solutions, examined by a combination of IR spectroscopic and quantum chemical studies. Furthermore, the investigation of DMSO/FA- and water/FA systems by 1H- and 13C-NMR spectroscopy revealed that only 1 : 1 aggregates are formed in the DMSO solutions of FA in a broad concentration range, while the stoichiometry and the number of the FA–water aggregates essentially depend on the concentration of aqueous solutions.
- This article is part of the themed collection: Bunsentagung 2016: Basic Mechanisms in Energy Conversion

 Please wait while we load your content...
Please wait while we load your content...