Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Understanding the electromagnetic interaction of metal organic framework reactants in aqueous solution at microwave frequencies†
Andrea
Laybourn
ab,
Juliano
Katrib
b,
Paula A.
Palade
b,
Timothy L.
Easun
 ac,
Neil R.
Champness
a,
Martin
Schröder
*ad and
Samuel W.
Kingman
*b
ac,
Neil R.
Champness
a,
Martin
Schröder
*ad and
Samuel W.
Kingman
*b
aSchool of Chemistry, University of Nottingham, Nottingham NG7 2RD, UK
bFaculty of Engineering, University of Nottingham, Nottingham NG7 2RD, UK. E-mail: Sam.Kingman@nottingham.ac.uk
cSchool of Chemistry, Cardiff University, Main Building, Park Place, Cardiff, CF10 3AT, UK
dSchool of Chemistry, University of Manchester, Oxford Road, Manchester M13 9PL, UK. E-mail: M.Schroder@manchester.ac.uk
First published on 29th January 2016
Abstract
Preparation of metal organic frameworks (MOFs) via microwave heating is becoming increasingly popular due to reduced reaction times and enhanced control of MOF particle size. However, there is little understanding about the detailed interaction of the electric field portion of the wave with reactants during the synthesis of MOFs. In order to overcome this lack of fundamental understanding, information about the dielectric properties of the reactants is required. In this work the dielectric constants (ε′) and loss factors (ε′′) of benzene-1,4-dicarboxylic acid (H2BDC; also known as terephthalic acid) and a number of M(III) (M = metal) salts dissolved in deionized water were measured as a function of frequency, temperature and concentration and with varying anions and cations. Dielectric data confirm the aqueous M(III) salts to be strong microwave absorbers, particularly at 915 MHz. M(III) salts with mono-anionic ligands (for example chlorides and nitrates) exhibit higher losses than di-anionic salts (sulfates) demonstrating that the former are heated more effectively in an applied microwave field. Of the M(III) salts containing either singly- or doubly-charged anions, those containing Fe(III) have the highest loss indicating that they will heat more efficiently than other M(III) salts such as Cr(III) and Al(III). Interestingly, H2BDC exhibits little interaction with the electric field at microwave frequencies.
Introduction
Metal organic frameworks (MOFs) have been the subject of significant research and commercial interest due to their high surface areas1,2 tunability and functionalities.3,4 Owing to their exceptional porosity, MOFs are ideal candidates for a wide range of applications such as gas storage and separation,5,6 catalysis,6,7 supercapacitors,8 sensors9,10 and as electrolytes in fuel cells.6,8 MOFs are crystalline materials constructed from metal ions or clusters11 and organic linkers,12 and the variability and availability of different metal centres and clusters and organic linkers has led to the preparation of many structural variants with a variety of materials properties. A major hurdle for the development of MOFs is the long time typically required for their synthesis and inability to scale-up routes to manufacture. Although synthesis by slow evaporation methods can require up to 7 months,13 the current predominant synthetic route involves solvothermal reactions (hydrothermal when water is the solvent) in sealed vessels. In these reactions, the reactants are heated above the boiling point of the solvent and retained under autogeneous pressure for up to one week.13 A variety of other methods have been investigated including electrochemical,14 mechanochemical,15 and sonochemical16 approaches. However, the most rapid route to date involves microwave irradiation.17 For example, IRMOF1, IRMOF2 and IRMOF3 have been prepared by Ni and Masel in as little as 25 seconds via microwave-assisted solvothermal reactions (150 W, microwave synthesizer model 520A from Resonance Instrument Inc.).17 Importantly they noted that the materials prepared using microwave methods were of the same quality as those prepared using conventional solvothermal routes.In addition to reducing reaction times, work on the synthesis of MOFs using microwaves has focused on investigating phase-selectivity,18 crystal nucleation rates19 and controlling particle size.17 For example, Khan and Jhung demonstrated phase-selective microwave synthesis of MIL-53 and MIL-101 (MIL = Materials Institute Lavoisier), with the latter isolated after reaction times of ca. 1–4 h and the former requiring longer times.18 This was ascribed to the conversion between the kinetically (MIL-101) and thermodynamically (MIL-53) favourable phases of these Cr(III) materials.19 Furthermore, phase-selective synthesis of MOFs using microwave irradiation has demonstrated the possibility of producing alternative MOF structures that may not be accessible through conventional solvothermal routes.18,20
A wide number of frameworks have been synthesised using microwaves.13 Despite this activity there is a paucity of information regarding the interaction between microwave irradiation and MOF reactants and intermediates. Current examples of microwave-assisted synthesis of MOFs are all carried out without any analysis of the interaction(s) of reactants with the microwave field; this paper aims to overcome this lack of fundamental understanding.
In order to understand the effects of microwave irradiation during the synthesis of MOFs, information about the dielectric properties (specifically the dielectric constant, ε′, and dielectric loss, ε′′) of the reactants is required. Such information is essential for the optimum design and safe scaling-up of microwave processes as these values quantify how effectively a material will be heated in an electric field. The bulk dielectric permittivity, ε*, is a measure of the extent to which a material can be polarised by an external electric field, and can be expressed in its complex form:
| ε* = ε′ − jε′′ |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ, where tan
δ, where tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = ε′′/ε′. Low loss materials are those with tan
δ = ε′′/ε′. Low loss materials are those with tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ < 3 × 10−4, medium loss materials have tan
δ < 3 × 10−4, medium loss materials have tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ values in the range 3 × 10−4 ≤ tan
δ values in the range 3 × 10−4 ≤ tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ < 3 × 10−2 and high loss materials have tan
δ < 3 × 10−2 and high loss materials have tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ ≥ 3 × 10−2.24
δ ≥ 3 × 10−2.24
| Materials that heat well in the presence of an electric field | Materials that heat poorly in the presence of an electric field | ||||
|---|---|---|---|---|---|
| Example | ε′ | ε′′ | Example | ε′ | ε′′ |
| a23, b20, c27, d22, e23 °C. All data from ref. 23 except b63 and e.64 | |||||
| Raw potato | 53.7 | 15.7 | Plexiglass, perspexc | 2.25 | 0.0007 |
| Distilled water | 76.7 | 12.0 | Polytetrafluoro-ethylene (Teflon)d | 2.1 | 0.0003 |
| Distilled water + 0.5 M NaCl | 67.0 | 41.48 | Fused silica glass | 3.78 | 0.0002 |
| Rapeseed, frost resistanta | 40.0 | 11.0 | Ruby mica, muscovite | 5.4 | 0.0016 |
| Ethanol 99.8% purityb (containing <0.2% water) | 5.99 | 5.48 | Titanium dioxide (Ticon T)e | 100 | 0.04 |
As well as providing insight into whether a material will heat in an electric field, ε′ and ε′′ also provide information about the mechanisms by which a material interacts with an electric field. For molecules in solution, ε* reflects the frequency dependent polarisation processes that occur as a result of the interaction of charges with the oscillating external electric field. In the frequency range of our study (0.5 to 10.0 GHz, which is in the range of domestic and commercially available microwave heating systems) two processes take place, namely ionic and dipolar polarisation mechanisms. Ionic mechanisms occur in ionic solutions, whereby comparative displacement of cations and anions by the electric field leads to net polarisation of the solution.21,25 Below 2.0 GHz, the dielectric permittivity of ionic solutions is greatly affected by the ion mobility as it moves with the oscillating electric field.26 The mobility of ions in solution is retarded by surrounding solvation spheres with a concentration-dependent reduction in ε′, i.e. the more ions (and therefore solvation spheres) present in solution, the lower the value of ε′. For example, at 1.0 GHz NiSO4 dissolved in distilled water at 25 °C exhibits ε′ values of ca. 80 and 70 for the total solution at concentrations of 0.15 and 1.40 M, respectively.27 Additionally, the retarding force exerted on the ion causes a significant lag that arises as a result of the ion being out of phase with the electric field.25 This lag results in high dielectric losses (ε′′) and leads to heating of the solution. For example, a 500 mM solution of NaCl heats well in an electric field since it has an extremely high dielectric loss, ε′′, of 269.0 at 1.0 GHz.23
Dipolar mechanisms occur for molecules that possess a permanent dipole, such as water.26 Dipolar molecules experience a torque causing them to re-orient in the direction of the applied electric field,25 and as the dipoles re-orient they experience frictional forces from neighbouring molecules.25 The kinetic energy causing friction is lost as heat giving rise to moderate values of ε′′ (e.g. at 3 GHz distilled water has a dielectric loss, ε′′, of 12.00).23
In this study we examine the dielectric constant (ε′) and loss (ε′′) of reactants commonly used to prepare the MIL-53 and MIL-47 series of MOFs.28,29 These targets were chosen for two reasons. Firstly, variation of starting metal salt enables MOFs containing different metal nodes to be synthesised using the same organic linker, in this case benzene-1,4-dicarboxylate (BDC2−). Secondly, the reaction kinetics30,31 and both the structure28 and properties29 of these MOFs have been established and are well understood. While it is perhaps unsurprising that the dielectric properties of H2BDC have not been studied in solution, we were surprised to find that values of ε′ and ε′′ for simple metal salts dissolved in aqueous solution have not been widely reported previously. Such data are fundamental to understanding the interaction of electromagnetic waves with such chemistries. Dielectric analysis of inorganic electrolytes, specifically metal salts dissolved in water, is of course a long-standing area of research.21,32
The dielectric properties of salt-water solutions depend greatly upon water–water interactions,33 the nature of hydrogen bonding networks,26,33,34 solute and solvent concentrations,35 ionic charge,36 ion-pairs,37 and the size effects of the ions36,38 and their hydration spheres.36 By considering the effect of frequency, concentration, temperature, the choice of cation and anion on the dielectric constant (ε′) and dielectric loss (ε′′) of metal salts and H2BDC, we have assessed directly the contributions of the above factors on the synthesis of MIL-53 and MIL-47 in an electric field. In this way, we aimed to identify the precise interactions of the electric field taking place in a typical reaction leading to the formation of MOF product(s).
Experimental methods
Chemicals
Al(NO3)3·9H2O, Cr2(SO4)3·H2O and Fe(NO3)3·9H2O were purchased from Sigma-Aldrich. FeCl3 was obtained from Merck. Al2(SO4)3·8H2O, CrCl3·6H2O, Cr(NO3)3·9H2O, Fe2(SO4)3·5H2O and VCl3 were purchased from Acros Organics. Terephthalic acid was acquired from Alfa Aesar. All chemicals had a purity of 97% or greater and were used as received.Dielectric analyses
The values of ε′ and ε′′ were measured using an Agilent 85070E Dielectric Probe Kit. The experimental set-up consisted of a performance coaxial probe (2.4 mm male connector and 1.6 mm tip) equipped with an electronic calibration (ECal) module. The coaxial probe was connected to an Agilent N5232A PNA-L (Purpose Network Analyser) operating at 300 kHz–20 GHz via a high quality coaxial cable. The probe was made of T316L stainless steel and had a nickel plated tungsten central conductor with a borosilicate glass seal at the tip. The probe is operational over a temperature range of −40 °C to +200 °C, and a typical measurement involved immersing the probe tip into the test solution and a swept frequency signal (500 MHz–10 GHz) was transmitted from the PNA into the solution via the coaxial line. Measurements were carried out over a temperature range of 20–60 °C. The dielectric constant (ε′) and dielectric loss (ε′′) were recorded using Agilent Technologies 85070 software and processed in Origin 8.0. Values of ε′ and ε′′ were measured for three separate test solutions of the same concentration at temperatures of 20, 40 and 60 °C. The value of ε′ and ε′′ at each point was also measured three times such that each data point represents an average value of 9 measurements. Error bars are plotted ± one standard deviation. The resultant data points (Fig. 1–3 and 5) are so close together that they impact upon the visual output of each data line such that the error bars appear as a continuous line; therefore, the thickness of the line is indicative of the error.Test solutions were prepared by dissolving the required amount of analyte (metal salt or organic linker) in deionized water to the desired concentration for a 200 mL volume. This volume was used as it was experimentally determined as being sufficiently large enough to ensure that the signal from the probe tip was being transmitted through the test solution only and not the surroundings, i.e. the sample is assumed to be of an infinite volume with respect to the transmitted signal. Concentrations were based upon a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 ratio of metal cation
80 ratio of metal cation![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BDC2−
BDC2−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, commonly used in the synthesis of MIL-53.28 This ratio represents a concentration of BDC2− of 25.0 mM. For the M(III) chlorides and nitrates, a concentration of 50.0 mM was required. As the M(III) sulfates contain two metal cations per molecule, only half the number of moles were required for each test solution such that the corresponding concentration remains 25.0 mM. In order to facilitate complete dissolution of BDC2− in water either sodium hydroxide or ammonium hydroxide were added in a ratio of 1
H2O, commonly used in the synthesis of MIL-53.28 This ratio represents a concentration of BDC2− of 25.0 mM. For the M(III) chlorides and nitrates, a concentration of 50.0 mM was required. As the M(III) sulfates contain two metal cations per molecule, only half the number of moles were required for each test solution such that the corresponding concentration remains 25.0 mM. In order to facilitate complete dissolution of BDC2− in water either sodium hydroxide or ammonium hydroxide were added in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of acid
2 of acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) base. Additional concentrations at factors of ×0.1 and ×10 with respect to the ratio of water were also prepared so that concentrations above and below that required for the synthesis of MIL-53 could be analysed. All test solutions were left for a minimum of 24 h before dielectric measurements to allow complete equilibration to their hydrolysed species in solution (as confirmed by UV-visible absorption spectroscopy, see ESI†).
base. Additional concentrations at factors of ×0.1 and ×10 with respect to the ratio of water were also prepared so that concentrations above and below that required for the synthesis of MIL-53 could be analysed. All test solutions were left for a minimum of 24 h before dielectric measurements to allow complete equilibration to their hydrolysed species in solution (as confirmed by UV-visible absorption spectroscopy, see ESI†).
UV-visible absorption spectroscopy
UV-visible absorption spectra were measured using an Ocean Optics USB2000+ UV-VIS-ES spectrometer with a DT-MINI-2GS light source. A typical experiment involved pipetting the analyte into a quartz cuvette and placing the cuvette in a 1 cm holder equipped with a P200-2 transmission fibre optic. Spectra were collected at time intervals of 0, 2 and 24 hours for each analyte. Spectra were recorded using Ocean Optics SpectraSuite 2.0 software and processed in Origin 8.0. Each spectrum was baseline corrected and intensities were normalized with respect to the highest peak. Peak maxima and shoulder positions are accurate to ±5.0 nm.Results and discussion
In this work the dielectric constants (ε′) and dielectric loss factors (ε′′) of BDC2− and a number of M(III) salts dissolved in deionized water were measured as a function of frequency, temperature and concentration in the presence of various anions. To our knowledge, there are no reports of the dielectric properties of aqueous Fe, Cr and V salts in the literature. Solutions of Al2(SO4)3 have been investigated at concentrations of 0.012–0.65 M39 and 0.1 M40 at 25 °C only. Also, Al(NO3)3·H2O at concentrations of 0.5 M to saturation have been examined at 25 °C.41The dielectric properties of M(III) salts
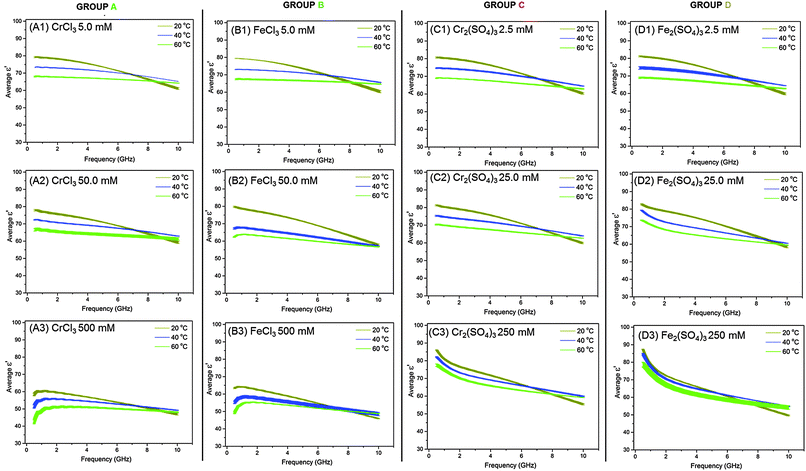
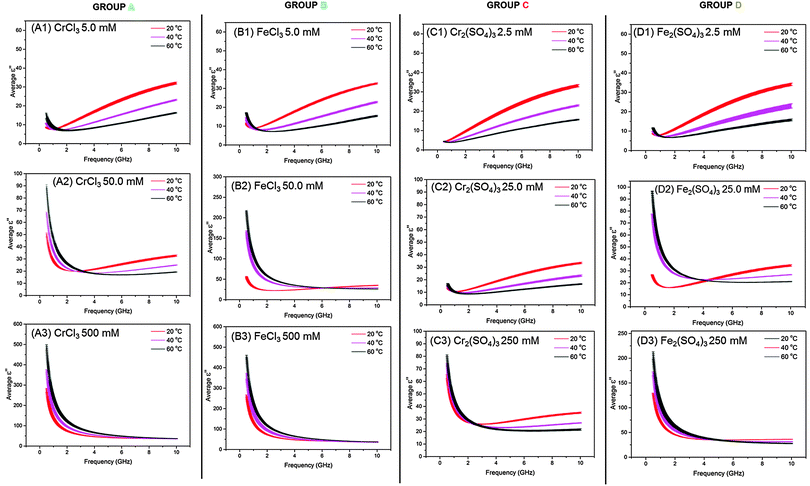
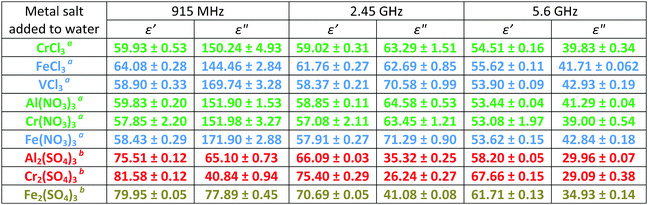
We discuss first the dielectric properties (ε′ and ε′′) of simple M(III) salts, which we can group into four types. The salts studied fall into those containing either mono- or di-charged anions, and based upon their dielectric properties we can divide both types into two separate sub-groups giving 4 categories (A, B, C and D) as shown in Table 2. Group A consists of CrCl3, Cr(NO3)3 and Al(NO3)3 all of which contain singly-charged anions and have the same trends in dielectric behaviour. Group B consists of FeCl3, Fe(NO3)3 and VCl3 which all similarly contain singly-charged anions and are categorized by their trends in dielectric behaviour. Group C comprises Al2(SO4)3 and Cr2(SO4)3, both of which contain doubly-charged anions and exhibit the same trends in dielectric properties. Finally, Group D contains Fe2(SO4)3 which displays unique dielectric behaviour compared to the other metal salts in this study. We describe here the dielectric data (ε′ and ε′′) for one illustrative M(III) salt from each group (Table 2). For Groups A, B and C and D, Fig. 1 and 2 show plots of dielectric constant (ε′) vs. frequency and of dielectric loss (ε′′) vs. frequency, respectively. Dielectric data for all other M(III) salts (i.e. those that are not italicized in Table 2) are given in ESI.†| Group A | Group B | Group C | Group D |
|---|---|---|---|
| Italics denotes species explicitly discussed in the main text. | |||
| CrCl 3 | FeCl 3 | Al2(SO4)3 | Fe 2 (SO 4 ) 3 |
| Cr(NO3)3 | VCl3 | Cr 2 (SO 4 ) 3 | |
| Al(NO3)3 | Fe(NO3)3 | ||
At the same concentration and temperature the dielectric loss, ε′′, (Fig. 2: A2 red line) displays slightly more complex behaviour than ε′. Within the frequency range 0.5–1.0 GHz ε′′ decreases rapidly as frequency increases, from 49.9 ± 1.55 to 28.4 ± 0.63 (Fig. 2: A2 red line). Above 1.0 GHz a non-linear increase in ε′′ up to 32.7 ± 0.65 at 10 GHz is observed with increasing frequency (Fig. 2: A2 red line). These trends in ε′ and ε′′ with frequency are consistent with those observed for all of the M(III) salt solutions in Groups A, B, C and D in this work and those described in the literature for aqueous electrolytic solutions and water.21,32,34,39,40,42 Such trends are governed by the polarisability of ions and water molecules in solution in response to the electric field.21,42c As mentioned in the introduction, ionic polarizations are dominant in the frequency range 0.5–2.0 GHz and dipolar mechanisms dominate in the range 2.0–10.0 GHz. However, perhaps the most interesting observation from these data is that the dielectric loss (ε′′) is significantly higher at low frequency (<1.0 GHz) than at frequencies above 1.0 GHz, suggesting that the metal salts from Groups A, B, C and D will heat more if the electric field applied is at a lower frequency (below 1.0 GHz).
Next we discuss the effect of temperature and concentration on the dielectric constant (ε′) and loss (ε′′) of the M(III) salts. Variation of these parameters indirectly probes water–water interactions,33 influence of the hydrogen bonding network,26,33,34 solute and solvent concentrations.35 A solution may have a stronger or weaker interaction with the electric field at different temperatures; for example, distilled water exhibits a reduction in dielectric constant (ε′) and loss (ε′′) with increasing temperature at 3.0 GHz, as shown in Table 3.21
| Temperature (°C) | 915 MHz | 2.45 GHz | 5.6 GHz | |||
|---|---|---|---|---|---|---|
| ε′ | ε′′ | ε′ | ε′′ | ε′ | ε′′ | |
| 20 | 80.00 | 3.98 | 78.68 | 10.61 | 72.91 | 22.17 |
| 30 | 76.44 | 2.97 | 75.66 | 7.96 | 72.17 | 17.15 |
| 40 | 73.1 | 2.3 | 72.62 | 6.21 | 70.39 | 13.62 |
| 50 | 69.84 | 1.79 | 69.53 | 4.83 | 68.1 | 10.71 |
Fig. 1(A1) and 2(A1) show the effect of temperature upon ε′ and ε′′, respectively, for a 5.0 mM solution of CrCl3 in water, and is representative of the M(III) salts in Group A of this study. At this concentration of M(III) salt the dielectric properties of the solution are dominated by those of the bulk water.42c At frequencies above 9.0 GHz ε′ increases with increasing temperature in the order 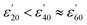 (Fig. 1: A1, brown < blue ≈ green line). At frequencies lower than 6.0 GHz the opposite effect is observed, i.e. ε′ decreases with increasing temperature in the order
(Fig. 1: A1, brown < blue ≈ green line). At frequencies lower than 6.0 GHz the opposite effect is observed, i.e. ε′ decreases with increasing temperature in the order 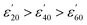 (Fig. 1: A1 brown, blue and green lines). For example, at 915 MHz ε′ decreases from 78.96 ± 0.43 at 20 °C to 73.25 ± 0.25 at 40 °C and 67.92 ± 0.23 at 60 °C (Fig. 1: A1 brown, blue and green lines). This behaviour has previously been reported for distilled water and is due to a decrease in the extent of water–water hydrogen bonding with increasing temperature as a result of a shift in the Boltzmann distribution of the water molecules, i.e. more water molecules have the energy required to overcome hydrogen bonding with increasing temperature.42c,44 The detrimental effect of increasing temperature on the extent of hydrogen bonding is further evidenced by a reduction in ε′′ with increasing temperature for frequencies above 1.4 GHz. For example, at 5.6 GHz ε′′ decreases from 22.02 ± 0.60 at 20 °C to 14.63 ± 0.21 at 40 °C and 10.32 ± 0.21 at 60 °C (Fig. 2: A1 red > pink > black line). A decrease in hydrogen bonding with increasing temperature enables the water molecules to move more freely in solution, thus lowering the rotational drag and ultimately the friction between water molecules.42c,44 By decreasing the amount of friction between water molecules, less kinetic energy is lost as heat and thus a reduction the value of ε′′ is observed.42c,44 The trends in ε′ and ε′′ with temperature exhibited by 5.0 mM solutions of Group A salts are also observed for M(III) salt solutions from Groups B, C and D at concentrations of 5.0 (Group B) and 2.5 mM (Group C and D) (see Fig. 1 and 2: B1, C1 and D1). A key feature of the decrease in ε′′ with temperature is that at frequencies above 1.4 GHz, all of the 2.5 and 5.0 mM M(III) salt solutions absorb less microwave power as the solution gets hotter.
(Fig. 1: A1 brown, blue and green lines). For example, at 915 MHz ε′ decreases from 78.96 ± 0.43 at 20 °C to 73.25 ± 0.25 at 40 °C and 67.92 ± 0.23 at 60 °C (Fig. 1: A1 brown, blue and green lines). This behaviour has previously been reported for distilled water and is due to a decrease in the extent of water–water hydrogen bonding with increasing temperature as a result of a shift in the Boltzmann distribution of the water molecules, i.e. more water molecules have the energy required to overcome hydrogen bonding with increasing temperature.42c,44 The detrimental effect of increasing temperature on the extent of hydrogen bonding is further evidenced by a reduction in ε′′ with increasing temperature for frequencies above 1.4 GHz. For example, at 5.6 GHz ε′′ decreases from 22.02 ± 0.60 at 20 °C to 14.63 ± 0.21 at 40 °C and 10.32 ± 0.21 at 60 °C (Fig. 2: A1 red > pink > black line). A decrease in hydrogen bonding with increasing temperature enables the water molecules to move more freely in solution, thus lowering the rotational drag and ultimately the friction between water molecules.42c,44 By decreasing the amount of friction between water molecules, less kinetic energy is lost as heat and thus a reduction the value of ε′′ is observed.42c,44 The trends in ε′ and ε′′ with temperature exhibited by 5.0 mM solutions of Group A salts are also observed for M(III) salt solutions from Groups B, C and D at concentrations of 5.0 (Group B) and 2.5 mM (Group C and D) (see Fig. 1 and 2: B1, C1 and D1). A key feature of the decrease in ε′′ with temperature is that at frequencies above 1.4 GHz, all of the 2.5 and 5.0 mM M(III) salt solutions absorb less microwave power as the solution gets hotter.
While the major component of the dielectric properties (ε′ and ε′′) for 5.0 mM and 2.5 mM solutions of M(III) salts from Groups A, B, C and D arises from bulk water, at frequencies below 1.0 GHz there is some evidence in the ε′′ data of ionic polarisation mechanisms (see Fig. 2: A1 and B1 and D1; red, pink and black lines). In this frequency range a slight increase in dielectric loss (ε′′) is observed with increasing temperature in the order; 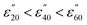 (Fig. 2: A1, B1 and D1; red, pink and black lines). For example, a 5.0 mM solution of CrCl3 (Group A) at 915 MHz shows an increase in ε′′ from 7.49 ± 0.10 at 20 °C to 7.60 ± 0.04 at 40 °C and 9.35 ± 0.80 at 60 °C. We attribute this increase in ε′′ with temperature to two factors; a shift in the ratios of ion-pairs owing to temperature-dependent perturbation of ion-pair association–dissociation equilibrium45,46 and a rise in frictional heat as a result of augmented ion mobility.42c,44a The effect of temperature on ε′′ is different for pure water and aqueous ionic solutions as the water molecules in the hydration sphere do not experience the same rotational restrictions as bulk/free water.47 Most importantly, below 1.0 GHz, solutions of M(III) salt from Groups A, B and D at concentrations of 5.0 mM (A and B) and 2.5 mM (D) absorb more microwave power as the solution gets hotter.
(Fig. 2: A1, B1 and D1; red, pink and black lines). For example, a 5.0 mM solution of CrCl3 (Group A) at 915 MHz shows an increase in ε′′ from 7.49 ± 0.10 at 20 °C to 7.60 ± 0.04 at 40 °C and 9.35 ± 0.80 at 60 °C. We attribute this increase in ε′′ with temperature to two factors; a shift in the ratios of ion-pairs owing to temperature-dependent perturbation of ion-pair association–dissociation equilibrium45,46 and a rise in frictional heat as a result of augmented ion mobility.42c,44a The effect of temperature on ε′′ is different for pure water and aqueous ionic solutions as the water molecules in the hydration sphere do not experience the same rotational restrictions as bulk/free water.47 Most importantly, below 1.0 GHz, solutions of M(III) salt from Groups A, B and D at concentrations of 5.0 mM (A and B) and 2.5 mM (D) absorb more microwave power as the solution gets hotter.
Significantly, the frequency range in which the metal salt solutions absorb more or less microwave power with temperature varies with concentration. These changes can be observed as points of inflexion in the plot of ε′′ vs. concentration (Fig. 2). At concentrations of 5.0, 50.0 mM (Group A and B) and 2.5, 25.0 and 250 mM (Groups C and D), the frequency at which the points of inflexion occur are unique for each individual M(III) salt. However, the frequency at which the inflexion point is observed increases with increasing concentration. At 500 mM solutions of salts containing singly-charged anions (Group A and B) show no points of inflexion in the ε′′ data, rather the values of ε′′ at each temperature converge with increasing frequency. This result confirms that the amount of ions in solution is sufficient under these conditions for the dielectric properties to be dominated by the interaction of ionic species with the electric field. Importantly, at 500 mM, the M(III) salt solutions of Group A and B absorb more microwave power as the solution gets hotter at frequencies of 0.5–10.0 GHz. The frequency range in which solutions of Group C and D absorb more or less microwave energy can be determined from the inflexion points. At frequencies below the inflexion point (low frequency) solutions of Group C and D absorb more microwave power as the solution heats. Above the inflexion point (high frequency) solutions of Group C and D absorb less microwave power as the solution heats.
At concentrations of 5.0 and 2.5 mM bulk water dominates the dielectric properties (ε′ and ε′′) of the M(III) salt solutions.44b All M(III) salt solutions exhibit a decrease in ε′ with increasing concentration over a frequency range of 1.5–10 GHz (Fig. 3(a)–(d) brown > blue > green lines). A reduction in ε′ for solutions with increasing concentration is commonly known as “dielectric decrement”,21,42c which arises from the mutual dependence of the mobilities of ions and their corresponding hydration spheres. The ions dictate the orientation of surrounding water molecules thereby reducing the ability of the water molecules to orient in the electric field. This in turn causes the hydration spheres to exert a retarding force on the ion as the sphere is less mobile than the ion.21,47
Indeed, dielectric decrement has been observed for many other electrolytic solutions such as KF, NaCl, NaCl, Al(NO3)3 (at 25 °C only),32 MgCl248 and MgSO4.49 While dielectric decrement does not affect the heating of a material within an electric field, it is important to be aware of changes in ε′ with increasing concentration as this has an impact on the propagation of the electric field through a material and is essential for microwave cell design.
Below 1.5 GHz differences in the trends in ε′ are observed for Groups A and B compared with Groups C and D. These differences are most obvious at the highest concentration. For example a 500 mM solution of CrCl3 (Group A) displays an increase in ε′ from 58.58 ± 0.68 at 0.5 GHz to 60.44 ± 0.39 at 1.3 GHz at 20 °C (Fig. 3(a)). A 250 mM solution of Cr2(SO4)3 (Group C) exhibits a decrease in ε′ from 85.90 ± 0.11 at 0.5 GHz to 79.41 ± 0.21 at 1.3 GHz at 20 °C (Fig. 3(c)). A possible explanation for this observation is the poor solubility of the salts at concentrations >250 mM. However, further investigations are required to confirm the validity of measurements in this frequency range.
All M(III) salt solutions exhibit the same behaviour in ε′′ with concentration over the 0.5–10.0 GHz frequency range. Below 2.0 GHz a large rise in ε′′ with concentration is observed as a result of increasing ionic polarisation mechanisms (Fig. 3(a)–(d) black > pink > red lines). For example, 5.0, 50.0 and 500 mM solutions of CrCl3 (Group A) exhibit a rise in ε′′ from 7.49 ± 0.0091 to 30.20 ± 0.68 to 150.24 ± 4.93, respectively, at 915 MHz and 20 °C (Fig. 3(a)). We attribute the increase in ε′′ with molarity to the greater concentration of ions and their corresponding hydration spheres in solution; therefore, more viscous lag is taking place. These observations are consistent with experimental data reported for other electrolytic solutions35,50 and theoretical dielectric models.51,52 These results suggest that below 2.0 GHz the most concentrated salt solutions from Groups A, B and D absorb more microwave power than less concentrated solutions owing to selective heating of the ions in solution.
From 2.0–10.0 GHz a smaller increase in ε′′ is exhibited by all M(III) salts compared with that observed below 2.0 GHz (Fig. 3(a)–(d)). For example, 5.0, 50.0 and 500 mM solutions of CrCl3 (Group A) exhibit a rise in ε′′ from 22.02 ± 0.60 to 24.83 ± 0.62 to 39.83 ± 0.34, respectively, at 5.6 GHz and 20 °C (Fig. 3(a)). These results suggest that at 5.6 GHz, the most concentrated salt solutions from Groups A, B and D absorb more microwave power than less concentrated solutions; however, they will not absorb as much power as that observed below 2.0 GHz.
At 10.0 GHz the ε′′ values for each concentration of M(III) salt begin to diverge (Fig. 3(a)–(d)), suggesting that unrestricted water molecules are able to interact with the electric field despite the increased presence of ions and their hydration spheres in solution, i.e. each concentration still contains un-associated ‘bulk’ water molecules.50 At this frequency range it would, therefore, be possible to selectively heat the bulk water molecules only. These results are in agreement with theoretical models used to interpret and/or predict the dielectric properties (ε′ and ε′′) of dilute electrolytic solutions of concentration less than 1000 mM.50
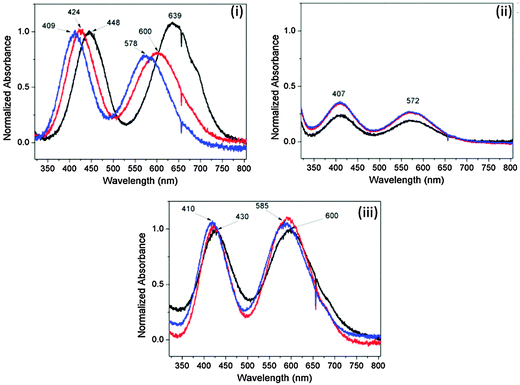
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53 are observed in the absorption spectra of Cr(NO3)3 in deionized water (Group A) at all times owing to the much faster dissociation of the NO3− anion compared with Cl− and SO42−.54 A thorough assessment of the dielectric properties of the species formed during the initial equilibration phase is beyond the scope of this study and will be addressed in future work. We can reasonably consider the fully equilibrated/hydrated species to be representative of the reactants in a typical MOF synthesis, but it is worth noting that the time taken for full equilibration is significant in the first two examples.
53 are observed in the absorption spectra of Cr(NO3)3 in deionized water (Group A) at all times owing to the much faster dissociation of the NO3− anion compared with Cl− and SO42−.54 A thorough assessment of the dielectric properties of the species formed during the initial equilibration phase is beyond the scope of this study and will be addressed in future work. We can reasonably consider the fully equilibrated/hydrated species to be representative of the reactants in a typical MOF synthesis, but it is worth noting that the time taken for full equilibration is significant in the first two examples.
Analyses of the absolute magnitudes of ε′ and ε′′ at three ISM (industrial, scientific and medical) frequencies for the most concentrated solutions allows assessment of the cationic and anionic contributions to the dielectric properties (ε′ and ε′′) of each at a specific temperature (Table 4). The most concentrated solutions are likely to contain a mixture of ion pair types (see below). For example, at 20 °C and a given concentration of 500 mM, all of the Group A M(III) salts [CrCl3, Cr(NO3)3 and Al(NO3)3] exhibit the same values of ε′ and ε′′ at 915 MHz and 2.45 GHz, within experimental error (Table 4). This observation suggests that changing either the cation or anion for these particular M(III) salts has a minor effect on the bulk dielectric properties (ε′ and ε′′) at these frequencies. This result is unsurprising as there is little difference between the cationic radii of Al(III) and Cr(III) (0.5055 and 0.62 Å,56 respectively) and the anionic radii of NO3− and Cl− (approx. 3.45 and 3.18 Å, respectively).54 Additionally, all of these cations adopt the same octahedral hexa-aquo complexes in water53 as confirmed by UV-visible absorption spectroscopy (ESI†). Differences in dielectric properties (ε′ and ε′′) as a result of changing the cation have only been observed for cations with large differences in their radii, for example between alkali earth cations.42a,d Separate reports have shown that the anionic contribution towards the bulk dielectric properties (ε′ and ε′′) of an aqueous electrolyte is much smaller than that of the cation.57–60 These results were ascribed to a difference in hydrogen bonding of water–cation complexes compared to water–anion complexes. For water–cation complexes, the water molecule interacts via the oxygen atom, thus preventing the water molecule from undergoing electric dipole rotation.42d However, in the case of water–anion complexes interaction occurs through one of the water hydrogen atoms such that electric dipole rotation, although restricted, is still possible.42d Ultimately, data in Table 4 suggest that each M(III) salt from Group A is likely to absorb the same amount of microwave power at 915 MHz and 2.45 GHz, indicating that that the individual effects of both the anion and cation on ε′ and ε′′ for the bulk solution for these M(III) salts are small, in agreement with the literature. Unlike Group A, M(III) salts from Group B exhibit small variations in their absolute values of ε′ and ε′′ at frequencies of 915 MHz and 2.45 GHz (Table 4). Out of the M(III) salts in Group B, and indeed all of the M(III) salts in Group A, FeCl3 exhibits the largest ε′ at 915 MHz and 2.45 GHz for a concentration of 500 mM. This observation, coupled with the UV-visible absorption spectra (ESI†), is indicative of the formation of ion pairs as solutions containing ion pairs exhibit higher values of ε′.50 Three types of ion pairs have been identified for electrolytic solutions: solvent sharing ionic-pairs (SSIPs); two solvent sharing ionic pairs (2SIPs); and contact ion pairs (CIPs).35 The presence of CIPs, particularly [FeCl4]−, is not uncommon for solutions of FeCl3 at pH < 2.53 Many solutions containing ion pairs have been reported, including for Al2(SO4)3,39 CuSO4,46 ZnCl2,35 and MSO4 (M = Mg, Ni, Co).27
The largest values of ε′′ are observed for the Group B salts VCl3 and Fe(NO3)3 at 915 MHz indicating that these M(III) salts will absorb more power than all of the M(III) salts in this study at this particular frequency at a given concentration/temperature. High values of ε′′ (Table 4) may be a consequence of the formation of hydroxy species of Fe and V. Solutions of [M(H2O)6]3+ (M = Fe, V) are known to react with water to form hydroxyl complexes such as [M(H2O)5(OH)]2+.53 These data may also arise as a result of the formation of CIPs. Solutions of VCl3 contain CIPs at high concentration;53 however, solutions of Fe(NO3)3 are less likely to contain CIPs as NO3− ions are more weakly associative than Cl− ions.
At 2.45 GHz any variation in ε′ and ε′′ for M(III) salt solutions in Group A and B of the same concentration is less pronounced than at 915 MHz, i.e. the values are converging (Table 4). At 5.6 GHz values of ε′ and ε′′ are the same for each M(III) salt solution for Groups A and B (Table 4). If the values of ε′ and ε′′ are the same for each metal salt, then they should absorb the same amount of microwave power. Therefore, at 2.45 GHz and 5.6 GHz there should be no difference between the heating for each M(III) salt. However, the large differences in ε′′ at 915 MHz indicate that it may be possible to selectively heat the ions in metal salt solutions at low frequency.
M(III) salt solutions from Groups C and D exhibit a large variation in ε′ and ε′′ with M(III) salt and frequency (Table 4). Again, the Fe(III) salt exhibits the highest value of ε′′ in these two groups and will be heated better in a microwave field than Cr(III) and Al(III) salts. This result is likely a consequence of the formation of CIPs and/or hydroxy species in water, as discussed earlier.53 Differences between the values of ε′ and ε′′ of Groups C and D compared with Groups A and B are due to the variance in concentration.
Dielectric properties of the terephthalate ligand (BDC2−)
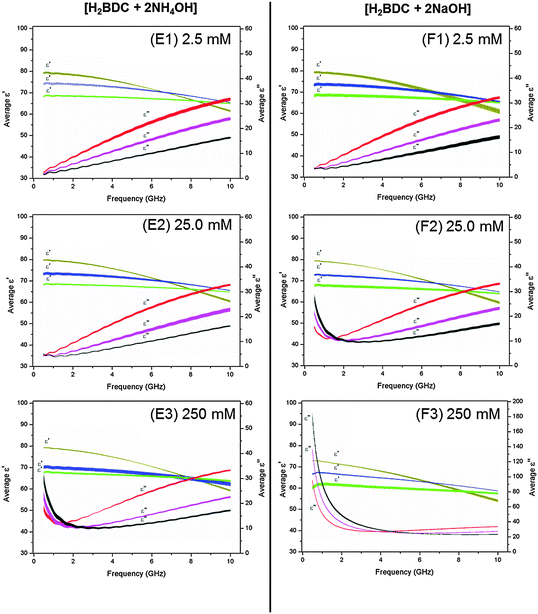
Dissolution of H2BDC in water was facilitated by addition of two equivalents of base (see Experimental). In order to assess the relative contributions of the base and terephthalate (deprotonated anion of BDC2−) to the overall dielectric properties (ε′ and ε′′) of the solution, two bases, NaOH and NH4OH were investigated. Na+ and NH4+ cations have been shown to exhibit very different dielectric behaviour from each other, which therefore facilitates identification of their contribution to the observed dielectric properties.42a,d,61,62 Moreover, comparison of dielectric data of BDC2− with cations and of the cations in the absence of BDC2− allows extraction of the constant contribution of BDC2− to the overall dielectric properties (Fig. 5: E1–3 and F1–3). Plots of dielectric constant (ε′) and loss (ε′′) versus frequency for bases in the absence of H2BDC are given in the ESI.† All data at three ISM frequencies are summarized in Table 5.| Entry no. | Test solution | 915 MHz | 2.45 GHz | 5.6 GHz | |||
|---|---|---|---|---|---|---|---|
| ε′ | ε′′ | ε′ | ε′′ | ε′ | ε′′ | ||
a Data collected at 20 °C with concentrations of 250 mM.
b Data collected at 20 °C with concentrations of 500 mM. Solutions containing mixtures of acid and base have a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 BDC 2 BDC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) base, respectively.
c Data collected at 20 °C on a solid sample using the cavity perturbation method.66 Values and errors of ε′ and ε′′ written to two decimal figures. base, respectively.
c Data collected at 20 °C on a solid sample using the cavity perturbation method.66 Values and errors of ε′ and ε′′ written to two decimal figures. |
|||||||
| 1 | [H2BDC + 2NH4OH]a | 78.95 ± 0.15 | 13.27 ± 0.57 | 77.49 ± 0.14 | 14.24 ± 0.08 | 71.45 ± 0.01 | 23.81 ± 0.23 |
| 2 | [H2BDC + 2NaOH]a | 72.66 ± 0.10 | 55.19 ± 0.24 | 70.53 ± 0.11 | 29.33 ± 0.21 | 64.74 ± 0.27 | 28.57 ± 0.29 |
| 3 | NH4OHb | 79.13 ± 0.05 | 5.70 ± 0.05 | 77.80 ± 0.04 | 11.46 ± 0.02 | 71.66 ± 0.04 | 22.84 ± 0.02 |
| 4 | NaOHb | 67.62 ± 0.49 | 173.76 ± 5.29 | 67.89 ± 0.36 | 73.06 ± 1.91 | 62.84 ± 0.29 | 47.48 ± 0.77 |
| 5 | H2BDCc | 2.50 ± 0.14 | 0.02 ± 0.00 | 2.53 ± 0.16 | 0.03 ± 0.00 | — | — |
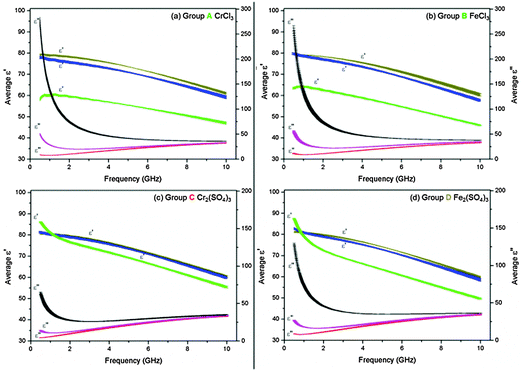
At concentrations of 25.0 and 250 mM, stark differences between the dielectric properties of solutions containing NH4+ and Na+ cations can be seen in the ε′′ data (Fig. 5: E2 and 3, F2 and 3 for BDC2− with cations; ESI† for cations in the absence of BDC2). For these concentrations at frequencies of 915 MHz a significant increase in ε′′ with increasing temperature is observed for BDC2− solutions containing Na+ cations (Fig. 5: F2 and F3, black line > pink line > red line). For example, a 250 mM solution of [H2BDC + 2NaOH] at 915 MHz shows an increase in ε′′ from 55.17 ± 0.24 at 20 °C to 76.04 ± 0.74 at 40 °C and 99.52 ± 2.07 at 60 °C. This trend of increasing ε′′ with temperature at frequencies below 2.0 GHz is consistent with that observed for the M(III) salts from Groups A, B, C and D and shows that the [H2BDC + 2NaOH] solution will absorb more microwave power as it is heated. However, this trend is not observed for the BDC2− solutions containing NH4+ cations. Only at a concentration of 250 mM does a solution of BDC2− containing NH4+ cations exhibit values of ε′′ below 2.0 GHz consistent with ionic polarisation mechanisms (Fig. 5: E3 red line > pink line > black line). For example, a 250 mM solution of [H2BDC + 2NH4OH] at 915 MHz exhibits ε′′ values of 13.27 ± 0.57 at 20 °C, 14.61 ± 0.98 at 40 °C and 18.10 ± 1.07 at 60 °C. Differences between the dielectric behaviour of Na+ and NH4+ cations arise from differences in their ionic radii,57,60 the tetrahedral geometry of NH4+, and the ability of NH4+ to act as a hydrogen bonding partner with water.59,61 In contrast, Na+ cations, like the M(III) salts in Group A, B, C and D, disrupt the hydrogen bonding in bulk water due to the restricted mobility and preferential orientation of water molecules in the hydration sphere of the cation.47,42d Importantly, these results confirm that solutions containing Na+ cations will absorb more power and therefore will be heated more effectively than solutions containing NH4+ cations at frequencies below 2.0 GHz.
Further differences between NH4+ and Na+ are evident in the ε′ data (Fig. 5 E1–3, F1–3 for BDC2− with cations; ESI† for cations in the absence of BDC2−). For example, as for the M(III) salts in Groups A, B, C and D, an increase in concentration of metal cations at fixed temperature leads to a reduction in ε′, i.e. dielectric decrement.21,42c The dielectric decrement displayed by Na+ cations is less than for M(III) salts owing to the smaller ionic charge and differences in size of hydration spheres. Solutions containing NH4+ ions do not display dielectric decrement with increasing concentration (Fig. 5: E1–3, brown lines). A larger reduction in ε′ with increasing temperature is observed for NH4+ than for solutions containing NaOH (Fig. 5: E1–3 compared to F1–3, respectively). For example, a 250 mM solution of [H2BDC + 2NH4OH] exhibits a reduction in ε′ at 915 MHz from 78.95 ± 0.15 at 20 °C to 69.94 ± 0.65 at 40 °C, whereas a 250 mM solution of [H2BDC + 2NaOH] at 915 MHz shows a smaller reduction of 72.66 ± 0.10 at 20 °C to 67.26 ± 0.19 at 40 °C. A reduction in ε′ with increasing temperature is due to a decrease in the extent of water–water hydrogen bonding as a result of a shift in the Boltzmann distribution of the water molecules with increasing temperature (as described above).42c,44a,b
Conclusions
Variation of metal salts enables the synthesis of MIL-53 and MIL-47 analogues containing different metal nodes with the same organic linker, BDC2−.28,29 In this work the dielectric constants (ε′) and loss factors (ε′′) of a number of M(III) salts and salts of terephthalic acid (H2BDC) dissolved in deionized water were measured as a function of frequency, temperature, concentration and with several anion and cations. In the frequency range of this study, test solutions have been shown to interact with the electric field via ionic and dipolar polarisation mechanisms.All solutions exhibit dielectric behaviour (values of ε′ and ε′′) consistent with bulk deionized water at concentrations of 5.0 mM (Group A and B M(III) salts) and 2.5 mM (Group C and D M(III) salts and BDC2− solutions). Above these concentrations the M(III) salts (Group A, B, C and D) show higher dielectric losses (ε′′) and smaller dielectric constants (ε′) than the BDC2− solutions at 915 MHz and 2.45 GHz as a result of ionic polarisation mechanisms. These data indicate that the M(III) salts will absorb more power and therefore be heated more effectively than the ligand. Owing to stoichiometry-driven differences in concentration required for MOF synthesis, M(III) salts with mono-anions (for example with Cl− and NO3−) exhibit higher ε′′ than di-anionic salts (SO42−) indicating that they will be heated better in an electric field. Of the M(III) salts studied, those containing Fe(III) have the highest ε′′ indicating that they will be heated better in an electric field than M(III) salts containing other metal cations, such as Cr(III) and Al(III).
This work has shown that [H2BDC + 2NaOH] exhibits much higher dielectric losses (ε′′) than [H2BDC + 2NH4OH] and H2BDC (solid) owing to interaction of Na+ with the electric field. Therefore solutions containing [2Na+ + BDC2−] are likely to heat better than those containing H2BDC. Also, M(III) salts in Group A, B, C and D show higher dielectric losses (ε′′) at a frequency of 915 MHz than at 2.45 GHz. Thus, heating may be achieved more effectively at 915 MHz than at 2.45 GHz. Information on the dielectric properties (ε′ and ε′′) is essential for the optimum design and safe scaling-up of microwave processes and also in understanding why microwave heated processes are far more rapid than those heated conventionally.
Acknowledgements
We thank the EPSRC for support. MS gratefully acknowledges support from the ERC for an Advanced Grant. NRC gratefully acknowledges a Royal Society Wolfson Merit Award.References
- J. L. C. Rowsell and O. M. Yaghi, Microporous Mesoporous Mater., 2004, 73, 3–14 CrossRef CAS; Y. Yan, X. Lin, S. Yang, A. J. Blake, A. Dailly, N. R. Champness, P. Hubberstey and M. Schröder, Chem. Commun., 2009, 1025–1027 RSC.
- O. K. Farha, I. Eryazici, N. C. Jeong, B. G. Hauser, C. E. Wilmer, A. A. Sarjeant, R. Q. Snurr, S. T. Nguyen, A. Ö. Yazaydın and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 15016–15021 CrossRef CAS PubMed; Y. Yan, S. Yang, A. J. Blake and M. Schröder, Acc. Chem. Res., 2014, 47, 296–307 CrossRef PubMed; X. Lin, I. Telepeni, A. J. Blake, A. Dailly, C. M. Brown, J. M. Simmons, M. Zoppi, G. S. Walker, K. M. Thomas, T. J. Mays, P. Hubberstey, N. R. Champness and M. Schröder, J. Am. Chem. Soc., 2009, 131, 2159–2171 CrossRef PubMed.
- V. Guillerm, D. Kim, J. F. Eubank, R. Luebke, X. Liu, K. Adil, M. S. Lah and M. Eddaoudl, Chem. Soc. Rev., 2014, 43, 6141–6172 RSC.
- W. Lu, Z. Wei, Z.-Y. Gu, T.-F. Liu, J. Park, J. Park, J. Tian, M. Zhang, Q. Zhang, T. Gentle III, M. Bosch and H.-C. Zhou, Chem. Soc. Rev., 2014, 43, 5561–5593 RSC.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC; S. Yang, A. J. Ramirez-Cuesta, R. Newby, V. Garcia-Sakai, P. Manuel, S. K. Callear, S. I. Campbell, C. C. Tang and M. Schröder, Nat. Chem., 2015, 7, 121–129 CrossRef CAS PubMed; W. Yang, A. J. Davies, X. Lin, M. Suyetin, R. Matsuda, A. J. Blake, C. Wilson, W. Lewis, J. E. Parker, C. C. Tang, M. W. George, P. Hubberstey, S. Kitagawa, H. Sakamoto, E. Bichoutskaia, N. R. Champness, S. Yang and M. Schröder, Chem. Sci., 2012, 3, 2993–2999 RSC.
- D. Farrusseng, Metal-Organic Frameworks Applications from Catalysis to Gas Storage, Wiley-VCH, Weinheim Germany, 2011 Search PubMed.
- D. Farrusseng, S. Aguado and C. Pinel, Angew. Chem., Int. Ed., 2009, 48, 7502–7513 CrossRef CAS PubMed.
- A. Morozan and F. Jaouen, Energy Environ. Sci., 2012, 5, 9269–9290 CAS.
- Y.-W. Li, J.-R. Li, L.-F. Wang, B.-Y. Zhou, Q. Chen and X.-H. Bu, J. Mater. Chem. A, 2013, 1, 495–499 CAS.
- M. Dan-Hardi, C. Serre, T. Frot, L. Rozes, G. Maurin, C. Sanchez and G. Férey, J. Am. Chem. Soc., 2009, 131, 10857–10859 CrossRef CAS PubMed.
- M. Eddaoudi, D. B. Moler, H. L. Li, B. L. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319–330 CrossRef CAS PubMed.
- G. Ferey, Chem. Soc. Rev., 2008, 37, 191–214 RSC.
- N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933–969 CrossRef CAS PubMed.
- A. M. Joaristi, J. Juan-Alcaniz, P. Serra-Crespo, F. Kapteijn and J. Gascon, Cryst. Growth Des., 2012, 12, 3489–3498 Search PubMed.
- M. Klimakow, P. Klobes, A. F. Thuenemann, K. Rademann and F. Emmerling, Chem. Mater., 2010, 22, 5216–5221 CrossRef CAS.
- N. A. Khan and S. H. Jhung, Coord. Chem. Rev., 2015, 285, 11–23 CrossRef CAS.
- Z. Ni and R. I. Masel, J. Am. Chem. Soc., 2006, 128, 12394–12395 CrossRef CAS PubMed.
- N. A. Khan and S. H. Jhung, Cryst. Growth Des., 2010, 10, 1860–1865 Search PubMed.
- E. Haque and S. H. Jhung, Chem. Eng. J., 2011, 173, 866–872 CrossRef CAS.
- S. H. Jhung, J.-H. Lee, P. M. Forster, G. Férey, A. K. Cheetham and J.-S. Chang, Chem. – Eur. J., 2006, 12, 7899–7905 CrossRef PubMed.
- J. B. Hasted, Aqueous Dielectrics, Chapman and Hall Ltd., London, 1973 Search PubMed.
- J. Krupka, Meas. Sci. Technol., 2006, 17, R55–R70 CrossRef CAS.
- A. C. Metaxas and R. J. Meredith, Industrial Microwave Heating, The Institution of Engineering and Technology, 2008 Search PubMed.
- R. N. Clarke, A. P. Gregory, D. Cannell, M. Patrick, S. Wylie, I. Youngs and G. Hill, A guide to the characterisation of dielectric materials at RF and microwave frequencies, Institute of Measurement and Control/National Physical Laboratory, 2003 Search PubMed.
- G. B. Dudley, R. Richert and A. E. Stiegman, Chem. Sci., 2015, 6, 2144–2152 RSC.
- U. Kaatze and R. Behrends, Tech. Mess., 2002, 69, 5–11 CAS.
- T. Chen, G. Hefter and R. Buchner, J. Solution Chem., 2005, 34, 1045–1066 CrossRef CAS.
- C. Serre, F. Millange, C. Thouvenot, M. Nogues, G. Marsolier, D. Louer and G. Férey, J. Am. Chem. Soc., 2002, 124, 13519–13526 CrossRef CAS PubMed; T. Loiseau, C. Serre, C. Huguenard, G. Fink, F. Taulelle, M. Henry, T. Bataille and G. Férey, Chem. – Eur. J., 2004, 10, 1373–1382 CrossRef PubMed.
- S. Bourrelly, P. L. Llewellyn, C. Serre, F. Millange, T. Loiseau and G. Férey, J. Am. Chem. Soc., 2005, 127, 13519–13521 CrossRef CAS PubMed; K. Barthelet, J. Marrot, D. Riou and G. Férey, Angew. Chem., Int. Ed., 2002, 41, 281–284 CrossRef.
- E. Haque, J. H. Jeong and S. H. Jhung, CrystEngComm, 2010, 12, 2749–2754 RSC.
- E. Haque, N. A. Khan, J. H. Park and S. H. Jhung, Chem. – Eur. J., 2010, 16, 1046–1052 CrossRef CAS PubMed.
- Y. Marcus, J. Solution Chem., 2013, 42, 2354–2363 CrossRef CAS.
- U. Kaatze, J. Solution Chem., 1997, 26, 1049–1112 CrossRef CAS; A. K. Lyashchenko and A. Lileev, J. Chem. Eng. Data, 2010, 55, 2008–2016 CrossRef.
- A. K. Lyashchenko and I. M. Karataeva, Russ. J. Phys. Chem. A, 2010, 84, 320–328 CrossRef CAS.
- U. Kaatze, V. Lonnecke and R. Pottel, J. Mol. Liq., 1987, 34, 241–255 CrossRef CAS.
- H. Falkenhagen, Rev. Mod. Phys., 1931, 3, 412–426 CrossRef CAS.
- J. Barthel, H. Hetzenauer and R. Buchner, Ber. Bunsen-Ges., 1992, 96, 1424–1432 CrossRef CAS.
- U. Kaatze, Z. Phys. Chem. Neue Fol., 1983, 135, 51–75 CrossRef CAS.
- S. Schrodle, W. W. Rudolph, G. Hefter and R. Buchner, Geochim. Cosmochim. Acta, 2007, 71, 5287–5300 CrossRef CAS.
- U. Kaatze and K. Giese, J. Mol. Liq., 1987, 36, 15–35 CrossRef CAS.
- F. E. Harris and C. T. Okonski, J. Phys. Chem., 1957, 61, 310–319 CrossRef CAS.
- (a) A. S. Lileev, Z. A. Filimonova and A. K. Lyashchenko, J. Mol. Liq., 2003, 103, 299–308 CrossRef; (b) U. Kaatze and R. Pottel, Z. Phys. Chem. Neue Fol., 1984, 141, 1–13 CrossRef CAS; (c) J. B. Hasted, Liquid water: dielectric properties, Water A comprehensive treatise, Plenum Press, New York, 1972 Search PubMed; (d) K. Giese, U. Kaatze and R. Pottel, J. Phys. Chem., 1970, 74, 3718 CrossRef CAS.
- J. Monzó-Cabrera, J. M. Catala-Civera, A. Díaz-Morcillo, D. Sánchez-Hernández, P. J. Plaza-González and E. de los Reyes, Microwave Opt. Technol. Lett., 2002, 32(6), 465–469 CrossRef; P. J. Plaza-González, J. Monzó-Cabrera, J. M. Catalá-Civera and D. Sánchez-Hernández, IEEE Trans. Microwave Theory Tech., 2005, 53(5), 1699–1706 CrossRef.
- (a) R. Buchner, J. Barthel and J. Stauber, Chem. Phys. Lett., 1999, 306, 57–63 CrossRef CAS; (b) U. Kaatze, J. Chem. Eng. Data, 1989, 34, 371–374 CrossRef CAS.
- C. Akilan, N. Rohman, G. Hefter and R. Buchner, ChemPhysChem, 2006, 7, 2319–2330 CrossRef CAS PubMed.
- C. Akilan, G. Hefter, N. Rohman and R. Buchner, J. Phys. Chem. B, 2006, 110, 14961–14970 CrossRef CAS PubMed.
- U. Kaatze R. Behrends and R. Pottel, J. Non-Cryst. Solids, 2002, 305, 19–28 CrossRef.
- E. Glueckauf, Trans. Faraday Soc., 1964, 60, 1637 RSC.
- R. Buchner, T. Chen and G. Hefter, J. Phys. Chem. B, 2004, 108, 2365–2375 CrossRef CAS.
- N. Gavish and K. Promislow, Chem. Phys., 2012 Search PubMed , arXiv:1208.5169.
- A. Levy, D. Andelman and H. Orland, Phys. Rev. Lett., 2012, 108(22), 227801 CrossRef PubMed.
- A. Chandra and B. Bagchi, J. Chem. Phys., 2000, 112, 1876–1886 CrossRef CAS.
- D. Nicholls, Complexes and first-row transition elements, Macmillan Education Ltd, Basingstoke and London, 1974 Search PubMed.
- P. J. Elving and B. Zemel, J. Am. Chem. Soc., 1957, 79, 1281–1285 CrossRef CAS.
- Y. Marcus, Chem. Rev., 1988, 88, 1475–1498 CrossRef CAS.
- R. D. Shannon, Acta Crystallogr., Sect. A: Found. Crystallogr., 1976, 32, 751–767 CrossRef.
- J. B. Hubbard, P. Colonomos and P. G. Wolynes, J. Chem. Phys., 1979, 71, 2652 CrossRef CAS.
- P. G. Kusalik and G. N. Patey, J. Chem. Phys., 1983, 79, 4468 CrossRef CAS.
- U. Kaatze, J. Solution Chem., 1997, 26, 1049–1112 CrossRef CAS.
- J. B. Hubbard and R. F. Kayser, J. Chem. Phys., 1981, 74, 3535 CrossRef CAS.
- U. Kaatze, J. Phys. Chem. B, 2013, 117, 12252–12260 CrossRef CAS PubMed.
- U. Kaatze, Radiat. Phys. Chem., 1995, 45, 549–566 CrossRef CAS.
- A. P. Gregory and R. N. Clarke, Tables of the Complex Permittivity of Dielectric Reference Liquids at Frequencies up to 5 GHz, 2012, Report ISSN 1754-2979.
- A. von Hippel, R. G. Breckenridge, F. G. Chesley and L. Tisza, Ind. Eng. Chem., 1946, 38, 1097–1109 CrossRef CAS.
- U. Kaatze, J. Solution Chem., 1997, 26, 1049–1112 CrossRef CAS.
- A. von Hippel, Dielectrics and waves, Wiley, Massachusetts, 3rd edn, 1954 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cp05426e |
| This journal is © the Owner Societies 2016 |