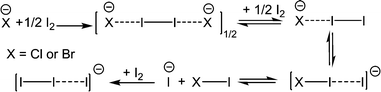Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
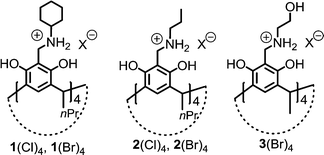
N-Alkyl ammonium resorcinarene polyiodides†
Fangfang
Pan
a,
Ngong Kodiah
Beyeh
*b,
Robin H. A.
Ras
b and
Kari
Rissanen
*c
aCollege of Chemistry, Central China Normal University, 152 Luoyu Road, Wuhan, Hubei 430079, China
bDepartment of Applied Physics, Aalto University, Puumiehenkuja 2, FI-02150, Espoo, Finland. E-mail: Kodiah.beyeh@aalto.fi
cDepartment of Chemistry, Nanoscience Center, University of Jyvaskyla, P.O. Box 35, FI-40014 Jyvaskyla, Finland. E-mail: Kari.t.rissanen@jyu.fi
First published on 17th June 2016
Abstract
Four N-alkyl ammonium resorcinarene halides incorporating polyiodides were obtained and structurally analyzed by single crystal X-ray crystallography. The unexpected formation of triiodides and pentaiodide anions in these structures was assumed to be the result of the heterolytic dissociation of molecular iodine (I2) in the presence of electron donors in the N-alkyl ammonium resorcinarene halide system, from which I− further binds one or two I2 molecules resulting in I3− or I5− species, respectively.
Halogen bonding,1 as a non-covalent interaction, is analogous to hydrogen bonding in both strength and directionality. Halogen bonding is widely used in the design and construction of small and large supramolecular assemblies with novel applications in crystal engineering, materials science and biological systems.2–8 Within the broad area of halogen bonding, polyhalides assembled through halogen⋯halogen interactions are particularly interesting. Charge transfers between the halides and the molecular halogen stabilize the polyhalide species and endow the final polyhalides with potential electronic and photophysical properties.9–11
The chemistry of N-alkyl ammonium resorcinarene halides (NARXs) as receptors and supramolecular synthons has been recently developed.12 The bowl-shaped C4v symmetry of the resorcinarene core in the NARXs is maintained by intramolecular hydrogen bonds between adjacent hydroxyl groups. The large organic salt molecules feature a hydrogen bonded cyclic geometry with the aromatic groups providing the pre-organized deep electron-rich interior cavities. The halides in the circular hydrogen bond seam ((⋯NArRH2+⋯X−⋯)4) with hydrogen and halogen bond acceptor ability introduce the possibility of extending the cavity size in the presence of suitable donors.13 This broadens the ability of the NARXs to act as receptors for a larger variety of guest molecules as well as increases their potential as synthons for larger supramolecular networks and assemblies.13 Molecular iodine (I2) as a halogen bond donor was successfully used to construct a halogen-bonded dimeric capsule with an NARX, where [Cl⋯I–I⋯Cl]2− species was detected in the solid state as the main factor linking the two capsule halves.9 To further investigate the nucleophilicity of the halides in NARXs towards I2, in this contribution, I2 was introduced into four NARXs: N-cyclohexyl ammonium resorcinarene chloride (1(Cl4)) and bromide (1(Br4)), N-propyl ammonium resorcinarene chloride (2(Cl4)) and bromide (2(Br4)), and the water soluble N-ethanol ammonium resorcinarene bromide (3(Br4)) (Scheme 1). Surprisingly, triiodide anions were observed from the mixture of I2 with 1(Cl4), 1(Br4) and 2(Br4), while the rare linear pentaiodide anion was detected in the system containing I2 and 3(Br4) in MeOH.
Crystals of 1,4-dioxane@1(Cl3·I3) were obtained by slow evaporation of the methanol solution from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar mixture of 1(Cl4) and I2 in the presence of 1,4-dioxane. Unlike in the reported capsular assembly between an analogue of 1(Cl4) with a shorter lower rim in which three ordered 1,4-dioxane molecules were entrapped in the cavity with the [Cl⋯I–I⋯Cl]2− species stabilizing the two capsule halves, in the new assembly, a stable triiodide (I3−) anion was formed, which replaced one of the chlorides from the cation–anion seam. The triiodide does not form part of the cation–anion hydrogen-bonded seam; instead, it is located above the resorcinarene skeleton. The bowl shape of the resorcinarene frame is still preserved with one 1,4-dioxane molecule trapped in the cavity via two N–H⋯O hydrogen bonds, although only two chlorides are positioned in the cation–anion seam. However, due to the steric hindrance between the large I3− and the four N-cyclohexyl ammonium arms, one of the arms rotates 180° with the ammonium hydrogens pointing outwards and is hydrogen-bonded to one additional exo-1,4-dioxane molecule (Fig. 1A). The exo-cavity-1,4-dioxane molecule simultaneously hydrogen bonds to two adjacent resorcinarene molecules forming a dimer ([1,4-dioxane@1(Cl3·I3)]·1,4-dioxane·[1,4-dioxane@1(Cl3·I3)]) (Fig. 1C). In addition to the linkage of 1,4-dioxane, the dimer is also connected by the third Cl− and a disordered water molecule via multiple hydrogen bonds (Fig. 1C). Along the crystallographic b-axis, the resorcinarene framework is arranged in a head-to-tail manner, with the I3− squeezing into the space between the upper rim of one resorcinarene and the lower rim of another. This kind of arrangement causes two of the four propyl groups at the lower rim to fold in gauche conformation. Intermolecular O–H⋯O hydrogen bonds are responsible for the structural extension along the a-axis (Fig. S1†).
2 molar mixture of 1(Cl4) and I2 in the presence of 1,4-dioxane. Unlike in the reported capsular assembly between an analogue of 1(Cl4) with a shorter lower rim in which three ordered 1,4-dioxane molecules were entrapped in the cavity with the [Cl⋯I–I⋯Cl]2− species stabilizing the two capsule halves, in the new assembly, a stable triiodide (I3−) anion was formed, which replaced one of the chlorides from the cation–anion seam. The triiodide does not form part of the cation–anion hydrogen-bonded seam; instead, it is located above the resorcinarene skeleton. The bowl shape of the resorcinarene frame is still preserved with one 1,4-dioxane molecule trapped in the cavity via two N–H⋯O hydrogen bonds, although only two chlorides are positioned in the cation–anion seam. However, due to the steric hindrance between the large I3− and the four N-cyclohexyl ammonium arms, one of the arms rotates 180° with the ammonium hydrogens pointing outwards and is hydrogen-bonded to one additional exo-1,4-dioxane molecule (Fig. 1A). The exo-cavity-1,4-dioxane molecule simultaneously hydrogen bonds to two adjacent resorcinarene molecules forming a dimer ([1,4-dioxane@1(Cl3·I3)]·1,4-dioxane·[1,4-dioxane@1(Cl3·I3)]) (Fig. 1C). In addition to the linkage of 1,4-dioxane, the dimer is also connected by the third Cl− and a disordered water molecule via multiple hydrogen bonds (Fig. 1C). Along the crystallographic b-axis, the resorcinarene framework is arranged in a head-to-tail manner, with the I3− squeezing into the space between the upper rim of one resorcinarene and the lower rim of another. This kind of arrangement causes two of the four propyl groups at the lower rim to fold in gauche conformation. Intermolecular O–H⋯O hydrogen bonds are responsible for the structural extension along the a-axis (Fig. S1†).
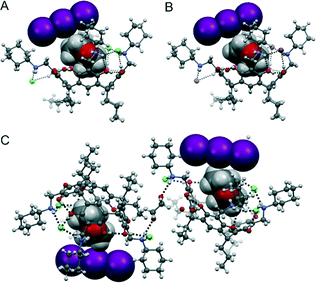 | ||
| Fig. 1 The structural units in 1(Cl3·I3) (A) and 1(Br3·I3) (B) as well as the dimer [1,4-dioxane@1(Cl3·I3)]·1,4-dioxane·[1,4-dioxane@1(Cl3·I3)] (C). | ||
The formation of the triiodide anion in the structure 1,4-dioxane@1(Cl3·I3) is unexpected. We assume that it is the result of the heterolytic dissociation of I2 in the presence of an electron donor of either Cl− or the solvent methanol molecule. The produced I−, further interacts with one I2 molecule giving rise to the I3− anion (Scheme 2). It is noteworthy that the difference between triiodide and chlorodiiodide is evident from the difference Fourier map; therefore, the species of I3− can undoubtedly be confirmed by single crystal X-ray analysis.
To further investigate this process, we extended the study to 1(Br4), an analogue of 1(Cl4), and to the more flexible N-propyl groups 2(Cl4) and 2(Br4). Single crystal analysis of 1(Br4), which is the bromide analogue of 1(Cl4), reveals an isomorphic structure (1,4-dioxane@1(Br3·I3)). The single crystals were obtained by slowly evaporating the methanol solvent from the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of 1(Br4) and I2 under the same conditions as for 1(Cl4) (Fig. 1B). Unfortunately, we could not isolate any suitable single crystals from the reaction of 2(Cl4) with I2. However, the reaction of the bromide analogue 2(Br4) with I2 resulted in a self-included dimer with six bromides and two triiodides.
2 mixture of 1(Br4) and I2 under the same conditions as for 1(Cl4) (Fig. 1B). Unfortunately, we could not isolate any suitable single crystals from the reaction of 2(Cl4) with I2. However, the reaction of the bromide analogue 2(Br4) with I2 resulted in a self-included dimer with six bromides and two triiodides.
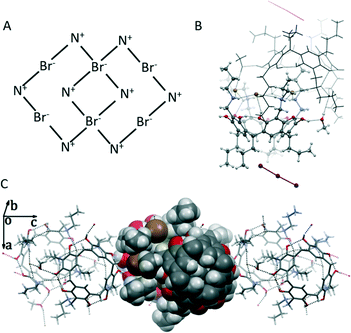
The formation of the self-included dimer is not unusual. We have previously reported a self-included dimeric assembly of other N-propyl ammonium halides.12a,14 Usually, one of the N-propyl ammonium arms from one NARX resides in the cavity of the second molecule with the help of six halide anions, which further bind the two resorcinarene dimer halves via multiple inter- and intramolecular hydrogen bonds. In this structure, for each resorcinarene cation, four Br− anions are situated between the ammonium arms, among which two are shared by the two receptors with four NH⋯Br− hydrogen bonds for each Br− as depicted in the schematic of Fig. 2A. The other four Br− anions are all involved in one OH⋯Br and two NH⋯Br interactions. The dimer formally shows two positive charges, and as a result, two triiodide anions appear in between the four propyl groups at the lower rim. It is worth noting that although 1,4-dioxane was used in the crystal growth process, it was not found in the[2(Br3·I3)]2 assembly. The stability of the self-included dimer overrides the possible host–guest binding process of 1,4-dioxane. The formation of such a dimer involves six of the eight anions, thus releasing the two “free” anions to be involved in the triiodide formation.
In the reaction of NARX 3(Br4) with I2 in methanol, we surprisingly found a very rare linear I5− anion in the crystal structure. The water soluble NARX 3(Br4) was synthesized according to recently reported procedures.15 Therein, the chloride analogues were shown to bind a series of alkanes, haloalkanes and arenes in water via hydrophobic interactions. The crystal structure of one chloride analogue showed a dimeric capsule with two 1,4-dioxane molecules trapped within the interior cavity similar to Rebek's classical hydrogen bonded cylindrical capsule.16 Here, the dimeric capsule [3(Br3.5)]2·I5 is formed in which the two salt molecules are arranged in a staggered pair. The two capsule halves are held together by eight OH⋯Br intermolecular hydrogen bonds. Due to the high symmetry of the structure, each Br− anion has 0.9 occupancy with the whole capsule bearing one positive charge, which is counter-balanced by one I5− anion. Highly disordered solvent molecules were observed in the cavity of the capsule. In general, the I5− anion formed can be regarded as an adduct of the I3− with an additional I2, or I−, and two I2 molecules. The structures of the I5− anion have been intensively reported;10 however, in most cases, they display a “V” or an “L” shaped geometry. The linear I5− species is rarely reported.17,18 In [3(Br3.5)]2·I5, the formally centrosymmetric I5− is considered as a halogen-bonded adduct of I− and two I2, with the central I⋯I⋯I halogen bond distance of 3.191 Å (×2, RXB = 0.81; ref. 19) and the terminal I–I length of 2.841 Å (×2). A strong charge transfer interaction occurs from the I2 and the central I− anion, elongating the terminal I–I bonds. The linear geometry of the I5− here is also due to the molecular packing in the crystal lattice (Fig. 3B).
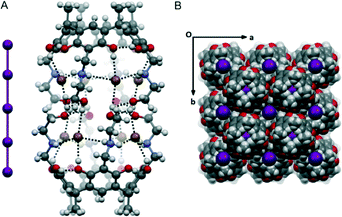 | ||
| Fig. 3 The hydrogen bonded dimeric capsule in [3(Br3.5)]2·I5 (A) and its solid state packing in CPK mode (B). | ||
Conclusions
In summary, we present four NARX3In (n = 3 or 5 and X = Cl or Br) compounds, three triiodides and one with a linear pentaiodide anion. The single crystal X-ray diffraction study confirms that in two N-cyclohexyl compounds and one N-propyl NARX compound a triiodide anion is formed which then replaces one of the original halides. The N-ethanol NARX 3(Br4) resulted in a rare linear pentaiodide entrapped within the crystal lattice. The formation of polyiodides in this system is assumed to be the result of the heterolytic dissociation of I2 in the presence of NARX halide anions. The electron donor properties of the NARX halides and/or the solvent methanol molecule are probably responsible for this process. The pentaiodide is described as a halogen bonded adduct of I− and two I2. In addition to their anion exchange properties, this study further highlights that the N-alkyl ammonium resorcinarene halides are versatile molecular building blocks for the design and construction of larger supramolecular assemblies.Acknowledgements
The authors gratefully acknowledge the Academy of Finland (KR: grant no. 263256 and 292746; NKB: grant no. 258653; RHAR: grant no. 272579), Central China Normal University, University of Jyvaskyla, Finland and Aalto University, Finland for financial support.References
- G. R. Desiraju, P. S. Ho, L. Kloo, A. C. Legon, R. Marquardt, P. Metrangolo, P. Politzer, G. Resnati and K. Rissanen, Pure Appl. Chem., 2013, 85, 1711–1713 CrossRef CAS
.
- E. Corradi, S. C. Meille, M. T. Messina, P. Metrangolo and G. Resnati, Angew. Chem., Int. Ed., 2000, 39, 1782–1786 CrossRef PubMed
.
- M. Erdelyi, Chem. Soc. Rev., 2012, 41, 3547–3557 RSC
.
- T. M. Beale, M. G. Chudzinski, M. G. Sawar and M. S. Taylor, Chem. Soc. Rev., 2013, 42, 1667–1680 RSC
.
- M. R. Scholfield, C. M. Vander Zanden, M. Carter and P. S. Ho, Protein Sci., 2013, 22, 139–152 CrossRef PubMed
.
- L. C. Gilday, S. W. Robinson, T. A. Barendt, M. J. Langton, B. R. Mullaney and P. D. Beer, Chem. Rev., 2015, 115, 7118–7195 CrossRef PubMed
.
- G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati and G. Terraneo, Chem. Rev., 2016, 116, 2478–2601 CrossRef PubMed
.
- A. Bertolani, L. Pirrie, L. Stefan, N. Houbenov, J. S. Haataja, L. Catalano, G. Terraneo, G. Giancane, L. Valli, R. Milani, O. Ikkala, G. Resnati and P. Metrangolo, Nat. Commun., 2015, 6, 754 Search PubMed
.
- N. K. Beyeh, F. Pan and K. Rissanen, Angew. Chem., Int. Ed., 2015, 54, 7303–7307 CrossRef CAS PubMed
.
- P. H. Svensson and L. Kloo, Chem. Rev., 2003, 103, 1649–1684 CrossRef PubMed
.
- C. Walbaum, M. Richter, U. Sachs, I. Pantenburg, S. Riedel, A. Mudring and G. Meyer, Angew. Chem., Int. Ed., 2013, 52, 12732–12735 CrossRef CAS PubMed
.
-
(a) N. K. Beyeh, M. Cetina, M. Lofman, M. Luostarinen, A. Shivanyuk and K. Rissanen, Supramol. Chem., 2010, 22, 737–750 CrossRef CAS
; (b) N. K. Beyeh, A. Ala-Korpi, F. Pan, H. H. Jo, E. V. Anslyn and K. Rissanen, Chem. – Eur. J., 2015, 21, 9556–9562 CrossRef CAS PubMed
; (c) F. Pan, N. K. Beyeh and K. Rissanen, RSC Adv., 2015, 5, 57912–57916 RSC
; (d) F. Pan, N. K. Beyeh, S. Bertella and K. Rissanen, Chem. – Asian J., 2016, 11, 782–788 CrossRef CAS PubMed
.
-
(a) F. Pan, N. K. Beyeh and K. Rissanen, J. Am. Chem. Soc., 2015, 137, 10406–10413 CrossRef CAS PubMed
; (b) N. K. Beyeh, A. Valkonen, S. Bhowmik, F. Pan and K. Rissanen, Org. Chem. Front., 2015, 2, 340–345 RSC
; (c) N. K. Beyeh, F. Pan, S. Bhowmik, T. Makela, R. H. A. Ras and K. Rissanen, Chem. – Eur. J., 2016, 22, 1355–1361 CrossRef CAS PubMed
.
- N. K. Beyeh, A. Ala-Korpi, M. Cetina, A. Valkonen and K. Rissanen, Chem. – Eur. J., 2014, 20, 15144–15150 CrossRef CAS PubMed
.
- N. K. Beyeh, F. Pan and R. H. A. Ras, Asian J. Org. Chem., 2016 DOI:10.1002/ajoc.201600187
.
- T. Amaya and J. J. Rebek, J. Am. Chem. Soc., 2004, 126, 14149–14156 CrossRef CAS PubMed
.
- T. Akutagawa, Y. Abe, Y. Nezu, T. Nakamura, M. Kataoka, A. Yamanaka, K. Inoue, T. Inabe, C. A. Christensen and J. Becher, Inorg. Chem., 1998, 37, 2330–2331 CrossRef CAS
.
- T.-Y. Dong, H.-M. Lin, M.-Y. Hwang, T.-Y. Lee, L.-H. Tseng, S.-M. Peng and G. Lee, J. Organomet. Chem., 1991, 414, 227–244 CrossRef CAS
.
-
(a) J. P. M. Lommerse, A. J. Stone, R. Taylor and F. H. J. Allen, J. Am. Chem. Soc., 1996, 118, 3108–3116 CrossRef CAS
; (b) L. Brammer, E. A. Bruton and P. Sherwood, Cryst. Growth Des., 2001, 1, 277–290 CrossRef CAS
; (c) F. Zordan, L. Brammer and L. P. Sherwood, J. Am. Chem. Soc., 2005, 127, 5979–5989 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and details of the crystallographic study. CCDC 1481996–1481999. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ce01229a |
| This journal is © The Royal Society of Chemistry 2016 |