 Open Access Article
Open Access ArticleComment on “Fullerene-based materials for solar cell applications: design of novel acceptors for efficient polymer solar cells – a DFT study” by A. Mohajeri and A. Omidvar, Phys. Chem. Chem. Phys., 2015, 17, 22367†
Denis Sh.
Sabirov
*a,
Anton O.
Terentyev
 a and
Igor S.
Shepelevich
b
a and
Igor S.
Shepelevich
b
aInstitute of Petrochemistry and Catalysis, Russian Academy of Sciences, 450075 Ufa, Russia. E-mail: diozno@mail.ru; Fax: +7 347 2842750
bBashkir State University, 450076 Ufa, Russia
First published on 4th January 2016
Abstract
Though a linear correlation has been recently reported between mean polarizabilities of the fullerene derivatives and open-circuit voltages of organic solar cells based on them, we demonstrate that there is no general dependence between these two values and some related quantities (anisotropy of polarizability and LUMO levels).
1 Introduction
A recent theoretical study1 reports on the correlations between the open-circuit voltage (VOC) of organic solar cells (OSCs) and physicochemical parameters of the fullerene derivatives used as electron acceptors in OSCs. Mohajeri and Omidvar1 have performed accurate quantum-chemical calculations (using appropriate DFT methods) and compared their computational results with the experimental VOC values from the review.2 It is important that all open-circuit voltages were measured under the same experimental conditions, so their joint use in the frame of one correlation is justified.One of the correlations reported in ref. 1 is a linear dependence of VOC on the mean polarizability of the corresponding fullerene derivative acceptors (R2 = 0.914). The authors concluded that “the most efficient fullerene-based acceptors are characterized by… maximum dipole polarizability”. We consider that the mentioned conclusion is overoptimistic and poorly justified. In the comment, we provide pro et contra arguments regarding the citation above.
As is known, polarizability is a tensor quantity that describes molecule's behavior in the external electric fields. It has wide applications in physical chemistry and chemical physics as it defines dispersion interactions, chemical properties, dielectric permittivity, refractivity, etc. As previously pointed in the reviews3–5 and original studies,6–19 polarizability has high importance for fullerene chemistry and materials science because fullerenes and their derivatives are highly polarizable species. We have illustrated how polarizability can be applied to such a current hot fullerene topic as organic solar cells.4 Polarizability plays an important role in OSCs due to (1) its relation to dielectric permittivity (described by the Clausius–Mossoti formula; see also a very recent review5), (2) extremely high polarizability of the charge-transfer complexes generated when OSCs act, and (3) influence of polarizability on wetting processes (the de Gennes equation), which define miscibility of polymer donors with fullerene acceptors and, therefore, the OSC output parameters. The aforementioned arguments are in favor of the importance of polarizability for OSCs. All of them are qualitative.
In contrast, Mohajeri and Omidvar1 propose a linear correlation between VOC and mean polarizability α. They operate in their work with 9 derivatives of the C60 fullerene, viz. substituted cyclopropa[60]fullerenes. Note that 6 of them were monoadducts whereas other 3 compounds were bis- and trisadducts. The latter, as known, have multiple regioisomers. This leads to the first mistake of ref. 1 using the experimental results for fullerene multiadducts corresponding to the mixtures of the isomers while the computational data correspond to one selected isomer. This mistake is not decisive for polarizability considerations because we have previously shown that regioisomeric fullerene multiadducts have almost the same mean polarizabilities (with very rare exceptions).8,10,11,13–15,17 However, it may be more pronounced for hardness, LUMO levels and other parameters calculated by Mohajeri and Omidvar.
The second weak spot is that ref. 1 covers only 9 fullerene derivatives to build up a correlation. We think it is not enough to substantiate a linear dependence. To demonstrate this, we have performed similar calculations with the extended set of fullerene derivatives. The experimental data (VOC and power conversion efficiency η) have been taken from the Troshin et al. work on OSCs with substituted cyclopropafullerenes20 (all of them are monoadducts; our set includes 6 compounds from the Mohajeri and Omidvar calculations). We have calculated their mean polarizabilities by the PBE/3ζ method in terms of the finite field approach. This method was previously used for accurate calculations of diverse fullerene derivatives.8,10,11,13–15,17 Unfortunately, we have found no correlations between the calculated α values and measured VOC and η (Fig. 1).
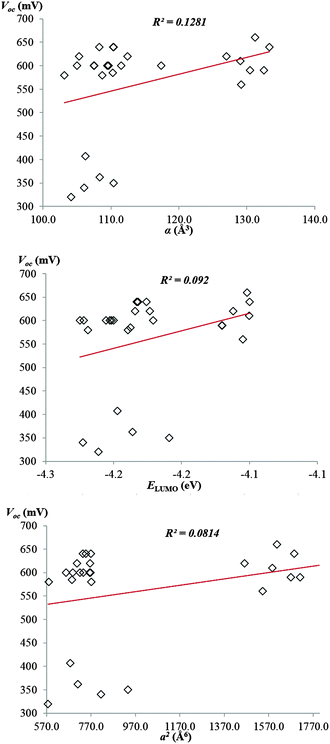 | ||
| Fig. 1 Plots of open-circuit voltages against mean polarizability, anisotropy of polarizability, and LUMO energy of fullerene adducts. | ||
Additional arguments against the reported correlation arise from comparative experimental studies21–24 applying the purified regioisomeric fullerene bisadducts to OSCs. As these studies discovered, isomerism of fullerene derivatives affects the key output parameters of OSCs. However, we have shown on a large set of fullerene adducts that their positional isomers have almost equal mean polarizabilities (only some isomers of chlorofullerenes,10 C60 oligomers,13 and C70 dimers17 reveal a significant difference; these compounds are not present in our set). Hence, Mohajeri and Omidvar's correlation contradicts the known experimental and theoretical data.
It is not an easy task to find out correlations between the physicochemical parameters and device performances. Indeed, we have previously obtained a correlation between the experimental VOC and η values and calculated anisotropy of polarizability a2 of isomeric fullerene bisadducts.15 However, it works only if the isomeric compounds are considered. In this comment in the context of Mohajeri and Omidvar's paper, we report on no correlation between the VOC (η) and a2 values (Fig. 1). Finally, ref. 1 provides a correlation between VOC and LUMO levels of fullerene derivatives. Therefore, we have calculated LUMO energies of 27 compounds from ref. 20 and plotted these against the experimental data (Fig. 1). No obvious correlation has been also found.
Thus, we explain the reported correlations1 by insufficiency of the studied set of fullerene derivatives (9 compounds). Extending the set to 27 samples breaks the declared regularities. As a consequence, only polarizability or only LUMO energies are insufficient to predict novel acceptor structures for OSCs. The situation may be corrected if a multi-parameter correlation will be proposed like models in QSAR and QSPR studies (quantitative structure–activity relationship and quantitative structure–property relationship).
Acknowledgements
The work was financially supported by Russian Foundation for Basic Research (project 16-03-00822 A).Notes and references
- A. Mohajeri and A. Omidvar, Phys. Chem. Chem. Phys., 2015, 17, 22367 RSC.
- Y. He and Y. Li, Phys. Chem. Chem. Phys., 2011, 13, 1970 RSC.
- M. Broyer, R. Antoine, E. Benichou, I. Compagnon, P. Dugourd and D. Rayane, C. R. Phys., 2002, 3, 301 CrossRef CAS.
- D. S. Sabirov, RSC Adv., 2014, 4, 44996 RSC.
- S. Few, J. M. Frost and J. Nelson, Phys. Chem. Chem. Phys., 2015, 17, 2311 RSC.
- C. R. Zhang, H. S. Chen, Y. H. Chen, Z. Q. Wei and Z. S. Pu, Acta Phys.-Chim. Sin., 2008, 24, 1353 CrossRef CAS.
- K. Hornberger, S. Gerlich, H. Ulbricht, L. Hackermüller, S. Nimmrichter, I. V. Goldt, O. Boltalina and M. Arndt, New J. Phys., 2009, 11, 043032 CrossRef.
- D. S. Sabirov, R. G. Bulgakov, S. L. Khursan and U. M. Dzhemilev, Dokl. Phys. Chem., 2009, 425, 54 CrossRef.
- R. Rivelino, T. Malaspina and E. E. Fileti, Phys. Rev. A: At., Mol., Opt. Phys., 2009, 79, 013201 CrossRef.
- D. S. Sabirov, R. R. Garipova and R. G. Bulgakov, Chem. Phys. Lett., 2012, 523, 92 CrossRef CAS.
- D. S. Sabirov, A. A. Tukhbatullina and R. G. Bulgakov, Comput. Theor. Chem., 2012, 993, 113 CrossRef CAS.
- C.-R. Zhang, L.-H. Han, J.-W. Zhe, N.-Z. Jin, Y.-L. Shen, L.-H. Yuan, Y.-Z. Wu and Z.-J. Liu, J. Nanomater., 2013, 612153 Search PubMed.
- D. S. Sabirov, RSC Adv., 2013, 3, 19430 RSC.
- D. S. Sabirov, R. R. Garipova and R. G. Bulgakov, J. Phys. Chem. A, 2013, 117, 13176 CrossRef CAS PubMed.
- D. S. Sabirov, J. Phys. Chem. C, 2013, 117, 9148 CAS.
- K. Akhtari, K. Hassanzadeh, B. Fakhraei, H. Hassanzadeh, G. Akhtari and S. A. Zarei, Comput. Theor. Chem., 2014, 1038, 1 CrossRef CAS.
- D. S. Sabirov, A. O. Terentyev and R. G. Bulgakov, Phys. Chem. Chem. Phys., 2014, 16, 14594 RSC.
- B. Bernardo, D. Cheyns, B. Verreet, R. D. Schaller, B. P. Rand and N. C. Giebink, Nat. Commun., 2014, 5, 3245 CAS.
- A.-R. Nekoei and S. Haghgoo, Comput. Theor. Chem., 2015, 1067, 148 CrossRef CAS.
- P. A. Troshin, H. Hoppe, J. Renz, M. Egginger, J. Y. Mayorova, A. E. Goryachev, A. S. Peregudov, R. N. Lyubovskaya, G. Gobsch, N. S. Sariciftci and V. F. Razumov, Adv. Funct. Mater., 2009, 19, 779 CrossRef CAS.
- S. Kitaura, K. Kurotobi, M. Sato, Y. Takano, T. Umeyama and H. Imahori, Chem. Commun., 2012, 48, 8550 RSC.
- R. Tao, T. Umeyama, K. Kurotobi and H. Imahori, ACS Appl. Mater. Interfaces, 2014, 6, 17313 CAS.
- T. Mikie, A. Saeki, N. Ikuma, K. Kokubo and S. Seki, ACS Appl. Mater. Interfaces, 2015, 7, 12894 CAS.
- F. Zhao, X. Meng, Y. Feng, Z. Jin, Q. Zhou, H. Li, L. Jiang, J. Wang, Y. Li and C. Wang, J. Mater. Chem. A, 2015, 3, 14991 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Numerical data associated with Fig. 1, the plot and structural formulas of 27 studied fullerene adducts. See DOI: 10.1039/c5cp05408g |
| This journal is © the Owner Societies 2016 |
