Novel approaches to study the crystal assembly in banded spherulites of poly(trimethylene terephthalate)†
Graecia
Lugito
ab and
Eamor M.
Woo
*a
aDepartment of Chemical Engineering, National Cheng Kung University, Tainan, 701-01, Taiwan. E-mail: emwoo@mail.ncku.edu.tw
bDepartment of Chemical Engineering, Faculty of Industrial Technology, Bandung Institute of Technology, Bandung, 40132 Indonesia
First published on 6th July 2016
Abstract
Poly(trimethylene terephthalate) (PTT), with large, well-defined, and strongly birefringent spherulites upon crystallization, has been chosen as a model to study the correlation between the interior lamellae assembly and the top surface banding structures. Approaches to investigate the bulk interior of the banded spherulites are needed to shed new light on the crystallization mechanism of polymers. Two etching methods (with two different etchants: methylamine and permanganate) were applied to two identical samples to enhance the contrast of crystal arrangements in three-dimensional perspectives. Methylamine, under mild conditions, was found to etch out lamellae bundles on ridges and leave discrete crevice-separated circumferential crystal layers. On the other hand, the oxidizing potassium permanganate was found to dissolve the circumferential crystal layers between the successive bands, exposing circular patterns. These two etching results mutually support the discontinuity of radial growth of PTT spherulites, providing valuable hints as to the actual crystal assembly of PTT.
Introduction
The crystallization of polymers is both a thermodynamically and kinetically driven ordering process of long polymer chains into chain-folded lamellae at first, and then into a more complex and hierarchical form of polycrystals, such as spherulites. For many kinds of spherulite morphologies, banded spherulites, in particular, have aroused considerable interest for over a century, because of their beautiful periodic patterns and orderly arrangements. Banded spherulites have been frequently observed in the crystallization of many natural and synthetic materials; not only in long-chain polymers but also in low-molecular-weight compounds (either organic or inorganic), as well as in combinations of those two.1–27 Banded spherulites used to be generalized as spherulites with periodic ring bands that are arranged concentrically. However, they could also be single- and/or double-spiral banded spherulites, as observed in poly(trimethylene terephthalate) (PTT),16,17 poly(nonamethylene terephthalate) (PNT),18 poly(lactic acid) (PLA),19 and many others.Great efforts have been made by many investigators to explain the formation mechanisms of banded polymer spherulites.1–15 Some studies proposed continuous helical twisting lamellae induced by unbalanced stresses;1–9 nevertheless, direct evidence of continuous spiral lamellae with pitches matching those of optical pitches is still lacking. Others proposed periodical rhythmic depletion due to competition between nonlinear interface kinetics and the supply of materials by diffusion.10–13 The extant classical concept of a continuous spiral twist to explain the formation of banded spherulites, however, rather over-simplifies the actual issues of repetitive crystal assembly and may be deficient in accounting for the bands in spherulites of small-molecule compounds with no chain-fold induced surface stresses. The model of rhythmic depletion may be fine in accounting for the bands in spherulites with periodic depletion zones; however, for most other types of bands with dual birefringent crystals (i.e., “double bands”), again, this approach may face a dilemma. In the most recent 10 years, novel and revolutionary models on the formation mechanisms and lamellar assembly of polymers' spherulites have been proposed by Woo et al.,14,15,17–19 with solid evidence of an interior lamellar assembly showing corrugate-board layer-structures, with distinct discontinuity between the successive bands. Furthermore, in these novel models, evidence has proved that each of the boards or layers comprises crystals of opposed orientation with the layer thickness exactly matching the optical band spacing. Although critical evidence has been gradually and successfully discovered and better visualizations have been made available through interior dissection, as demonstrated in some recent studies,14,15,17–19 more in-depth details, however, have yet to be expounded. Corrugated-board structures of two perpendicular lamellar arrangements (radial and tangential) through bending and/or branching account for the manifestation of two alternating contrast interference colours in PEA ring-banded spherulites.14 Various approaches have been conducted to enhance the contrast of the interior lamellar arrangement, such as the use of additional diluents [either amorphous or crystalline polymer] and/or etching agents. Dissection to reveal the interior lamellar assembly is a necessary approach to elucidate the true inner anatomy of the crystal assembly in spherulites hidden under the top surface.
An achiral aromatic polyester of poly(trimethylene terephthalate) (PTT), with its unique three types of banded morphology (concentric-ring, single-spiral, and double-spiral)17 was chosen as an ideal model for studying polymer crystallization and self-assembly. PTT consists of rigid terephthalate units and semi-flexible propyl units, resulting an outstanding combination of strength and stability. In fact, there is a lot of work focusing on the crystallization of PTT, their banded morphologies,20–26 intrinsic birefringence,28–30 and kinetics.31,32 Li et al.23,24 previously reported the use of methylamine (MA) to etch out PTT amorphous segments from the top surface of PTT banded spherulites and attempted to correlate the results with the lamellar twisting model. Recently, Rosenthal et al.25,26 attempted to explain the origin of crystalline lamellae twists and how the chirality of hybrid left–right crystalline helicoids was built from the achiral PTT from an extreme approach. The concept was based on the molecular level of a single lamella, assumptions were taken to interpret the results, and models were built to demonstrate how the lamellae twist in opposite handedness in opposing hemispheres of PTT spherulites. They stated that the value of 4° chain tilt did not appear to be sufficient for generation of the surface stresses required for twisted lamellar growth. Instead, they suggested the inclination of the terminal segment of crystalline stem protruding the lamellar surface to be the key factor controlling the surface stresses. Therefore, in this work, we attempted to take an alternative approach from bulk dissection to perceive the behaviour of PTT crystals, and to view whether or not the continuous lamellae twist might be justified.
Morphological transformations of spherulites with regard to the crystallization temperature (Tc) observed under a polarized optical microscope in PTT thin film samples are shown as ESI† Fig. S1. The PTT spherulitic morphology transforms from subtle bands at low Tc (<150 °C) to microscopic observable bands at a lower medium Tc (150–170 °C), then to radial lamellar splay at a higher medium Tc (180–190 °C), and finally to irregular bands at high Tc (200–210 °C). In an earlier work on spherulites in PTT thin films,17 the top surface morphologies of different banded structures in PTT crystallization at 165 °C were reported and discussed thoroughly from the views of crystal assembly in thin films. The concentric-ring-PTT banded spherulite has initial sheaf-like crystals equally grown towards all radial directions without obstacles [with a core diameter equal to its band-spacing]. The spiral-banded spherulite has its initial grown sheaf-like crystals curved around its long axis, making an S-shape. When both arms of the S-shaped crystal are equally grown, a double-spiral banded spherulite appears [where the core diameter is double the band-spacing]; however, when one of the arms gets retarded or grows much slower than the other, a single spiral banded spherulite appears [where the core diameter is equal to the band-spacing]. On the growing arms, the lamellae are initially aligned with their long axes parallel to the spiral direction, then gradually become oriented in the perpendicular direction as the spiral wave propagates toward the outer edge, as previously reported by Okabe et al.10 On the area between the growing arms, fibril lamellae are found to be aligned parallel to the spiral direction. These two perpendicular alignments of the crystal bands make the spherulite show a spiral banded pattern with two distinct colours under POM. Crystals situated in the interior underneath the top surface, nevertheless, can differ dramatically as the crystals emerge from the interior to the top.
This present study aimed at extending conventional analyses from thin-film top surface analyses in previous work to the interior of spherulites in bulk states. Three-dimensional interior architectures of PTT banded spherulites in bulk samples were analysed through a combination of dissection and etching using methylamine vapour or potassium permanganate. The main objectives of this study were to probe the questions regarding crystallization in further depth, and to analyse complex issues by novel approaches, namely: (1) examining the interior structures of PTT banded spherulites, (2) correlating the interior lamellar assembly with the banded pattern which appears on the top surface, (3) tracing the formation mechanisms of the three different banded patterns from the interior vs. the exterior lamellar arrangements of the etched samples, and (4) analysing the plausible key factors in the formation of those banded structures in the PTT spherulites.
Experimental
Aromatic polyester, PTT, was obtained from Industrial Technology Research Institute (ITRI) (Taiwan). The glass transition (Tg) and melting temperatures (Tm) of PTT were measured to be 45 °C and 228 °C, respectively. Thick bulk samples of PTT (thickness ca. 100 μm) were prepared by directly pressing PTT pellets on a glass substrate at 260 °C. Each of the samples was firstly melted at 260 °C for 5 minutes to erase any prior thermal history and then quenched to at 165 °C for isothermal crystallization.After crystallization, the bulk samples were randomly fractured so that the interior lamellae could be observed from different angles of viewpoint and perspective. These fractured samples were then exposed to methylamine vapour (from 40% w/w aqueous solution) at ambient temperature for a period of time (2 to 24 hours) to disclose the intricate lamellar structure of PTT crystals that was covered up by the amorphous PTT. After etching, the MA-etched PTT samples were dipped into de-mineralized water to rinse off all the condensed methylamine and the unwanted by-products. For comparison, the permanganate etching technique was also applied on comparable samples. 2 wt% potassium permanganate (KMnO4) dissolved in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of H3PO4
1 mixture of H3PO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2SO4 was placed in a glass bottle. The etching takes place inside the bottle, supported in an ultrasonic bath for 20 minutes at ambient temperature. Etching was then followed by a series of washing treatments with a H2O
H2SO4 was placed in a glass bottle. The etching takes place inside the bottle, supported in an ultrasonic bath for 20 minutes at ambient temperature. Etching was then followed by a series of washing treatments with a H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2SO4 (7
H2SO4 (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) solution in ice bath, H2O2, water, and acetone. A degassing process was performed at 40 °C in a vacuum oven for 48 h to get rid all of the liquids properly.
2) solution in ice bath, H2O2, water, and acetone. A degassing process was performed at 40 °C in a vacuum oven for 48 h to get rid all of the liquids properly.
A light-polarized optical microscope (POM) (Nikon Optiphot-2), equipped with a Nikon Digital camera system for microscopy (Digital Sight (DS-U1)) and a microscope hot stage (Linkam THMS-600 with T95 temperature program) was used to observe the crystal morphology of bulk PTT samples during crystallization, before, and after the etching treatment. Scanning electron microscopy (SEM) (FEI Quanta-400F) was used to characterize the detailed lamellar arrangement of the fracture surfaces of samples in correlation to their top free surfaces before and after the methylamine etching process. The samples were coated by gold vapour deposition using vacuum sputtering prior to the SEM characterization.
Results and discussion
In studying the interior of crystal morphology and diversified banding patterns, it is essential to verify that the banding patterns obtained in thin films are obtainable in bulk samples. Fig. 1 shows OM (a) and POM (b) micrographs as well as a scheme (c) displaying the simultaneous appearance of three different types of banded spherulites in a ∼100 μm thickness PTT bulk sample. This evidences that multiple types of ring bands are able to maintain their simultaneous existence not only in thin-film samples (with thickness ∼1 μm) but also in bulkier thicker PTT samples (thickness ∼100 μm). Each of the spherulites appears in hazy blue (indicating high order of retardation color) under POM, indicating intricate lamellar arrangements inside the spherulites. Concentric-ring, clockwise single-spiral, and counter-clockwise double-spiral banded spherulites are found side by side with 7 to 14 μm band-spacing. One may then speculate that the clockwise single-spiral spherulites perhaps induce the double-helicoid spherulites to rotate reversely or vice versa, and together they restrict the middle spherulite to grow concentrically. That is to say, perhaps invisible interactions exist among the neighboring spherulites, even starting at the very beginning of the crystallization process. However, the possibility of these external factors will not be further discussed in this study. | ||
| Fig. 1 OM micrograph (a), POM micrograph (b), and schemes (c) of three different types of banded spherulites that appear simultaneously in a PTT bulk sample. | ||
In order to get better resolution, morphological analyses have been performed using SEM. Fig. 2 shows SEM micrographs of a PTT spherulite before the etching process: (a) shows the top surface and (b) shows the top vs. the fractured surface. After being fully crystallized at 165 °C, the sample was directly quenched to room temperature (below the Tg of PTT). This rapid transfer from a crystalline to a glassy state coupled with the thermal expansion difference between PTT and glass induces cracks on the spherulites.33 Correlations between the top and fractured surfaces could be obtained by tilting the fractured sample to a certain angle, and thus, the electron beam could hit both top and fractured surfaces at once. The wavy structures of lamellae are seen as ridges and valleys on the top surface as well as the fracture surface of the bulk sample. White dashed lines are drawn along the valleys on the top and fracture surfaces, to show the unbroken three-dimensional spiralling pattern from the centre to the outer layer of the ring-banded spherulite.
Two commonly used etching methods, using methylamine and alternatively potassium permanganate (in acidic solution), are utilized to get better morphological contrasts on PTT spherulites. The results have been analysed and compared to interpret the actual lamellar arrangement of PTT crystals that construct the banded spherulites. The discussions will be limited to the morphological changes of PTT banded spherulites as the final results of etching, while the detailed processes of chemical and physical degradations during etching will be discussed elsewhere in the future.
Dissected PTT spherulites were exposed to methylamine vapour at ambient temperature for various etching periods from 2 to 24 hours prior to microscopic characterization. SEM micrographs of the top surface of PTT spherulites after various periods of MA-etching are shown as ESI† Fig. S2. The intermittent development of crevices starts within 2–4 h; after 8 h of MA-exposure, crevices significantly increase in number. A longer etching period of 24 h yields results where the size (dimension) of those crevices is enlarged, although the number of crevices does not significantly increase. PTT samples with 16 h of MA-etching were thus chosen to represent the observed morphology because these samples yielded the optimum results. POM images of PTT spherulites after MA-etching for 16 h observed in thin film samples in comparison with bulk samples are shown as ESI† Fig. S3. From the thin-film observation, the MA-etching-induced crevices cross the high-interference-colour region and part of the low-interference-colour region, indicating that lamellar orientation is not the only factor influencing the changes of interference colour in this system. Lamella thickness, crystal density, and/or compactness may also enhance the retardation, resulting a higher interference color in one region compared to the other. In the bulk, the MA-etched PTT spherulites appear in grayscale with traces of cracks and crevices.
Fig. 3 shows SEM micrographs of the top surface of 16 h-MA-etched PTT banded spherulites (a) before and (b–d) after being washed and dried; parts (b–d) show concentric-ring, single-helicoid, and double-helicoid spherulites, respectively. Cracks and crevices are observed, indicating the areas in which PTT has been removed from the spherulites through the chemical reaction with methylamine vapor.34 In Fig. 3(a), spindle-like crystals of methyl-ammonium salt appear as a by-product of the reaction, they get dissolved into the condensed vapour and re-crystallized on the top of the sample. After being washed and dried [Fig. 3(b–d)], the samples show clearer dimensions and curvatures of crevices. Each crevice is of 1–2 μm in width [hundreds of times larger than the thickness of virgin PTT lamellae, approx. 8 nm]. On the concentric-ring-banded spherulite, the crevices are either biconvex or stripe. On the single- and double-spiral-banded spherulites, they curve slightly into C or  shapes with regard to the spiral direction. When viewed from the top, these crevices could be radially interconnected by lines; hence, the remaining crystals appeared as twisted giant lamellae.23,24 However, by considering the narrow crystal layer between the crevices on two successive bands [indicated by the dashed lines], the twist is confined to discrete layers and is not continuous like a single strand of crystals.
shapes with regard to the spiral direction. When viewed from the top, these crevices could be radially interconnected by lines; hence, the remaining crystals appeared as twisted giant lamellae.23,24 However, by considering the narrow crystal layer between the crevices on two successive bands [indicated by the dashed lines], the twist is confined to discrete layers and is not continuous like a single strand of crystals.
Dissection was carried out to give views of the interior architectures of PTT banded spherulites from different angles. For discussion, these different dissection angles are classified into (1) lateral dissection along the radial direction crossing the nucleus and (2) circumferential dissection. Fig. 4 shows SEM micrographs of ring-banded spherulites of a PTT sample being laterally fractured across their nucleus or being circumferentially fractured across their ridge or valley, followed by etching by MA for 16 h. When the nucleation occurs near the boundary between the sample and air (on the top surface), as shown in Fig. 4(a), the upward growth of the up-half sphere is highly restricted. On the other hand, when nucleation occurs near the boundary between the sample and the glass substrate (on the bottom surface), as shown in Fig. 4(b), the downward growth of the crystal is entirely hindered by the glass surface. Three-dimensional waving structures of lamellae can be observed throughout the two samples regardless their nuclei positions. The wave goes upward (ridge) and downward (valley), frontward (out of the paper) and backward (into the paper).
Unlike the single-spiral spherulite in Fig. 1(b), the concentric-ring-banded spherulite in Fig. 4(a) has an opposite correlation between the top and fractured surfaces. The upward bands (ridges) on the top surface connect with the backward bands (valleys) on the fractured surface, and vice versa; the downward bands (valleys) on the top surface connect with the frontward bands (ridges) on the fractured surface. At ridges, the lamellae are radially arranged, and in contrast, at valleys, the lamellae are circumferentially arranged [along its latitude on the top surface or along its longitude on the fractured surface]. For this typical concentric-banded spherulite with the nucleus on the top surface, the band-spacing on both the top and fractured surfaces are perfectly matched (8 μm). For a spherulite whose nucleus is on the bottom (Fig. 4(b)), the band spacing on the top surface (∼10 μm) is larger than that on the fractured surface (∼8 μm) due to the top-surface confinement, yet their correlations of ridges and valleys on the top vs. the fractured surface remain opposite. Along the peripheral surface (Fig. 4(c)) where the impingement with other spherulites occurs, the crystal lamellae beneath the ridges are stacked along its longitude [squares in red]. By contrast, under the valleys, the lamellae are stacked along its latitude [squares in yellow]. Truncated banded structures on the peripheral surface indicate the discontinuous development of the bands. Similar phenomena could also be observed from the internal point of view (Fig. 4(d)).
The contrast is even more pronounced on randomly-fractured PTT spherulites, as shown in Fig. 5. Instead of radially spiralling lamellae similar to what other investigators found, we observed a circumferentially spiralling shell inside the 3D MA-etched PTT spherulite [as marked by the white dashed line], further strengthening the case for intermittent growth of lamellae in the radial direction. Lamellae at two successive ridges are interconnected by this shell. The lamellae at the next ridge are most likely fanned out from the lamellae of the previous shell (valley) and slightly curved with respect to the spiral direction of the shell [as found in the skeleton of the nautilus shell] to fill the space of each band discretely. Summarizing from all points of view, a scheme has been made to illustrate the 3D spiral development of the spherulite. C or  -shaped crevices are generated due to the reaction of methylamine with PTT lamellae, justifying the arrangements and curvatures of the crystals. An onion bulb with intermittent growing scales is the perfect illustration for PTT banded spherulites.
-shaped crevices are generated due to the reaction of methylamine with PTT lamellae, justifying the arrangements and curvatures of the crystals. An onion bulb with intermittent growing scales is the perfect illustration for PTT banded spherulites.
 | ||
| Fig. 5 SEM micrograph of ring-banded spherulites of 16 h MA-etched PTT after being randomly fractured. | ||
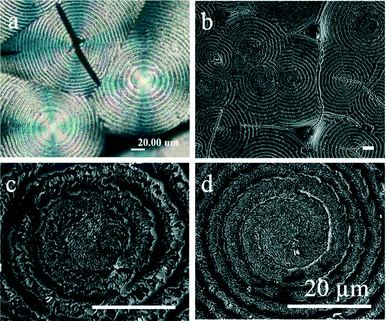
In comparison with methylamine etching, which is known to alter chemical and morphological structures, permanganate etching allows the systematic study of real microstructures with original crystals remaining more intact. Another set of PTT samples was prepared with exactly the same thermal treatment, but instead of being exposed to methylamine vapour, they were soaked in a bottle of permanganate enchant in ultrasonic condition. POM and SEM images of the top surface of KMnO4-etched PTT spherulites are shown in Fig. 6. The morphology of KMnO4-etched PTT banded spherulites is dramatically different from that of MA-etched PTT discussed earlier. Instead of sporadically etching out the lamellae at the ridges and causing crevices on the ridge, as MA does, the KMnO4, through oxidation, removes the lamellae at the junction and causes circumferential crevices between the successive bands. Higher magnifications of detailed lamellar arrangements on the top surface of (KMnO4-etched) PTT spherulites are shown as ESI† Fig. S4. The radially-arranged lamellae underneath the ridges are curved, as traced from the MA-etched PTT spherulites. Similarly, the lamellae are packed in layer-by-layer like compact onion bulbs, but instead of thin crystal layers of shells, they are separated by a narrow crevice. From the top, each band consists of crystal stacks and finer crystal fibrils that branch out and/or taper to thinner tips from the initial crystal stacks. Considering the band spacing of PTT spherulites (∼7 μm), both crystal stacks and fibrils are under the coverage of the ridge, while the narrow crevice between the bands is under the coverage of the valley. It is worth mentioning that the dimension of the crevices on KMnO4-etched spherulites is proportional to the crystal lamellae shells on MA-etched spherulites (ca. 1 μm). The results suggest that KMnO4 tends to attack the connections between the crystal fibrils of successive bands where the circumferentially arranged lamellae (shells in MA-etched spherulites) existed.
The discontinuity of spherulite growth in radial directions is further strengthened by analysing the SEM images of the top vs. the fractured surface of KMnO4-etched PTT spherulites, shown in Fig. 7. Both the fractured and top surfaces of PTT clearly show a periodic manner of crystal lamellae deposition along radial directions. The colour contrast observed in the graphs might be caused by either the crystal position (working distance) or by different conductive properties of the crystals. The SEM images indicate the uneven crystal density distributions along the radial directions. If the etching period is prolonged to one hour, the KMnO4 further erodes the crystal fibrils and therefore the crevice between bands is enlarged, as shown in Fig. S5.† The erosion causes further separation of the bands, starting with the central core and moving to the rest of the spherulite. The KMnO4-etched concentric-ring-banded spherulite (Fig. 7(a)) shows discrete bands resembling onion bulbs, interconnected by layers where crystal fibrils of the successive bands collide with each other. Single- and double-spiral banded spherulites (Fig. 7(b and c)) show interconnected 3D spiral arm(s) that continuously form from the core until they collide with other spherulites. Each arm is composed of crystal stacks (occupying the higher crystal density regions, appearing brighter) that branch and/or taper into crystal fibrils (occupying the lower crystal density regions, appearing darker). The arms are separated from one another by crevices on which crystals are most severely eroded.
 | ||
| Fig. 7 SEM images of fracture surfaces in correlation with the top surfaces of KMnO4-etched PTT spherulites: (a) concentric-ring, (b) single-spiral, (c) double-spiral. | ||
As a summary, Fig. 8 shows SEM graphs of the top surface (a) as well as SEM graphs (b) and schemes (c) of top vs. fracture surface for (i) unetched single-spiral, (ii) MA-etched concentric, and (iii) KMnO4-etched double-spiral banded spherulites. The unetched single-spiral banded spherulite shows wavy structures with valleys that can be interconnected by two unbroken 3D spiral separated ridges at both the top and fracture surfaces. The MA-etched concentric banded spherulite also shows a wavy structure with valleys and ridges on the top, yet they oppose the valleys and ridges on the fracture surface. Crevices are found after etching. The number of crevices increases to a certain extent, then a further increment of the etching period will cause the enlargement of crevices, indicating that MA attacks the exposed PTT amorphous layers on ridges before it, without exception, attacks the interfacial crystal layers. Discontinuous crevices and the intermittent growth of lamellae in radial directions suggest that instead of the conventional model of a continuous spiralling twist of long lamellae radiating from a common center, the ring-banded PTT spherulite is more likely composed of polycrystals, oriented perpendicularly and periodically, branching out and colliding among the neighboring bands. The KMnO4-etched double-spiral banded PTT spherulite justifies the spiral crevices between two continuously growing arms being the weakest part of the sphere where collisions of crystal fibrils takes place. Each arm consists of a higher crystal density region of crystal stacks and a lower crystal density regions of crystal fibrils that grow from the crystal stacks to fill the space, allowing maximum coverage of the spherulite.
Based on the MA-etched and KMnO4-etched PTT results, a replica model of discrete crystallization can be built to illustrate the formation of PTT banded spherulites. As shown by the results of KMnO4-etching, the variation of crystal density along the radial directions leads to the formation of periodic bands that are composed of crystal stacks and crystal fibrils. According to the results of MA-etching, the cavities are radially arranged, crossing over both higher and lower birefringence regions; thus, both crystal bundles composing each band are arranged in the radial direction. Between the two successive bands, a thin layer of lamellae form by the collision of crystal fibrils and they are arranged perpendicularly to the radial directions (i.e., circumferential). These impingement zones, owing to stresses, are the weakest part of the spherulites where circumferential crevices appear after KMnO4-etching.
Conclusions
Conventional analyses of banded PTT spherulites in the top surfaces of thin films are advanced one major step forward to 3D interior analyses on the crystal assembly in this study. The interior crystal morphology in correlation with the birefringence patterns of PTT were thoroughly probed, and mechanisms behind these diversified spherulite structures were investigated. By the novel interior observation coupled with selective etching, it is shown that the interior of both MA-etched and KMnO4-etched PTT spherulites show the discontinuity of growth in the radial directions.Each band in the 3D banded spheres is composed of radially-oriented branches from tangentially-oriented lamellae, resembling a corrugated board structure, and branching growth impingement between the two neighbouring bands creates the noted discontinuity in ring bands. The interior-dissected layer thickness (radial + tangential lamellae) of the banded PTT spherulites matches perfectly with the optical band spacing.
Acknowledgements
This work has been financially supported by a basic research grant (NSC 102-2221-E-006-268-MY3) for three consecutive years from the Taiwan National Science Council (NSC), now Ministry of Science and Technology (MOST), to which the authors express their gratitude. This research was also partially supported by the Ministry of Education, Taiwan, R. O. C., as part of the Top University Project for National Cheng Kung University (NCKU) for the fiscal years of 2015–16.Notes and references
- T. Mori, M. Kyotani and K. Akagi, Macromolecules, 2010, 43, 8363 CrossRef CAS.
- T. Mori and K. Akagi, Macromolecules, 2013, 46, 6699 CrossRef CAS.
- A. G. Shtukenberg, E. Gunn, M. Gazzano, J. Freudenthal, E. Camp, R. Sours, E. Rosseeva and B. Kahr, ChemPhysChem, 2011, 12, 1558 CrossRef CAS PubMed.
- H. D. Keith and F. J. Padden Jr., J. Polym. Sci., 1959, 39, 101 CrossRef CAS.
- H. D. Keith and F. J. Padden Jr., J. Polym. Sci., 1959, 39, 123 CrossRef CAS.
- A. Keller and H. H. Wills, J. Polym. Sci., 1959, 39, 151 CrossRef CAS.
- A. Lustiger, B. Lotz and T. S. Duff, J. Polym. Sci., Polym. Phys. Ed., 1989, 27, 561 CrossRef CAS.
- J. Xu, B. H. Guo, Z. M. Zang, J. J. Zhou, Y. Jiang, S. Yan, L. Li, Q. Wu, G. Q. Chen and J. M. Schultz, Macromolecules, 2004, 37, 4118 CrossRef CAS.
- B. Lotz and S. Z. D. Cheng, Polymer, 2005, 46, 577 CrossRef CAS.
- T. Kyu, H. W. Chiu, A. J. Guenthner, Y. Okabe, H. Saito and T. Inoue, Phys. Rev. Lett., 1999, 83, 2749 CrossRef CAS.
- J. M. Schultz, Polymer, 2003, 44, 433 CrossRef CAS.
- J. Chen and D. Yang, Macromolecules, 2005, 38, 3371 CrossRef CAS.
- Z. Wang, Z. Hu, Y. Chen, Y. Gong, H. Huang and T. He, Macromolecules, 2007, 40, 4381 CrossRef CAS.
- G. Lugito and E. M. Woo, Cryst. Growth Des., 2014, 14, 4929 CAS.
- Y. F. Chen and E. M. Woo, Macromol. Rapid Commun., 2009, 30, 1911 CrossRef CAS PubMed.
- B. Wang, C. Y. Li, J. Hanzlicek, S. Z. D. Cheng, P. H. Geil, J. Grebowicz and R. M. Ho, Polymer, 2001, 42, 7171 CrossRef CAS.
- G. Lugito and E. M. Woo, J. Polym. Sci., Part B: Polym. Phys., 2016, 54, 1207 CrossRef CAS.
- E. M. Woo, S. Nurkhamidah and Y. F. Chen, Phys. Chem. Chem. Phys., 2011, 13, 17841 RSC.
- E. M. Woo, G. Lugito, J. H. Tsai and A. J. Müller, Macromolecules, 2016, 49, 2698 CrossRef CAS.
- R. M. Ho, K. Z. Ke and M. Chen, Macromolecules, 2000, 33, 7529 CrossRef CAS.
- P. L. Wu and E. M. Woo, J. Polym. Sci., Part B: Polym. Phys., 2003, 41, 80 CrossRef CAS.
- H. B. Chen, L. Chen, Y. Zhang, J. J. Zhang and Y. Z. Wang, Phys. Chem. Chem. Phys., 2011, 13, 11067 RSC.
- J. Li, Z. Hu, Z. Wang, Q. Gu, Y. Wang and Y. Huang, Ind. Eng. Chem. Res., 2013, 52, 1892 CrossRef CAS.
- J. Li, Y. Li, J. Zhou, J. Yang, Z. Jiang, P. Chen, Y. Wang, Q. Gu and Z. Wang, Macromolecules, 2011, 44, 2918 CrossRef CAS.
- M. Rosenthal, G. Portale, M. Burghammer, G. Bar, E. T. Samulski and D. A. Ivanov, Macromolecules, 2012, 45, 7454 CrossRef CAS.
- M. Rosenthal, M. Burghammer, G. Bar, E. T. Samulski and D. A. Ivanov, Macromolecules, 2014, 47, 8295 CrossRef CAS.
- E. M. Woo, G. Lugito and C. E. Yang, CrystEngComm, 2016, 18, 997 RSC.
- P. D. Hong, W. T. Chung and C. F. Hsu, Polymer, 2002, 43, 3335 CrossRef CAS.
- W. T. Chuang, P. D. Hong and H. H. Chuah, Polymer, 2004, 45, 2413 CrossRef CAS.
- H. H. Chuah, J. Polym. Sci., Part B: Polym. Phys., 2002, 40, 1513 CrossRef CAS.
- J. H. Yun, K. Kuboyama and T. Ougizawa, Polymer, 2006, 47, 1715 CrossRef CAS.
- K. Kuboyama, Y. J. Hwa, T. Chiba and T. Ougizawa, Polymer, 2006, 47, 4831 CrossRef.
- K. Kuboyama and T. Ougizawa, Polym. J., 2008, 40, 1005 CrossRef CAS.
- G. Lugito, L. Y. Wang and E. M. Woo, Macromol. Chem. Phys., 2014, 215, 1297 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: POM micrographs of PTT at various Tc; SEM images of PTT spherulites top surface after various periods of MA-etching; POM images of 16 h MA-etched PTT in thin vs. bulk samples; HR-SEM images of 20 min-KMnO4-etched and 1 h-KMnO4-etched PTT. See DOI: 10.1039/c6ce01261b |
| This journal is © The Royal Society of Chemistry 2016 |