Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Uncommon halogen bond motifs in cocrystals of aromatic amines and 1,4-diiodotetrafluorobenzene†
Vinko
Nemec
and
Dominik
Cinčić
*
Department of Chemistry, Faculty of Science, University of Zagreb, Horvatovac 102a, HR-10000 Zagreb, Croatia. E-mail: dominik@chem.pmf.hr; Fax: +385 14606341; Tel: +385 14606362
First published on 17th August 2016
Abstract
A number of novel halogen-bonded aromatic amine cocrystals with 1,4-diiodotetrafluorobenzene have been synthesized via both mechanochemical and solution syntheses, offering valuable insight into potential halogen bond acceptor species.
Halogen bonding relies on the presence of an anisotropically polarized halogen atom, usually bromine or iodine, and its interaction with an electron-rich region on another atom, the halogen bond acceptor.1 The halogen bond strength is strongly dependent on the surroundings to which the halogen atom is bonded.2 Due to this fact, the halogen bond is, similarly to the well-researched hydrogen bond, significantly directional.3 Throughout the past decade, the halogen bond has played an increasingly prominent role in crystal engineering4 and is one of the most interesting non-covalent interactions used for constructing supramolecular assemblies.5 Additionally, halogen bonding is a growing area of research covering the fields of solution chemistry,6 biomedicine7 and biomolecular chemistry,8 as well as fundamental chemistry.9 In spite of the continuously expanding research on halogen bonds, the data available in the Cambridge Structural Database (CSD)10 are still insufficient for establishing a complete insight into the halogen bond hierarchy, i.e. how these interactions may compete with each other and with hydrogen bonds, as well as the flexibility and predictability of halogen bond synthons.
In this study, we set out to investigate the binding abilities of a classic halogen bond donor, 1,4-diiodotetrafluorobenzene (tfib),11 with acceptors containing various functional groups able to take part in halogen bonding. The acceptors we were interested in are aromatic amines: 4-aminoacetophenone (4aap), 4-aminobenzophenone (4ab), 4-nitroaniline (4noa), 3-aminopyridine (3ap), 3-aminobenzonitrile (3abn) and 5-amino-2-methoxypyridine (5a2mp) (Fig. 1a).
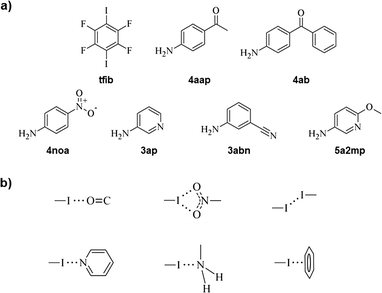 | ||
| Fig. 1 (a) Structures of 1,4-diiodotetrafluorobenzene (tfib) and aromatic amines used in this work and (b) halogen bond motifs present in our synthesized cocrystals. | ||
The selected amines substituted with halogen bond acceptor species (a nitro, a pyridine, a carbonyl, a nitrile or a methoxy group) are useful compounds from a crystal engineering standpoint, since not only can they participate in the formation of halogen bonds but also of hydrogen bonds with the donor amino group. Interestingly, while there is a solid amount of data in the CSD on halogen bonds between tfib and a pyridine nitrogen atom (61 hits), identifying this motif as a reliable one, there are significantly fewer results for halogen bonds between tfib and either the carbonyl oxygen atom (10 hits), the cyano nitrogen atom (5 hits), or the methoxy oxygen atom (0 hits). To the best of our knowledge, we report the first known cocrystal of tfib with a robust I(⋯O)2 halogen bond motif, containing a bifurcated halogen bond donor with nitro group oxygen atoms as halogen bond acceptors.12
In our work, we first attempted mechanochemical13 cocrystal screening experiments by liquid-assisted grinding (LAG)14 solid reactants with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 amine
1 amine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tfib molar ratio (see the ESI†). Milling was conducted in a Retsch MM200 mill using a stainless steel milling assembly under normal laboratory conditions (temperature of ca. 25 °C, 40–60% relative humidity). To observe the grinding experiments, as well as to facilitate the characterization of the new cocrystals by single-crystal X-ray diffraction, mechanochemical experiments were accompanied by crystallization from the solution. All reactants and products have been characterized by means of PXRD and DSC. In the solution experiments, single crystals were obtained either by dissolving reactant mixtures in a solvent or a solvent mixture or by recrystallizing LAG samples. The solutions were left at room temperature, and the product crystallized upon cooling (see the ESI†). A variety of experimental parameters have been tested for obtaining the (4noa)2(tfib) cocrystal (see Table S1 in the ESI†), but the desired product was only obtained by dissolving an equimolar mixture of the copper(II) chloride complex CuCl2(4noa)2 and tfib in acetone. The (4noa)2(tfib) product crystallized upon solvent evaporation and cooling at room temperature. The measured PXRD patterns of the cocrystals obtained by both methods, grinding and from the solution, are in good agreement with those calculated from single crystal data, thus confirming that all products were obtained as pure single phases (Fig. 2). Crystal structure determination of the prepared cocrystals revealed that the molecules are connected via halogen bonds and they form various uncommon halogen bond motifs (Fig. 1b).
tfib molar ratio (see the ESI†). Milling was conducted in a Retsch MM200 mill using a stainless steel milling assembly under normal laboratory conditions (temperature of ca. 25 °C, 40–60% relative humidity). To observe the grinding experiments, as well as to facilitate the characterization of the new cocrystals by single-crystal X-ray diffraction, mechanochemical experiments were accompanied by crystallization from the solution. All reactants and products have been characterized by means of PXRD and DSC. In the solution experiments, single crystals were obtained either by dissolving reactant mixtures in a solvent or a solvent mixture or by recrystallizing LAG samples. The solutions were left at room temperature, and the product crystallized upon cooling (see the ESI†). A variety of experimental parameters have been tested for obtaining the (4noa)2(tfib) cocrystal (see Table S1 in the ESI†), but the desired product was only obtained by dissolving an equimolar mixture of the copper(II) chloride complex CuCl2(4noa)2 and tfib in acetone. The (4noa)2(tfib) product crystallized upon solvent evaporation and cooling at room temperature. The measured PXRD patterns of the cocrystals obtained by both methods, grinding and from the solution, are in good agreement with those calculated from single crystal data, thus confirming that all products were obtained as pure single phases (Fig. 2). Crystal structure determination of the prepared cocrystals revealed that the molecules are connected via halogen bonds and they form various uncommon halogen bond motifs (Fig. 1b).
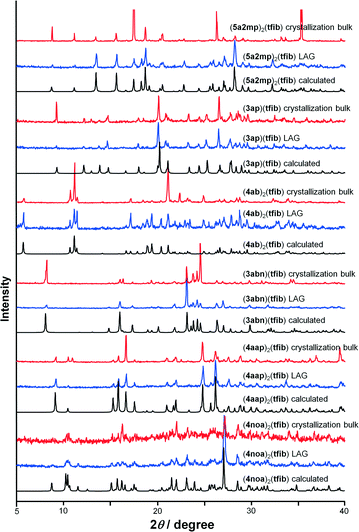 | ||
| Fig. 2 Comparison of diffraction patterns calculated from the obtained single crystal data and those of mechanochemical and bulk crystallization products. | ||
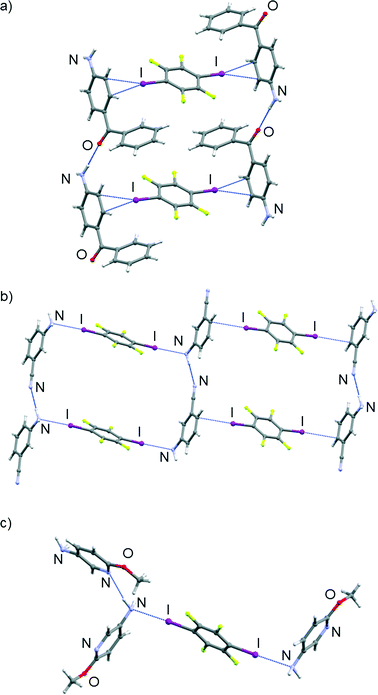
While both nitro group oxygen atoms in the (4noa)2(tfib) cocrystal participate in halogen bonding, the iodine atom being a bifurcated donor in this case, their participation in various hydrogen bonds results in different halogen bond geometries: one oxygen atom is more strongly bound to iodine (d(I⋯O) = 3.18 Å, ∠ (C–I⋯O) = 163°) and therefore participates only in a weak C–H⋯O hydrogen bond, while the other oxygen atom that is more distant (d(I⋯O) = 3.36 Å, ∠ (C–I⋯O) = 158°) participates in a strong N–H⋯O hydrogen bond (d(N2⋯O3) = 3.14 Å, ∠ (N2–H2N⋯O3) = 139°; d(N4⋯O2) = 3.15 Å, ∠ (N4–H3N⋯O2) = 142°). The combination of the mentioned interactions with N–H⋯F and C–H⋯F hydrogen bonds gives rise to layers (Fig. 3, see the ESI†). The 3D structure is a result of layer stacking.
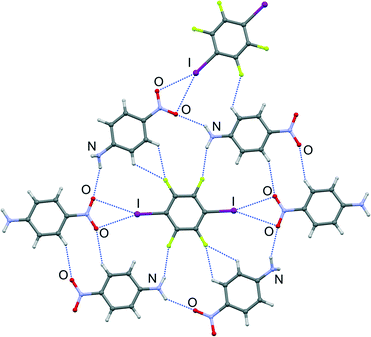 | ||
| Fig. 3 Part of the crystal structure of (4noa)2(tfib), showcasing supramolecular interactions present in a 2D layer. | ||
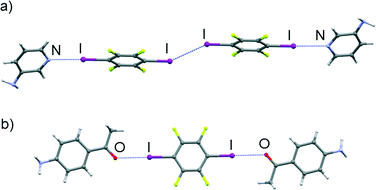
In the (3ap)(tfib) cocrystal, apart from the expected strong halogen bond between tfib iodine and a pyridine nitrogen atom (d(I⋯N) = 2.81 Å, ∠ (C–I⋯N) = 175°), the other iodine atom participates in an iodine–iodine halogen interaction (d(I⋯I) = 3.78 Å, ∠ (C–I⋯I) = 141°). The obtained structure infers that this halogen–halogen interaction is competitive enough with the iodine–pyridine halogen bond, although not exclusively preferred to it. These interactions give rise to a discrete halogen bonded structure (Fig. 4a). The 3D structure is the result of combining the mentioned halogen bonds with N–H⋯F and C–H⋯F hydrogen bonds (see the ESI†).
The (4aap)2(tfib) cocrystal is a good example for the potential of the carbonyl oxygen atom as a halogen bond acceptor: each iodine atom in a tfib molecule forms a strong halogen bond with an oxygen atom (d(I⋯O) = 2.89 Å, ∠ (C–I⋯O) = 178°), giving rise to a discrete molecular complex (Fig. 4b). The discrete molecular complexes form chains via weak C–H⋯F hydrogen bonds (d(C⋯F) = 3.29 Å, ∠ (C–H⋯F) = 140°). The 3D crystal structure results from stacking and N–H⋯F contacts (see the ESI†). Contrary to the above, in the (4ab)2(tfib) cocrystal, the carbonyl oxygen atom is a much better hydrogen bond acceptor, forming a strong N–H⋯O (d(N⋯O) = 2.92 Å, ∠ (N–H⋯O) = 175°) and a weak C–H⋯O hydrogen bond (d(C⋯O) = 3.30 Å, ∠ (C–H⋯O) = 131°). This leaves the π-system of the aromatic amine as the next best halogen bond acceptor, each tfib molecule forming halogen bonds with two 4ab molecules (d(I⋯Cπ) = 3.46 Å, ∠ (C–I⋯Cπ) = 167°). The combination of the 4ab bridging by tfib along the c crystallographic axis and hydrogen bonding between 4ab molecules along the a crystallographic axis gives rise to a ladder-like structural motif and a densely-packed crystal structure when hydrogen bonds along the b crystallographic axis are taken into account (Fig. 5a).
In (5a2mp)2(tfib) and (3abn)(tfib), the pyridine nitrogen atom in 5a2mp, and the cyano nitrogen atom in 3abn, turned out to possess much better hydrogen bond acceptor qualities than halogen bond ones, evident by preferentially forming strong N–H⋯N hydrogen bonds (in (3abn)(tfib) d(N⋯N) = 3.10 Å, ∠ (N–H⋯N) = 170°, while in (5a2mp)2(tfib) d(N⋯N) = 3.26 Å, ∠ (N–H⋯N) = 172°). Additionally, in both cases, the electron density on the amino group unexpectedly15 turned out to be a decent halogen bond acceptor (in (3abn)(tfib) d(I⋯N) = 2.97 Å, ∠ (C–I⋯N) = 179°, while in (5a2mp)2(tfib) d(I⋯N) = 3.04 Å, ∠ (C–I⋯N) = 177° – in both cases significantly shorter than the sum of the van der Waals radii of nitrogen and iodine, which amounts to 3.53 Å) (Fig. 5). The free methoxy group in 5a2mp, in keeping with the current CSD data, does not participate in halogen bonding with tfib. Furthermore, in (3abn)(tfib), while one molecule of tfib forms halogen bonds with amino groups of two 3abn molecules, another one forms I⋯Cπ halogen bonds with π-systems of two 3abn molecules. These interactions lead to the formation of chains that are connected in two dimensions via the aforementioned N–H⋯N hydrogen bonds. The 3D structure is again a result of stacking layers.
The thermal analysis experiments have shown that out of the six obtained cocrystals, three ((4aap)2(tfib), (3abn)(tfib) and (4noa)2(tfib)) have a melting point higher and the other three ((4ab)2(tfib), (3ap)(tfib) and (5a2mp)2(tfib)) lower than that of pure tfib (ESI,† Table S4). (4noa)2(tfib) has the highest melting point of all cocrystals, albeit a lower one than that of pure 4noa, while (3ap)(tfib) has the lowest melting point, lower than that of pure 3ap. This observation is in good agreement with the crystal and molecular structure of these compounds, seeing as in (4noa)2(tfib) numerous strong hydrogen and halogen bonds give rise to an intricate layer, while (3ap)(tfib) is a discrete complex that also features a combination of I⋯N and I⋯I halogen interactions.
To conclude, we have proven the potential of the nitro group to act as a halogen bond acceptor by synthesizing the (4noa)(tfib) cocrystal which is the first known cocrystal of tfib with a robust I(⋯O)2 halogen bond motif, containing a bifurcated halogen bond donor with nitro group oxygen atoms. By comparing the crystal structures of the obtained cocrystals to the crystal structures of pure halogen bond acceptors,16 we have found that only in two out of the five cases, (4noa)2(tfib) and (4ab)2(tfib), motifs of the strong hydrogen bonds (i.e. N–H⋯O or N–H⋯N) were very similar. In agreement with previous research in the field of halogen bonding, the pyridine nitrogen atom has again proven itself a strong halogen bond acceptor, although only when it is preferred to the formation of competing hydrogen bonds. The methoxy group oxygen atom and the cyano group nitrogen atom turned out to be poor halogen bond acceptors in this study, weaker than even the electron density on the amino group or that belonging to the π-system of the aromatic amine. Due to the fact that halogen bonds with cyano groups are known to exist, we propose that their absence in our cocrystal is a result of them preferentially functioning as hydrogen bond acceptors. The described results are an important step in the understanding of the halogen bond hierarchy and the competition between them and with hydrogen bonds and could have significant implications for the future design and synthesis of halogen bonded materials.
Acknowledgements
This research was supported by the Croatian Science Foundation under the project IP-2014-09-7367. We are grateful to Prof. Vladimir Stilinović for helpful suggestions and to Ulla Cinčić for help on the Graphical Abstract.Notes and references
- (a) G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimägi, G. Resnati and G. Terraneo, Chem. Rev., 2016, 116, 2478–2601 CrossRef CAS PubMed; (b) P. Politzer, J. S. Murray and T. Clark, Phys. Chem. Chem. Phys., 2010, 12, 7748–7757 RSC; (c) A. Priimägi, G. Cavallo, P. Metrangolo and G. Resnati, Acc. Chem. Res., 2013, 46, 2686–2695 CrossRef PubMed.
- (a) P. Metrangolo, H. Neukirch, T. Pilati and G. Resnati, Acc. Chem. Res., 2005, 38, 386–395 CrossRef CAS PubMed.
- (a) M. Saccone, G. Cavallo, P. Metrangolo, A. Pace, I. Pibiri, T. Pilati, G. Resnati and G. Terraneo, CrystEngComm, 2013, 15, 3102–3105 RSC; (b) D. Cinčić, T. Friščić and W. Jones, Chem. – Eur. J., 2008, 14, 747–753 CrossRef PubMed.
- (a) C. B. Aakeröy, J. Desper, M. Fasulo, I. Hussain, B. Levin and N. Schultheiss, CrystEngComm, 2008, 10, 1816–1821 RSC; (b) G. Lapadula, N. Judaš, T. Friščić and W. Jones, Chem. – Eur. J., 2010, 16, 7400–7403 CrossRef CAS PubMed; (c) P. Sgarbossa, R. Bertani, V. Di Noto, M. Piga, G. A. Giffin, G. Terraneo, T. Pilati, P. Metrangolo and G. Resnati, Cryst. Growth Des., 2012, 12, 297–305 CrossRef CAS; (d) M. T. Johnson, Z. Džolić, M. Cetina, O. F. Wendt, L. Öhrström and K. Rissanen, Cryst. Growth Des., 2012, 12, 362–368 CrossRef CAS PubMed; (e) K. Raatikainen and K. Rissanen, CrystEngComm, 2011, 13, 6972–6977 RSC; (f) C. B. Aakeröy, T. K. Wijethunga, J. Desper and M. Đaković, Cryst. Growth Des., 2016, 16, 2662–2670 CrossRef.
- (a) C. Merkens, F. Pan and U. Englert, CrystEngComm, 2013, 15, 8153–8158 RSC; (b) D. Cinčić, T. Friščić and W. Jones, Chem. Mater., 2008, 20, 6623–6626 CrossRef; (c) D. Cinčić, T. Friščić and W. Jones, New J. Chem., 2008, 32, 1776–1781 RSC; (d) O. S. Bushuyev, D. Tan, C. J. Barrett and T. Friščić, CrystEngComm, 2015, 17, 73–80 RSC; (e) D. Cinčić and T. Friščić, CrystEngComm, 2014, 16, 10169–10172 RSC; (f) R. W. Troff, T. Mäkelä, F. Topić, A. Valkonen, K. Raatikainen and K. Rissanen, Eur. J. Org. Chem., 2013, 1617–1637 CrossRef CAS; (g) D. Cinčić, T. Friščić and W. Jones, J. Am. Chem. Soc., 2008, 130, 7524–7525 CrossRef PubMed.
- (a) T. M. Beale, M. G. Chudzinski, M. G. Sarwar and M. S. Taylor, Chem. Soc. Rev., 2012, 134, 8260–8267 CrossRef PubMed; (b) M. Erdélyi, Chem. Soc. Rev., 2012, 41, 3547–3557 RSC; (c) M. Erdélyi, Chem. Soc. Rev., 2012, 41, 3547–3557 RSC.
- (a) Y. Lu, T. Shi, Y. Wang, H. Yang, X. Yan, X. Luo, H. Jiang and W. Zhu, J. Med. Chem., 2009, 52, 2854–2862 CrossRef CAS PubMed.
- (a) A. R. Voth, F. A. Hays and P. S. Ho, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 6188–6193 CrossRef CAS PubMed; (b) J. Fanfrlík, F. X. Ruiz, A. Kadlčíková, J. Řezáč, A. Cousido-Siah, A. Mitschler, S. Haldar, M. Lepšík, M. H. Kolář, P. Majer, A. D. Podjarny and P. Hobza, ACS Chem. Biol., 2016, 10, 1637–1642 CrossRef PubMed.
- (a) J. Fanfrlik, A. Prada, Z. Padelkova, A. Pecina, J. Machacek, M. Lepsik, J. Holub, A. Ruzicka, D. Hnyk and P. Hobza, Angew. Chem., 2014, 58, 10139–10142 CrossRef PubMed; (b) C. Wang, D. Danovich, Y. Mo and S. Shaik, J. Chem. Theory Comput., 2014, 10, 3726–3737 CrossRef CAS PubMed.
- F. H. Allen, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 380–388 CrossRef.
- An overview of the currently available literature reveals that studies of halogen bonding have largely focused on cocrystals of perfluorohalocarbons as classic halogen bond donors, see: (a) M. Fourmigué, Curr. Opin. Solid State Mater. Sci., 2009, 13, 36–45 CrossRef; (b) C. B. Aakeröy, T. K. Wijethunga, M. A. Haj, J. Desper and C. Moore, CrystEngComm, 2014, 16, 7218–7225 RSC; (c) D. Cinčić, T. Friščić and W. Jones, CrystEngComm, 2011, 13, 3224–3231 RSC.
- The nitro group oxygen atoms as halogen bond acceptors are well known in other halogen bonded systems and have been described in the recent literature. For examples, see: (a) J. C. Bennion, L. Vogt, M. E. Tuckerman and A. J. Matzger, Cryst. Growth Des., 2016, 16, 4688–4693 CrossRef CAS; (b) S. Saha, S. Ganguly and G. R. Desiraju, Aust. J. Chem., 2014, 67, 1840–1848 CrossRef CAS; (c) S. Tothadi, P. Sanphui and G. R. Desiraju, Cryst. Growth Des., 2014, 14, 5293–5302 CrossRef CAS; (d) S. Tothadi and G. R. Desiraju, Chem. Commun., 2013, 49, 7791–7793 RSC; (e) S. Ghosh and C. M. Reddy, CrystEngComm, 2012, 14, 2444–2453 RSC; (f) V. R. Thalladi, B. S. Goud, V. J. Hoy, F. H. Allen, J. A. K. Howard and G. R. Desiraju, Chem. Commun., 1996, 401–402 RSC; (g) S. J. Garden, F. R. da Cunha, C. Glidewell, J. N. Low, J. M. S. Skakle and J. L. Wardell, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2004, 60, o12–o14 Search PubMed.
- (a) S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC; (b) T. Friščić and W. Jones, Cryst. Growth Des., 2009, 9, 1621–1637 CrossRef; (c) J. Mavračić, D. Cinčić and B. Kaitner, CrystEngComm, 2016, 18, 3343–3346 RSC.
- The amounts of liquid corresponded to liquid-to-solid ratios ranging from η = 0.09 μL mg−1 to η = 0.38 μL mg−1, see: T. Friščić, S. L. Childs, S. A. A. Rizvi and W. Jones, CrystEngComm, 2009, 11, 418–426 RSC.
- The CSD search of tfib halogen bonded to an amino group returned only 3 crystal structures, refcodes JEFFUW, RUWVUB, and RUWWAI.
- Five out of the six used amines are solids, refcodes AMACPH01, VOFAN, BERTIB, NANILI21, and AMIPYR.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the mechanochemical and solution syntheses, instrumental analysis, PXRD, and DSC data. CCDC 1494268–1494273 contains crystallographic data for this paper. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ce01703g |
| This journal is © The Royal Society of Chemistry 2016 |