Effects of biradical deuteration on the performance of DNP: towards better performing polarizing agents†
Frédéric A.
Perras
a,
Regina R.
Reinig
ab,
Igor I.
Slowing
ab,
Aaron D.
Sadow
*ab and
Marek
Pruski
*ab
aU.S. DOE Ames Laboratory, Iowa State University, 230 Spedding Hall, Ames, IA 50011-3020, USA. E-mail: mpruski@iastate.edu; Fax: +1 515 294 4709; Tel: +1 515 294 2017
bDepartment of Chemistry, Iowa State University, 1605 Gilman Hall, Ames, IA 50011-3020, USA. E-mail: sadow@iastate.edu; Tel: +1 515 294 8069
First published on 20th November 2015
Abstract
We study the effects of the deuteration of biradical polarizing agents on the efficiency of dynamic nuclear polarization (DNP) via the cross-effect. To this end, we synthesized a series of bTbK and TOTAPol biradicals with systematically increased deuterium substitution. The deuteration increases the radicals' relaxation time, thus contributing to a higher saturation factor and larger DNP enhancement, and reduces the pool of protons within the so-called spin diffusion barrier. Notably, we report that full or partial deuteration leads to improved DNP enhancement factors in standard samples, but also slows down the build-up of hyperpolarization. Improvements in DNP enhancements factors of up to 70% and time savings of up to 38% are obtained upon full deuteration. It is foreseen that this approach may be applied to other DNP polarizing agents thus enabling further sensitivity improvements.
Dynamic nuclear polarization (DNP) is the most widely applicable hyperpolarization technique to enhance the sensitivity of nuclear magnetic resonance (NMR) experiments. In most DNP solid-state (SS)NMR applications, a sample is placed in contact with a source for unpaired electrons (usually an exogenous radical polarizing agent) and is irradiated, at low temperature (<110 K), with high power microwaves near the electron Larmor frequency.1 Electron polarization can then be transferred to the nuclei by a series of mechanisms, the cross-effect generally being the most efficient. The cross-effect is a process involving three coupled spins: two electrons and one nucleus.2–4
Large strides have been made in recent years towards the improvement of DNP for solids. Specifically, the development of stable high-power microwave sources (gyrotrons), and advances in probe technology for low temperature magic angle spinning (MAS) have enabled the application of DNP SSNMR to high magnetic field strengths (≥9.4 T).5–12 Another important line of inquiry, of relevance here, concerns the development of polarizing agents capable of generating larger NMR enhancement factors (ε) and operating at higher temperatures. A notable breakthrough was reached by Hu et al. who demonstrated that the cross-effect condition was easier to satisfy by tethering two nitroxide radicals together in a single biradical molecule.13,14 It was then discovered that further improvement could be obtained by using a rigid linker, thus fixing the relative orientation of the two electrons' g tensors.15,16 Most recently, it has been demonstrated that even larger enhancements may be obtained by using a biradical with a high molecular weight.17–19 It was hypothesized that the lessened molecular motions in these compounds slow the electrons' relaxation, thus enabling a higher electron saturation factor, and a larger DNP enhancement.17,20 These breakthroughs may be combined, thus multiplying the improvements available from each approach, as is the case for the TEKPol biradical.19 Note that such efforts may not be beneficial for DNP experiments performed at liquid helium temperatures, where the electron relaxation times can become much longer.21
Instead of hindering the molecular motions that are responsible for the electrons' relaxation, the size of the interactions contributing to relaxation may also be lessened. Given that dipolar coupling to protons is a leading cause of electron relaxation under the DNP conditions, these interactions may be reduced by a factor of 6.5 by simply perdeuterating the polarizing agent.22,23 This effect is thought to be particularly important for rapidly rotating methyl groups. Perdeuteration also eliminates the 1H spins that are nearest to the radical. These nuclei possess short relaxation times and thus become a polarization sink. Since spin diffusion from these nuclei to the bulk is also slowed by the presence of pseudocontact shifts, they are said to be within the so-called spin diffusion barrier.24–26 As a result of deuteration, less electron polarization will be spent repolarizing these rapidly relaxing 1H spins. It is important to note that the use of deuterated solvent, or sample, is also known to lead to an increase in DNP enhancement factor.27–29
We have prepared four versions of the benchmark organic-soluble polarizing agent bTbK15 using reagents at natural abundance (bTbK-d0), a deuterated pentaerythritol linker (bTbK-d8), deuterated nitroxides (bTbK-d32), as well as perdeuterated reagents (bTbK-d40), see Fig. 1.
All isotopologues of bTbK were dissolved in a 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 tetrachloroethane (TCE)
4 tetrachloroethane (TCE)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
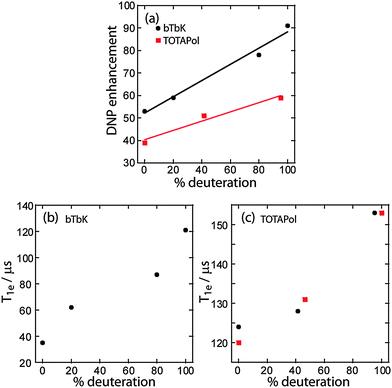
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol-d4 (CD3OD) solvent mixture to form 16 mM solutions, and 20 μL of these solutions was pipetted into 3.2 mm sapphire rotors. The solutions were degassed by repeatedly inserting and ejecting the sample, as previously described,19,30 until the DNP enhancement reached a plateau. As shown in Fig. 2 and Table 1, the maximum achievable DNP enhancements at 109 K, using a constant microwave power near 30 W, progressively increase with the level of deuteration, from 53 in the protonated bTbK to 91 in the fully perdeuterated version. This corresponds to a 70% increase in DNP enhancement by simply deuterating the polarizing agent. Surprisingly, the deuteration of the linker, containing only 8 hydrogen atoms, was particularly important, leading to a comparable improvement in enhancement per atom as the deuteration of the TEMPO moiety (1.4–2.1% vs. 1.5–1.7% higher enhancement per hydrogen atom). As shown in Table 1, this increase in DNP enhancement is also accompanied by an increase in the DNP buildup time (TDNP) from 3.5 s in the nonlabeled compound to 7.8 s in the perdeuterated compound. Because the signal to noise increases linearly as a function of the square of the number of scans, the relative sensitivity per unit of time (ε2/TDNP) is still 30% higher when using the perdeuterated radical. 13C and 29Si DNP-enhanced NMR experiments performed on 3-(3-phenylureido)propyl-functionalized mesoporous silica nanoparticles (PUP-MSNs) in fact demonstrate that larger enhancement factors are also obtained on solid samples of interest (see ESI†), although in PUP-MSNs the relative sensitivity per unit of time remains approximately the same. As can be seen in Fig. 2b (and 3b for TOTAPol-d0 and TOTAPol-d40, vide infra), the extent of the signal loss, thought to be related to both blanking and MAS-induced depolarization, does not increase by a measurable amount for the deuterated biradicals.31
methanol-d4 (CD3OD) solvent mixture to form 16 mM solutions, and 20 μL of these solutions was pipetted into 3.2 mm sapphire rotors. The solutions were degassed by repeatedly inserting and ejecting the sample, as previously described,19,30 until the DNP enhancement reached a plateau. As shown in Fig. 2 and Table 1, the maximum achievable DNP enhancements at 109 K, using a constant microwave power near 30 W, progressively increase with the level of deuteration, from 53 in the protonated bTbK to 91 in the fully perdeuterated version. This corresponds to a 70% increase in DNP enhancement by simply deuterating the polarizing agent. Surprisingly, the deuteration of the linker, containing only 8 hydrogen atoms, was particularly important, leading to a comparable improvement in enhancement per atom as the deuteration of the TEMPO moiety (1.4–2.1% vs. 1.5–1.7% higher enhancement per hydrogen atom). As shown in Table 1, this increase in DNP enhancement is also accompanied by an increase in the DNP buildup time (TDNP) from 3.5 s in the nonlabeled compound to 7.8 s in the perdeuterated compound. Because the signal to noise increases linearly as a function of the square of the number of scans, the relative sensitivity per unit of time (ε2/TDNP) is still 30% higher when using the perdeuterated radical. 13C and 29Si DNP-enhanced NMR experiments performed on 3-(3-phenylureido)propyl-functionalized mesoporous silica nanoparticles (PUP-MSNs) in fact demonstrate that larger enhancement factors are also obtained on solid samples of interest (see ESI†), although in PUP-MSNs the relative sensitivity per unit of time remains approximately the same. As can be seen in Fig. 2b (and 3b for TOTAPol-d0 and TOTAPol-d40, vide infra), the extent of the signal loss, thought to be related to both blanking and MAS-induced depolarization, does not increase by a measurable amount for the deuterated biradicals.31
| Polarizing agenta | Solventb | ε C,CP | T DNP/sd | ε 2/TDNP/s−1 | T 1e/μse |
|---|---|---|---|---|---|
| a Note that the true deuteration level of the TOTAPol biradicals in solution will differ as there are 2 exchangeable protons. b The concentrations used were 16 mM for bTbK and 10 mM for TOTAPol. c To reduce unnecessary human errors, the enhancement factors were measured by integrating the 13C CPMAS signals with the microwaves turned on and off. The uncertainties of the enhancement factors are then on the order of ±5%. d The proton T1 values were approximately equal to TDNP. e The T1e values were measured at 9 GHz. | |||||
| bTbK | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 TCE 4 TCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD CD3OD |
53 | 3.5 | 800 | 35 |
| bTbK-d8 | 59 | 3.9 | 890 | 62 | |
| bTbK-d32 | 78 | 5.8 | 1050 | 87 | |
| bTbK-d40 | 91 | 7.8 | 1060 | 121 | |
| TOTAPol-d0 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 glycerol-d8 10 glycerol-d8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O D2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
67 | 8.5 | 530 | 120 |
| TOTAPol-d17 | 85 | 11.0 | 660 | 131 | |
| TOTAPol-d40 | 83 | 23.5 | 290 | 153 | |
| TOTAPol-d0 | 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 glycerol 40 glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
39 | 6.1 | 250 | 124 |
| TOTAPol-d17 | 51 | 8.6 | 300 | 128 | |
| TOTAPol-d40 | 59 | 10.1 | 344 | 153 | |
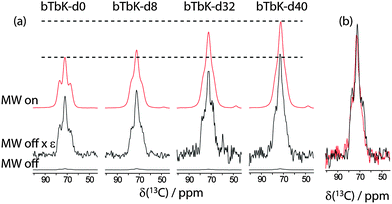 | ||
Fig. 2 (a) DNP enhancement of 16 mM 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 TCE 4 TCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3OD solutions of bTbK-dn radicals. The ‘MW off’ spectra are shown on the bottom along with the same spectra scaled by the enhancement factor listed in Table 1. The ‘MW on’ spectra are shown on the top of each figure and dashed lines highlight the relative intensities of the spectra acquired using bTbK-d0 and bTbK-d40. A comparison of ‘MW off’ spectra of equal-volume solutions of the non-deuterated (black) and per-deuterated (red) biradicals is shown in (b) highlighting the minute difference in depolarization for both radicals. CD3OD solutions of bTbK-dn radicals. The ‘MW off’ spectra are shown on the bottom along with the same spectra scaled by the enhancement factor listed in Table 1. The ‘MW on’ spectra are shown on the top of each figure and dashed lines highlight the relative intensities of the spectra acquired using bTbK-d0 and bTbK-d40. A comparison of ‘MW off’ spectra of equal-volume solutions of the non-deuterated (black) and per-deuterated (red) biradicals is shown in (b) highlighting the minute difference in depolarization for both radicals. | ||
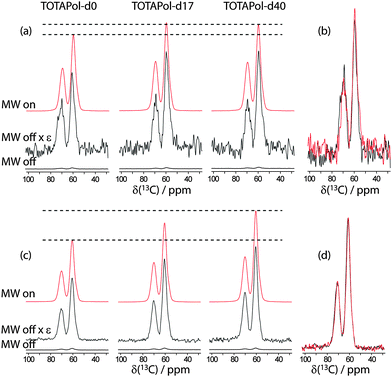 | ||
Fig. 3 DNP enhancement of 10 mM 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 glycerol-d8 10 glycerol-d8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O D2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (a) and 60 H2O (a) and 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 glycerol 40 glycerol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (c) solutions of various TOTAPol radicals. The ‘MW off’ spectra are shown at the bottom along with the same spectra scaled by the enhancement factors listed in Table 1. The ‘MW on’ spectra are shown at the top of each figure and dashed lines highlight the relative intensities of the spectra acquired using TOTAPol-d0 and TOTAPol-d40. A comparison of ‘MW off’ spectra of equal-volume solutions of the non-deuterated (black) and per-deuterated (red) biradicals is shown in (b and d) highlighting the minute difference in depolarization for both radicals. H2O (c) solutions of various TOTAPol radicals. The ‘MW off’ spectra are shown at the bottom along with the same spectra scaled by the enhancement factors listed in Table 1. The ‘MW on’ spectra are shown at the top of each figure and dashed lines highlight the relative intensities of the spectra acquired using TOTAPol-d0 and TOTAPol-d40. A comparison of ‘MW off’ spectra of equal-volume solutions of the non-deuterated (black) and per-deuterated (red) biradicals is shown in (b and d) highlighting the minute difference in depolarization for both radicals. | ||
To confirm that this is indeed a generally applicable strategy for improving the effectiveness of other DNP polarizing agents we have also synthesized and tested deuterated versions of the hydrophilic TOTAPol biradical.14 Natural abundance (TOTAPol-d0), perdeuterated (TOTAPol-d40), as well as a biradical with deuterium labeling on only the amine-terminated nitroxide (TOTAPol-d17) were prepared, see Fig. 1. These radicals were then dissolved in 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 glycerol-d8
10 glycerol-d8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D2O
D2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (the so-called ‘DNP juice’) and in a deuterium-free 60
H2O (the so-called ‘DNP juice’) and in a deuterium-free 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 mixture of glycerol and H2O at a concentration of 10 mM.32 The DNP enhancement measurements on these solutions were performed following the protocol described for the bTbK radicals.
40 mixture of glycerol and H2O at a concentration of 10 mM.32 The DNP enhancement measurements on these solutions were performed following the protocol described for the bTbK radicals.
As shown in Fig. 3a and Table 1, the DNP enhancement increases upon deuteration of TOTAPol from 67 to 85 for the d0 and d17 derivatives, respectively, when using DNP juice. The fully deuterated biradical, TOTAPol-d40, yields the same enhancement as partly-deuterated TOTAPol-d17, within uncertainty in the estimates (±5%). Since the DNP build-up time increases from 8.5 to 11.0 and 23.5 s upon deuteration, the sensitivity per time is affected accordingly; the reason for this will be expanded later in the text. As was expected, however, the enhancement increases steadily from 39 to 51 and 59 for the d0, d17, and d40 derivatives, respectively, when using the fully protonated solvent. The enhancement factor for TOTAPol then increases by 50% upon perdeuteration and the sensitivity per unit of time increases by 38%.
Using a model spin system consisting of two electrons and one nucleus, Thurber and Tycko demonstrated that, in theory, long T1e values prevent the loss of magnetization between the MAS-induced frequency crossing events that lead to cross-effect polarization transfer.26 The subsequent investigation of a series of functionalized nitroxide biradicals by Zagdoun et al.19 confirmed that electron relaxation properties were highly correlated with the DNP enhancements. Thus, both theoretical and experimental studies of the cross-effect under MAS demonstrate that longer T1e relaxation times of the electrons generally lead to higher enhancement factors;17,33,34 as was mentioned earlier, this hypothesis was the main motivation for the synthesis of the perdeuterated biradicals by the present authors. The results of T1e measurements performed using the very same solutions used to measure the DNP enhancements, as explained in the ESI,† are presented in Table 1 and Table S1 (ESI†). As can be seen from the plots shown in Fig. 4, the T1e and ε values indeed increase linearly as the biradical polarizing agents are progressively deuterated. The deuteration of the solvent, on the other hand, does not lead to an increase of T1e.35 We also note that, for bTbK, the observed increase in DNP enhancement is in agreement with the numerical calculations of Mance et al. for the increase in T1e that we observed.33 However, for TOTAPol, the modest increase in T1e does not appear to fully explain the observed 50% increase in DNP enhancement factor.33 This may be the result of the use of a much lower magnetic field for the EPR measurements (9 vs. 263 GHz). Another plausible mechanism contributing to an increase in ε values would be that, as mentioned earlier, there is a diminished loss of polarization within the so-called spin diffusion barrier when perdeuterated radicals are used.24 The elimination of 1H spins within the spin diffusion barrier also, unfortunately, has the effect of increasing the 1H DNP build-up time due to the reduced efficiency of DNP-assisted spin diffusion (see Table 1).36 Hovav et al. demonstrated that the rate at which hyperpolarization is transferred to the bulk nuclei depends on the strength of the dipolar coupling between the core nuclei and the electron and depends very little on the dipolar coupling between the bulk nuclei.36 The deuteration of the radicals then reduces the dipolar coupling between the core nuclei and the electrons and essentially slows down the spin diffusion of hyperpolarization to the bulk. In extreme cases when deuterated solvents are used this can lead to particularly long TDNP values and a loss of sensitivity.
In conclusion, we have explored a simple approach for improving the DNP enhancement afforded by biradical polarizing agents. Indeed, using the bTbK and TOTAPol biradicals, an increase in DNP enhancement factors of up to 70%, and an improved sensitivity per unit of time of up to 38% can be obtained by simply perdeuterating the polarizing agent. The deuteration of biradicals increases their T1e values, thus improving the saturation factors upon continuous wave microwave irradiation, and consequently contributing to higher enhancement factors. The use of deuterated polarizing agents also reduces the number of 1H spins within the spin diffusion barrier, which has the effect of reducing the polarization loss, but also leads to slower DNP-assisted spin diffusion and longer build-up times.36 The perdeuteration, or partial deuteration, of the current state-of-the-art polarizing agents (AMUPol18 and TEKPol19), which can already yield enhancement factors over 200 at 105 K, is likely to yield the next generation of biradicals giving enhancement factors well over 300; we are actively exploring this avenue. The results of this study may also help in understanding the mechanisms of the cross-effect in DNP of solids, particularly with respect to the polarization of remote spins.
Notes
During the preparation of this manuscript a paper was published by Kubicki and co-workers describing the performance of three methyl-deuterated biradicals: PyPol-diMe, bTurea-diMe and bTbK, where increased enhancement factors and build-up times were also observed.37Acknowledgements
We wish to thank Drs. S. Vega and T. Kobayashi for valuable discussions. Also, we are grateful to Dr S. Cady and K. Harrata in the ISU Chemistry Instrumentation Facility and Dr C. Klug and K. Schultz at the Medical College of Wisconsin for assistance with EPR and the LC/MS experiments. This research is supported by LDRD program at the U.S. Department of Energy (DOE), Office of Science, Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences. Support for F. P. is through a Spedding Fellowship. Ames Laboratory is operated for the DOE by Iowa State University under Contract No. DE-AC02-07CH11358.References
- T. Maly, G. T. Debelouchina, V. S. Bajaj, K.-N. Hu, C.-G. Joo, M. L. Mak-Jurkauskas, J. R. Sirigiri, P. C. A. van der Wel, J. Herzfeld, R. J. Temkin and R. G. Griffin, J. Chem. Phys., 2008, 128, 052211 CrossRef PubMed.
- C. F. Hwang and D. A. Hill, Phys. Rev. Lett., 1967, 19, 1011–1014 CrossRef CAS.
- Ü. Akbey, W. T. Franks, A. Linden, M. Orwick-Rydmark, S. Lange and H. Oschkinat, Top. Curr. Chem., 2013, 338, 181–228 CrossRef PubMed.
- K. Michaelis, T.-C. Ong, M. K. Kiesewetter, D. K. Frantz, J. J. Walish, E. Ravera, C. Luchinat, T. M. Swager and R. G. Griffin, Isr. J. Chem., 2014, 54, 207–221 CrossRef PubMed.
- L. R. Becerra, G. J. Gerfen, R. J. Temkin, D. J. Singel and R. G. Griffin, Phys. Rev. Lett., 1993, 71, 3561–3564 CrossRef CAS PubMed.
- M. Rosay, J. C. Lansing, K. C. Haddad, W. W. Bachovchin, J. Herzfeld, R. J. Temkin and R. G. Griffin, J. Am. Chem. Soc., 2003, 125, 13626–13627 CrossRef CAS PubMed.
- A. B. Barnes, M. L. Mak-Jurkauskas, Y. Matsuki, V. S. Bajaj, P. C. A. van der Wel, R. DeRocher, J. Bryant, J. R. Sirigiri, R. J. Temkin, J. Lugtenburg, J. Herzfeld and R. G. Griffin, J. Magn. Reson., 2009, 198, 261–270 CrossRef CAS PubMed.
- M. Rosay, L. Tometich, S. Pawsey, R. Bader, R. Schauwecker, M. Blank, P. M. Borchard, S. R. Cauffman, K. L. Felch, R. T. Weber, R. J. Temkin, R. G. Griffin and W. E. Maas, Phys. Chem. Chem. Phys., 2010, 12, 5850–5860 RSC.
- Y. Matsuki, H. Takahashi, K. Ueda, T. Idehara, I. Ogawa, M. Toda, H. Akutsu and T. Fujiwara, Phys. Chem. Chem. Phys., 2010, 12, 5799 RSC.
- A. B. Barnes, E. Markhasin, E. Daviso, V. K. Michaelis, E. A. Nanni, S. K. Jawla, E. L. Mena, R. DeRocher, A. Thakkar, P. P. Woskov, J. Herzfeld, R. J. Temkin and R. G. Griffin, J. Magn. Reson., 2012, 224, 1 CrossRef CAS PubMed.
- K. J. Pike, T. F. Kemp, H. Takahashi, R. Day, A. P. Howes, E. V. Kryukov, J. F. Macdonald, A. E. C. Collis, D. R. Bolton, R. J. Wylde, M. Orwick, K. Kosuga, A. J. Clark, T. Idehara, A. Watts, G. M. Smith, M. E. Newton, R. Dupree and M. E. Smith, J. Magn. Reson., 2012, 215, 1 CrossRef CAS PubMed.
- T. Idehara, Y. Tatematsu, Y. Yamaguchi, E. M. Khutoryan, A. N. Kuleshov, K. Ueda, Y. Matsuki and T. Fujiwara, J. Infrared, Millimeter, Terahertz Waves, 2015, 36, 613 CrossRef.
- K.-N. Hu, H.-h. Yu, T. M. Swager and R. G. Griffin, J. Am. Chem. Soc., 2004, 126, 10844–10845 CrossRef CAS PubMed.
- C. Song, K.-N. Hu, C.-G. Joo, T. M. Swager and R. G. Griffin, J. Am. Chem. Soc., 2006, 128, 11385–11390 CrossRef CAS PubMed.
- Y. Matsuki, T. Maly, O. Ouari, H. Karoui, F. Le Moigne, E. Rizzato, S. Lyubenova, J. Herzfeld, T. Prisner, P. Tordo and R. G. Griffin, Angew. Chem., Int. Ed., 2009, 48, 4996–5000 CrossRef CAS PubMed.
- M. K. Kiesewetter, B. Corzilius, A. A. Smith, R. G. Griffin and T. M. Swager, J. Am. Chem. Soc., 2012, 134, 4537–4540 CrossRef CAS PubMed.
- A. Zagdoun, G. Casano, O. Ouari, G. Lapadula, A. J. Rossini, M. Lelli, M. Baffert, D. Gajan, L. Veyre, W. E. Maas, M. Rosay, R. T. Weber, C. Thieuleux, C. Coperet, A. Lesage, P. Tordo and L. Emsley, J. Am. Chem. Soc., 2012, 134, 2284–2291 CrossRef CAS PubMed.
- C. Sauvée, M. Rosay, G. Casano, F. Aussenac, R. T. Weber, O. Ouari and P. Tordo, Angew. Chem., Int. Ed., 2013, 52, 10858–10861 CrossRef PubMed.
- A. Zagdoun, G. Casano, O. Ouari, M. Schwarzwälder, A. J. Rossini, F. Aussenac, M. Yulikov, G. Jeschke, C. Copéret, A. Lesage, P. Tordo and L. Emsley, J. Am. Chem. Soc., 2013, 135, 12790–12797 CrossRef CAS PubMed.
- V. Kathirvelu, C. Smith, C. Parks, M. A. Mannan, Y. Miura, K. Takeshita, S. S. Eaton and G. R. Eaton, Chem. Commun., 2009, 454–456 RSC.
- L. Lumata, M. E. Merritt, C. R. Malloy, A. D. Sherry and Z. Kovacs, J. Phys. Chem. A, 2012, 116, 5129–5138 CrossRef CAS PubMed.
- B. van den Brandt, E. I. Bunyatova, P. Hautle and J. A. Konter, Nucl. Instrum. Methods Phys. Res., Sect. A, 2004, 526, 53–55 CrossRef CAS.
- L. Lumata, A. K. Jindal, M. E. Merritt, C. R. Malloy, A. D. Sherry and Z. Kovacs, J. Am. Chem. Soc., 2011, 133, 8673–8680 CrossRef CAS PubMed.
- C. Ramanathan, Appl. Magn. Reson., 2008, 34, 409–421 CrossRef CAS.
- A. A. Smith, B. Corzilius, A. B. Barnes, T. Maly and R. G. Griffin, J. Chem. Phys., 2012, 136, 015101 CrossRef PubMed.
- K. R. Thurber and R. Tycko, J. Chem. Phys., 2012, 137, 084508 CrossRef PubMed.
- A. Kagawa, Y. Murokawa, K. Takeda and M. Kitagawa, J. Magn. Reson., 2009, 197, 9–13 CrossRef CAS PubMed.
- Ü. Akbey, W. T. Franks, A. Linden, S. Lange, R. G. Griffin, B.-J. van Rossum and H. Oschkinat, Angew. Chem., Int. Ed., 2010, 49, 7803–7806 CrossRef PubMed.
- L. Lumata, M. E. Merritt and Z. Kovacs, Phys. Chem. Chem. Phys., 2013, 15, 7032 RSC.
- D. J. Kubicki, A. J. Rossini, A. Purea, A. Zagdoun, O. Ouari, P. Tordo, F. Engelke, A. Lesage and L. Emsley, J. Am. Chem. Soc., 2014, 136, 15711–15718 CrossRef CAS PubMed.
- F. Mentink-Vigier, S. Paul, D. Lee, A. Feintuch, S. Hediger, S. Vega and G. De Paëpe, Phys. Chem. Chem. Phys., 2015, 17, 21824–21836 RSC.
- G. J. Gerfen, L. R. Becerra, D. A. Hall, R. G. Griffin, R. J. Temkin and D. J. Singel, J. Chem. Phys., 1995, 102, 9494–9497 CrossRef CAS.
- D. Mance, P. Gast, M. Huber, M. Baldus and K. L. Ivanov, J. Chem. Phys., 2015, 142, 234201 CrossRef PubMed.
- F. Mentink-Vigier, Ü. Akbey, H. Oschkinat, S. Vega and A. Feintuch, J. Magn. Reson., 2015, 258, 102 CrossRef CAS PubMed.
- A. Volkov, C. Codkter, T. Bund, H. Paulsen and G. Jeschke, Biophys. J., 2009, 96, 1124 CrossRef CAS PubMed.
- Y. Hovav, A. Feintuch and S. Vega, J. Chem. Phys., 2011, 134, 074509 CrossRef PubMed.
- D. Kubicki, G. Casano, M. Schwarzwälder, S. Abel, C. Sauvée, K. Ganesan, M. Yulikov, A. H. Rossini, G. Jeschke, C. Copéret, A. Lesage, P. Tordo, O. Ouari and L. Emsley, Chem. Sci., 2015 10.1039/C5SC02921J.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cp06505d |
| This journal is © the Owner Societies 2016 |