Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and characterisation of the porous zinc phosphonate [Zn2(H2PPB)(H2O)2]·xH2O†
N.
Hermer
a,
H.
Reinsch
a,
P.
Mayer
b and
N.
Stock
*a
aChristian-Albrechts-Universität, Max-Eyth-Straße 2, 24118 Kiel, Germany. E-mail: stock@ac.uni-kiel.de; Fax: +49 431 8801775; Tel: +49 431 8803261
bLudwig-Maximilians-Universität München, Department Chemie, Butenandtstr. 5-13, Haus F, 81377 München, Germany. E-mail: pemay@cup.uni-muenchen.de; Fax: +49 89 2180 77407; Tel: +49 89 2180 77399
First published on 8th August 2016
Abstract
The synthesis, structure and properties of the new microporous zinc phosphonate [Zn2(H2PPB)(H2O)2]·xH2O (denoted as CAU-25, CAU stands for Christian-Albrechts-University) are presented. The crystal structure was determined by combining single crystal and powder X-ray diffraction data. The final refinement was carried out by using the Rietveld method. The structure contains one-dimensional channels between dense, corrugated, hydrogen bonded layers with a diameter of approximately 5 Å. While N2 uptake was not observed at 77 K, CO2 and H2O uptakes were measured at 298 K.
The intensely investigated field of metal–organic frameworks (MOF) offers materials with exceptional properties and remarkable structural versatility.1 The possibility of tuning certain properties by isoreticular chemistry leads to a wide field of possible applications, for example in gas storage or separation, drug delivery, proton conductivity and catalysis.1 The majority of MOFs are metal carboxylates; however, their stability towards moisture and temperature is often insufficient for applications under real life conditions. Due to the higher charge of phosphonate groups and the larger number of atoms involved in coordination to the metal ions, stronger metal–linker bonding is observed, which results in more stable compounds.2 The various coordination modes and protonation states accessible to the phosphonate group also lead to large structural variability. A challenge in the synthesis of porous metal phosphonates is their tendency to form dense layered structures, and thus, the number of open framework metal phosphonates is very limited (Table S2a–d†).2
Different strategies have been used to prevent formation of the dense layered structures. For example, the use of methylphosphonic acid, which is too short to bridge the layers, led to porous compounds (Table S2a†).3 Other strategies are the insertion of another functional group (Table S2b†),4 which is coordinating to the metal, or using phosphonic acid monoester linkers, which could act like bidentate carboxylate-like groups (Table S2c†).5 A promising strategy is to disrupt the formation of a dense layered structure motif by adjustment of the geometry of the linker molecule (Table S2d†).6–9 The corresponding triphosphonic acids that have been successfully employed are listed in Fig. 1. 1,3,5-tris-(4-Phosphonophenyl)benzene (H6PPB) has already been shown to lead to five metal phosphonates with a 3D framework structure (Table S3†),7–10 and three of them were successfully tested for porosity, [Sr2(H2PPB)(CH3OH)(H2O)4],7 [Sn(H2PPB)]·4.5H2O,8 and [Zr(H2PPB)]·7H2O.9
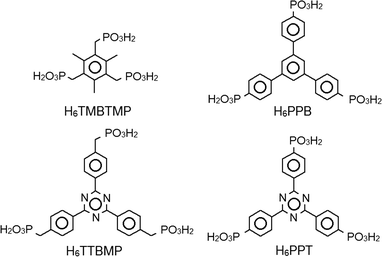 | ||
| Fig. 1 Tritopic phosphonic acids that build 3D crystalline porous metal phosphonates (see also Table S2d†). H6TMBTMP = (2,4,6-trimethylbenzene-1,3,5-triyl)tris(methylene)triphosphonic acid, H6TTBMP = 2,4,6-tris[4-(phosphonomethyl)phenyl]-1,3,5-triazine, H6PPB = 1,3,5-tris-(4-phosphonophenyl)benzene and H6PPT = 2,4,6-tris-(phenylene-4-phosphonic acid)-s-triazine. | ||
Here, we report systematic studies of the system Zn2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H6PPB
H6PPB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaOH
NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH. The synthesis of the phosphonic acid H6PPB was carried out in a three step reaction according to the literature,7,11 for which details can be found in the ESI.† First, 4-bromo-acetophenone was converted into tris(4-bromophenyl)benzene by cyclotrimerisation with thionylchloride.11 By nickel(II) chloride catalysed cross-coupling of this compound with triethylphosphite, 1,3,5-tris-(4-diethylphosphonophenyl)benzene was obtained.7 The hydrolysis of the diethylester to H6PPB was subsequently carried out in concentrated HCl.7
CH3OH. The synthesis of the phosphonic acid H6PPB was carried out in a three step reaction according to the literature,7,11 for which details can be found in the ESI.† First, 4-bromo-acetophenone was converted into tris(4-bromophenyl)benzene by cyclotrimerisation with thionylchloride.11 By nickel(II) chloride catalysed cross-coupling of this compound with triethylphosphite, 1,3,5-tris-(4-diethylphosphonophenyl)benzene was obtained.7 The hydrolysis of the diethylester to H6PPB was subsequently carried out in concentrated HCl.7
High-throughput studies led to the new microporous zinc phosphonate [Zn2(H2PPB)(H2O)2]·xH2O, hereafter denoted as CAU-25 (CAU stands for Christian-Albrechts-University). The system Zn2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H6PPB
H6PPB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaOH
NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O
H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH was studied using high-throughput methods,12 which allow a fast and efficient investigation of different reaction parameters. Varying the molar ratios of Zn2+
CH3OH was studied using high-throughput methods,12 which allow a fast and efficient investigation of different reaction parameters. Varying the molar ratios of Zn2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H6PPB
H6PPB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaOH led to the title compound CAU-25 and two other crystalline products of low long range order (see Fig. S3 and S4†), which were not further characterized. Optimization of the molar ratios of the starting materials, the solvent mixture (H2O
NaOH led to the title compound CAU-25 and two other crystalline products of low long range order (see Fig. S3 and S4†), which were not further characterized. Optimization of the molar ratios of the starting materials, the solvent mixture (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH) as well as the synthesis temperature and time resulted in a highly crystalline product of CAU-25 (Table S4†). The optimized synthesis procedure is as follows: H6PPB (30 mg, 55 mmol), aqueous 2 M Zn(NO3)2 (28 μL, 55 mmol), aqueous 2 M NaOH (55 μL, 110 mmol), 152 μL of deionised water and 366 μL of methanol were transferred to a Teflon-lined autoclave (Vmax = 2 mL) and sealed. The reactor was slowly heated to 120 °C within 24 h. The temperature was held for 24 h, and the reactor was subsequently cooled to room temperature within 16 h. The precipitate was filtered off and washed with water and methanol.
CH3OH) as well as the synthesis temperature and time resulted in a highly crystalline product of CAU-25 (Table S4†). The optimized synthesis procedure is as follows: H6PPB (30 mg, 55 mmol), aqueous 2 M Zn(NO3)2 (28 μL, 55 mmol), aqueous 2 M NaOH (55 μL, 110 mmol), 152 μL of deionised water and 366 μL of methanol were transferred to a Teflon-lined autoclave (Vmax = 2 mL) and sealed. The reactor was slowly heated to 120 °C within 24 h. The temperature was held for 24 h, and the reactor was subsequently cooled to room temperature within 16 h. The precipitate was filtered off and washed with water and methanol.
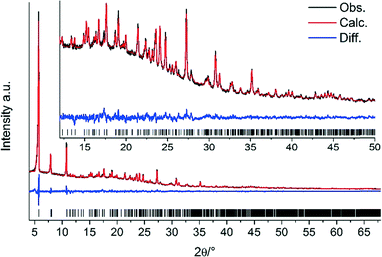
During synthesis optimisation, small single crystals (ca. 50 × 10 × 10 μm3) of the title compound were obtained. Single-crystal X-ray diffraction (SC-XRD) data were recorded using a Nonius Kappa-CCD diffractometer at 173 K with monochromatic MoKα1 radiation. However, due to the small size of the single crystals and the insufficient data quality, only a structure model could be determined. Thus, a combination of force-field calculations and Rietveld refinement was used to confirm and complete this model. In the first step, the approximate model obtained from SC-XRD was optimised using the universal force field as implemented in Materials Studio.13 The thus obtained model was subsequently refined by Rietveld methods using TOPAS.14 Additional electron density was observed in the difference Fourier map which was attributed to the presence of a non-coordinating water molecule. From thermogravimetric data and sorption measurements, the presence of a second water molecule is suggested, but it could not be assigned to electron density in the difference Fourier map, possibly as a result of disorder. Due to the large unit cell, the carbon backbone of the linker molecules was refined as a rigid body. The final Rietveld plot (Fig. 2), some relevant crystal data and the final figures of merit are provided in Table 1. Further details can be found in the ESI.†
| Formula sum | Zn2HO3PC6H4(C6H3)(C6H4PO3)C6H4PO3H |
| Crystal system | Monoclinic |
| a/Å, b/Å, c/Å | 44.673(4), 16.3110(8), 8.2967(3) |
| β/° | 95.301(8) |
| V/Å3 | 6019.6(6) |
| Space group | C2/c |
| Atoms | 39 |
| λ | CuKα1 |
| R wp, RBragg | 3.45, 1.09 |
| GoF | 2.71 |
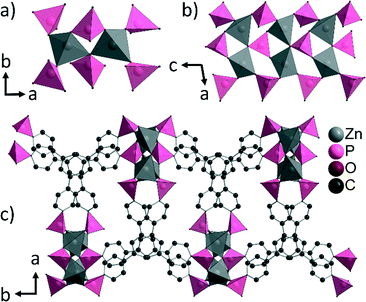
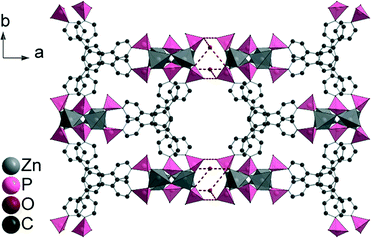
In the crystal structure of CAU-25, each Zn2+ ion exhibits a tetrahedral coordination environment consisting of four oxygen atoms of four different phosphonate groups. According to the Harris notation,15 the phosphonate groups exhibit [2.110] and [4.211] bonding modes. Corner-sharing of the ZnO4 polyhedra leads to the formation of dimeric Zn2O7 units (Fig. 3a). These units are further connected via the phosphonate groups to form ribbons along the c-axis (Fig. 3b). Each ribbon is connected to four adjacent inorganic building units via the linker molecules (Fig. 3c) resulting in corrugated double layers of linker connected chains.
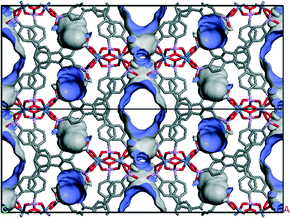
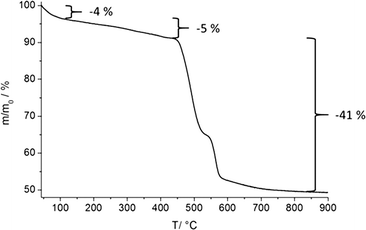
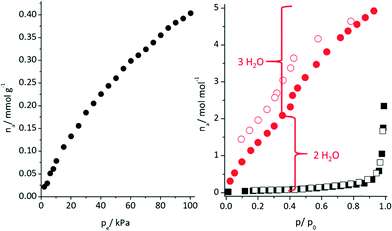
Based on O⋯O distances (Table S6†), possible hydrogen bonding can be anticipated which connects the double layers via P–O–H⋯O–P and P–O⋯H2O⋯O–P hydrogen bonds (Fig. 4 and S8†). The connection of adjacent corrugated double layers results in the formation of one-dimensional channels of approximately 5 Å in diameter along the c-axis (Fig. S9†) as determined using the van der Waals radii of the atoms lining the pore walls. The Connolly surface (Fig. 5) generated for a probe molecule with the kinetic diameter of CO2 (3.3 Å) indicates kinetically inaccessible intralayer cavities and accessible interlayer channels. In contrast to other porous metal phosphonates, the structure of CAU-25 is a remarkable example due to the presence of interlayer porosity. Thermogravimetric measurements (Fig. 6 and Table S7†) showed a weight loss of 4% up to 100 °C and of 5% from 100 to 430 °C which is probably due to the loss of solvent molecules (calc. −1.7H2O, 4%; −2H2O, 5%). From 430 °C to 900 °C, the oxidation of the organic part occurs (obs. −41%, calc. −41%). Hence, CAU-25 was activated prior to sorption experiments at 150 °C for 18 h under reduced pressure (10−2 kPa). CAU-25 is not porous towards N2 at 77 K (Fig. 7, right) but towards CO2 (0.4 mmol g−1 (1.8 wt%) at 100 kPa) (Fig. 7, left) and water (7.3 mmol g−1 (13.2 wt%), Fig. 7, right) at 298 K, which is due to the dipole–dipole interactions of the latter two adsorptives with the framework and the higher measurement temperature that allows for higher framework flexibility and faster diffusion. The water vapour isotherm shows two steps. Initially, two water molecules per formula sum are adsorbed up to p/p0 = 0.35, which corresponds to the two hydrogen bonded water molecules in between the double layers. At higher p/p0 values, an uptake of three additional water molecules per formula sum is observed. In theory, the interlayer channels should be accessible for larger N2 molecules. The reasons for this size selective porosity towards CO2 is probably due to the higher temperature at which CO2 sorption was measured. At lower temperatures, the mobility of gas molecules in narrow channels as in CAU-25 is often limited.7 The thermal stability of CAU-25 was evaluated by comparison of the PXRD patterns of the as-synthesized compound, the compound after activation and H2O sorption measurements and the simulated PXRD pattern (Fig. S10†). While a decrease in long range order can be observed, the structure itself is retained.
 | ||
| Fig. 4 Double layers of CAU-25, connected along the a-axis through hydrogen bonds (marked in red). Hydrogen bonds are postulated based on O⋯O distances. | ||
Conclusions
The strategy to create porous metal phosphonates by disrupting the formation of a dense layered structure motif by adjustment of the geometry of the linker molecule seems to be very promising. Using this concept, we have been able to synthesize a new zinc phosphonate employing the triphosphonic acid 1,3,5-tris-(4-phosphonophenyl)benzene. The resulting compound [Zn2(H2PPB)(H2O)2]·xH2O (CAU-25) crystallises in a layered structure, and hydrogen bonding between the layers leads to interlayer porosity. CAU-25 is permanently porous and thermally stable up to 400 °C. The structure determination was possible by a combination of single crystal X-ray diffraction, universal force-field calculations and Rietveld refinement. Although the compound is not porous towards N2, porosity was shown by CO2 and H2O sorption experiments.Notes and references
- Themed issue on Metal Organic Frameworks , Chem. Rev., 2012, 112, 673 CrossRef PubMed Themed issue on Metal Organic Frameworks , Chem. Soc. Rev., 2014, 43, 5405 RSC.
- A. Clearfield and K. Demandis, Metal Phosphonate Chemistry: From Synthesis to Applications, The Royal Society of Chemistry, Cambridge, UK, 2012 CrossRef CAS PubMed; K. J. Gagnon, H. P. Perry and A. Clearfield, Chem. Rev., 2012, 112, 1034 CrossRef CAS PubMed; G. K. H. Shimizu, R. Vaidhyanathan and J. M. Taylor, Chem. Soc. Rev., 2009, 38, 1430 RSC; K. Maeda, Microporous Mesoporous Mater., 2004, 73, 47 CrossRef; M. Taddei, F. Costantino and R. Vivani, Eur. J. Inorg. Chem., 2016 DOI:10.1002/ejic.201600207.
- K. Maeda, Y. Kiyozumi and F. Mizukami, Angew. Chem., Int. Ed. Engl., 1994, 33, 2335 CrossRef CAS; K. Maeda, J. Akimoto, Y. Kiyozumi and F. Mizukami, Angew. Chem., Int. Ed. Engl., 1995, 34, 1199 CrossRef.
- J. A. Groves, S. R. Miller, S. J. Warrender, C. Mellot-Draznieks, P. Lightfoot and P. A. Wright, Chem. Commun., 2006, 3305 RSC; C. Serre, J. A. Groves, P. Lightfoot, A. M. Z. Slawin, P. A. Wright, N. Stock, T. Bein, M. Haouas, F. Taulelle and G. Férey, Chem. Mater., 2006, 18, 1451 CrossRef CAS; Q. Yue, J. Yang, G.-H. Li, G.-D. Li and J.-S. Chen, Inorg. Chem., 2006, 45, 4431 CrossRef PubMed; M. T. Wharmby, S. R. Miller, J. A. Groves, I. Margiolaki, S. E. Ashbrook and P. A. Wright, Dalton Trans., 2010, 39, 6389 RSC; M. T. Wharmby, J. P. S. Mowat, S. P. Thompson and P. A. Wright, J. Am. Chem. Soc., 2011, 133, 1266 CrossRef PubMed; M. T. Wharmby, G. M. Pearce, J. P. S. Mowat, J. M. Griffin, S. E. Ashbrook, P. A. Wright, L.-H. Schilling, A. Lieb, N. Stock, S. Chavan, S. Bordiga, E. Garcia, G. D. Pirngruber, M. Vreeke and L. Gora, Microporous Mesoporous Mater., 2012, 157, 3 CrossRef; S. Begum, Z. Wang, A. Donnadio, F. Costantino, M. Casciola, R. Valiullin, C. Chmelik, M. Bertmer, J. Kärger, J. Haase and H. Krautscheid, Chem. – Eur. J., 2014, 20, 8862 CrossRef PubMed; S. Begum, S. Horike, S. Kitagawa and H. Krautscheid, Dalton Trans., 2015, 44, 18727 RSC.
- S. S. Iremonger, J. Liang, R. Vaidhyanathan, I. Martens, G. K. H. Shimizu, T. D. Daff, M. Z. Aghaji, S. Yeganegi and T. K. Woo, J. Am. Chem. Soc., 2011, 133, 20048 CrossRef CAS PubMed; S. S. Iremonger, J. Liang, R. Vaidhyanathan and G. K. H. Shimizu, Chem. Commun., 2011, 47, 4430 RSC; J. M. Taylor, R. Vaidhyanathan, S. S. Iremonger and G. K. H. Shimizu, J. Am. Chem. Soc., 2012, 134, 14338 CrossRef PubMed.
- J. M. Taylor, A. H. Mahmoudkhani and G. K. H. Shimizu, Angew. Chem., Int. Ed., 2007, 46, 795 CrossRef CAS PubMed; N. Hermer and N. Stock, Dalton Trans., 2015, 44, 3720 RSC; M. Taddei, F. Costantino, F. Marmottini, A. Comotti, P. Sozzani and R. Vivani, Chem. Commun., 2014, 50, 14831 RSC; S.-F. Tang, J.-J. Cai, L.-J. Li, X.-X. Lv, C. Wang and X.-B. Zhao, Dalton Trans., 2014, 43, 5970 RSC.
- R. Vaidhyanathan, A. H. Mahmoudkhani and G. K. H. Shimizu, Can. J. Chem., 2009, 87, 247 CrossRef CAS.
- R. K. Mah, M. W. Lui and G. K. H. Shimizu, Inorg. Chem., 2013, 52, 7311 CrossRef CAS PubMed.
- R. K. Mah, B. S. Gelfand, J. M. Taylor and G. K. H. Shimizu, Inorg. Chem. Front., 2015, 2, 273 RSC.
- W. Ouellette, G. Wang, H. Liu, G. T. Yee, C. J. O'Connor and J. Zubieta, Inorg. Chem., 2009, 48, 953 CrossRef CAS PubMed; M. Taddei, F. Costantino, R. Vivani, S. Sabatini, S.-H. Lim and S. M. Cohen, Chem. Commun., 2014, 50, 5737 RSC.
- H. Zhi Guo, J. Liu, L. Gong An and D. Zhi Bing, J. Chem. Res., 2003, 2003, 778 CrossRef.
- P. Maniam and N. Stock, in Metal Phosphonate Chemistry: From Synthesis to Applications, The Royal Society of Chemistry, 2012, p. 87 CrossRef CAS; N. Stock, Microporous Mesoporous Mater., 2010, 129, 287–295 CrossRef CAS.
- Materials Studio Version 5.0, Accelrys Inc., San Diego, CA, 2009 Search PubMed.
- Topas Academics 4.2, Coelho Software, 2007 Search PubMed.
- R. A. Coxall, S. G. Harris, D. K. Henderson, S. Parsons, P. A. Tasker and R. E. P. Winpenny, Dalton Trans., 2000, 2349 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1493141 contains the supplementary crystallographic data for CAU-25. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ce01580h |
| This journal is © The Royal Society of Chemistry 2016 |