Open Access Article
Open Access ArticleOne-dimensional supramolecular columnar structure of trans-syn-trans-dicyclohexano[18]crown-6 and organic ammonium cations†
Yu
Ohshima
a,
Kazuya
Kubo
*ab,
Takashi
Matsumoto
c,
Heng-Yun
Ye
d,
Shin-ichiro
Noro
ab,
Tomoyuki
Akutagawa
e and
Takayoshi
Nakamura
*ab
aGraduate School of Environmental Science, Hokkaido University, N10W5, Kita-ku, Sapporo, Hokkaido 060-0810, Japan. E-mail: kkubo@es.hokudai.ac.jp; tnaka@es.hokudai.ac.jp; Fax: +81 11 706 9420; Tel: +81 11 706 9419
bResearch Institute for Electronic Science, Hokkaido University, N20W10, Kita-ku, Sapporo, Hokkaido 001-0020, Japan
cApplication Laboratories, Rigaku Corporation, 3-9-12 Matsubara-cho, Akishima, Tokyo 196-8666, Japan
dOrdered Matter Science Research Center, Southeast University, Nanjing 211189, PR China
eInstitute of Multidisciplinary Research for Advanced Materials, Tohoku University, 1-1 Katahira, Aoba-ku, Sendai, Miyagi 980-8577, Japan
First published on 19th July 2016
Abstract
Supramolecular crystals having one-dimensional (1d) columnar structures were constructed by using supramolecules based on tst-DCH[18]crown-6 (tst-DCH = trans-syn-trans-dicyclohexano). In the crystal of (Ani+)(tst-DCH[18]crown-6)[Ni(dmit)2]− (1) and (m-FAni+)(tst-DCH[18]crown-6)[Ni(dmit)2]− (2) (Ani+ = anilinium+, m-FAni+ = m-fluoroanilinium+, dmit2− = 2-thioxo-1,3-dithiole-4,5-dithiolate), the tst-DCH[18]crown-6 formed supramolecular cations with Ani+ and m-FAni+, respectively, through N–H⋯O hydrogen bonds. The planar conformation of tst-DCH[18]crown-6 molecules in the crystals provided bidirectional hydrogen bonding from the upper and lower side of the molecule that were the driving forces for the construction of the 1d supramolecular architecture. The results provided a method to control arrangements of crown ether-based supramolecules in 1d columns that could be used for channels and/or molecular nanomachines such as molecular rotators.
Introduction
Supramolecular chemistry provides molecular assembly with a complicated structure, which traditional organic chemistry cannot achieve, and which exerts a function that a single molecule does not possess.1 The driving force for obtaining supramolecular architecture is non-covalent bonding soft interactions such as van der Waals, π–π, dipole–dipole and hydrogen-bonding interactions.2 Molecular assemblies such as catenane and rotaxane, which are typical examples of supramolecular architectures, are used as essential units to realize “molecular nanomachines”.3 Molecular nanomachines are distributed universally in biological organisms; for example, adenosine triphosphate (ATP) synthases achieve a highly efficient energy conversion by using molecular rotation.4 The conversion efficiency approaches nearly 100%, far exceeding the value of existing devices such as fuel cells. Based on this fact, molecular nanomachines composed of artificial organic molecules are expected to exhibit functions that simple organic molecules do not achieve. Crown ether is known as a typical example of a building block for supramolecular architectures. Crown ether has a cyclic structure in which a negative charge induced by polar oxygen atoms is gathered and captures cations that have corresponding sizes.5 The function of crown ether has provided intriguing molecular nanomachines such as a molecular shuttle, molecular elevator and molecular tweezer.6 Crown ether is one of the most important molecules in supramolecular chemistry.Previously, we reported on a solid-state molecular rotator constructed by a DB[18]crown-6 (DB = dibenzo) and m-FAni+ (m-FAni+ = m-fluoroanilinium+) cation. In a crystal of (m-FAni+)(DB[18]crown-6)[Ni(dmit)2]− (dmit2− = 2-thioxo-1,3-dithiole-4,5-dithiolate), a m-FAni+ cation and a DB[18]crown-6 molecule form a supramolecular cation through hydrogen bonds between an ammonium group and oxygen atoms.7 The supramolecular cation forms a two-dimensional (2d) layer through CH–π interactions between the DB[18]crown-6 molecules, decreasing the steric hindrance around the m-FAni+ cation by ejecting the counter anions out of the layer. As a result, the m-FAni+ cation could rotate around its C–N bond in the solid state. As the dipole moment induced by the F atom could follow an external electric field, the polarization of the crystal caused by the inversion of the dipole moment expresses ferroelectricity. The driving force for constructing the molecular rotator were two intermolecular interactions of DB[18]crown-6, namely, the hydrogen bonds and the CH–π interaction. Actually, 2d layers composed of supramolecular cations orientated similarly were observed in numerous crystals, such as (X)(DB[18]crown-6)[Ni(dmit)2]− (X = m-FAni+, anilinium+, adamantylammonium+, 4-methyl-3-fluoroanilinium+).8,9 However, if a crown ether except for DB[18]crown-6, was used, the supramolecular structures did not always form 2d-layer structures suitable for molecular rotation. For example, the (Ani+)([18]crown-6)[Ni(dmit)2]− (Ani+ = anilinium+) crystal was reported to have two polymorphs, A and B.10 In polymorph A, (Ani+)([18]crown-6) supramolecular cations with the same orientation were stacked one-dimensionally. In contrast, in polymorph B, two supramolecular cations aligned in an antiparallel manner, forming a 2d layer. In the crystal of (Ani+)(csc-DCH[18]crown-6)[Ni(dmit)2]− (csc-DCH = cis-syn-cis-dicyclohexano), the supramolecular cation formed a 2d layer.8 However, the (Ani+)(csc-DCH[18]crown-6) supramolecular cation was directed out of the layer, although the (Ani+)([18]crown-6) supramolecular cation was directed parallel to the layer in polymorph B of (Ani+)([18]crown-6)[Ni(dmit)2]−. Moreover, (adamantylammonium+)([18]crown-6)[Ni(dmit)2]− and (adamantylammonium+)(csc-DCH[18]crown-6)[Ni(dmit)2]− have round-shaped adamantylammonium cations and the supramolecular cations did not form 2d layers nor one-dimensional (1d) columns, they were just located in such a way as to fill the spaces between [Ni(dmit)2]− anion.8 The variation in arrangement in the latter case could be attributed to the absence of a driving force that is sufficient for the control of molecular arrangements. The control of driving forces is essential for constructing assembly structures of supramolecules in the solid state.
In this study, we report 1d columnar structures of supramolecules by using tst-DCH[18]crown-6 (tst-DCH = trans-syn-trans-dicyclohexano), a stereoisomer of csc-DCH[18]crown-6.11 The two cyclohexane rings of tst-DC[18]crown-6 were substituted at equatorial positions. As a result, tst-DCH[18]crown-6 takes a planar conformation belonging to the C2h point group, unlike csc-DCH[18]crown-6. Moreover, the tst-DCH[18]crown-6 has no particular sites for relatively strong intermolecular interactions such as π–π and CH–π interactions like DB[18]crown-6. These characteristics of the tst-DCH[18]crown-6 were the key to forming the supramolecular cation columns in the crystals of (Ani+)(tst-DCH[18]crown-6)[Ni(dmit)2]− (1) and (m-FAni+)(tst-DCH[18]crown-6)[Ni(dmit)2]− (2). We will describe the structures of 1 and 2, and discuss the 1d columnar structures of the supramolecular cations in terms of the conformation and symmetry of tst-DCH[18]crown-6.
Experimental
General
Elemental analysis was carried out using a J-Science Lab Micro Corder JM10 (for 1) and a Yanaco CHN Corder MT-6 (for 2) at the Instrumental Analysis Division, Equipment Management Center, Creative Research Institution, Hokkaido University. (Ani+)(BF4−), (m-FAni+)(BF4−), tst-DCH[18]crown-6 and (n-Bu4N+)[Ni(dmit)2]− were prepared according to a literature procedure (Scheme 1).8,11,12Preparation of (Ani+)(tst-DCH[18]crown-6)[Ni(dmit)2]− (1) and (m-FAni+)(tst-DCH[18]crown-6)[Ni(dmit)2]− (2)
Single crystals of 1 and 2 were obtained by a standard diffusion method in an H-shaped cell (50 mL). A solution of (n-Bu4N+)[Ni(dmit)2]− (25 mg, 48 μmol) in CH3CN (30 mL) was carefully poured into one side of the H-shaped cell, and the tst-DCH[18]crown-6 (140 mg, 376 μmol) and XBF4− (1: X = Ani+ (30 mg, 166 μmol), 2: X = m-FAni+ (30 mg, 151 μmol)) in CH3CN (20 mL) were added into the other side. After one week, black-coloured single crystals were obtained. Elemental analysis: 1: calc. for C32H44O6NNiS10 (918.00): C, 41.87; H, 4.83; N, 1.53%; found: C, 41.82; H, 4.69; N, 1.59%, 2: calc. for C32H43O6NFNiS10 (936.05): C, 41.06; H, 4.63; N, 1.50%; found: C, 40.89; H, 4.64; N, 1.42%.Crystal structure determination
Crystallographic data for single crystals of 1 and 2 were collected by using a Rigaku R-AXIS RAPID diffractometer with MoKα radiation (λ = 0.71075 Å) from a graphite monochromator at 173 K. Structural refinements were carried out using the full-matrix least-squares method on F2. The calculations were performed using the Crystal Structure software package and Yadokari-XG.13 The parameters were refined using anisotropic temperature factors, except for the m-FAni+ molecules and hydrogen atoms, which were refined using the riding model with a fixed C−H bond distance of 0.95 Å. The crystallographic data for 1 and 2 at 173 K are summarized in Table S1.† CCDC 1473486 (1) and 1473487 (2) contain the supplementary crystallographic data for this paper.Calculations
The overlap integrals (s) between the LUMOs of the [Ni(dmit)2]− anions were calculated with the tight-binding approximation by the extended Hückel method.14 Semi-empirical parameters for the Slater-type atomic orbitals were obtained from the literature.15 Transfer integrals (t) were obtained from the equation t = −10s.The relative energy of the model structures was calculated using the RHF/6-31(d) basis set. The nearest-neighbouring [Ni(dmit)2]− anions around (m-FAni+)(tst-DCH[18]crown-6) were included in the calculations of the potential energy curves. Atomic coordinates based on the X-ray crystal structural analysis were used for the calculations. The relative energy of the model structures was obtained by evaluating the rigid rotation of m-FAni+ around the C–N bond. Rotations were carried out at every 30°, and the relative energies were calculated using fixed atomic coordinates.
Magnetic susceptibility
The temperature-dependent magnetic susceptibilities of 1 and 2 were measured using a Quantum Design MPMS-XL SQUID magnetometer for the polycrystalline samples. The DC magnetic susceptibility was measured at temperatures from 2 to 300 K in an applied field of 1 T.Results and discussion
Crystal structure of 1
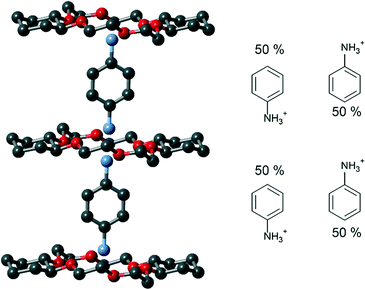
The crystal system and space group of 1 were triclinic and P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , respectively. In 1, a 1d columnar assembly along the a-axis was constructed by the alternative stack of the Ani+ cation and the tst-DCH[18]crown-6 molecule. Four [Ni(dmit)2]− anions surrounded the Ani+ cation, filling the spaces between the 1d supramolecular cations (Fig. 1b). Ani+ and the tst-DCH[18]crown-6 formed a supramolecular cation through hydrogen bonding in the [–(Ani+)-(tst-DCH[18]crown-6)–]n column (Fig. 1a). The Ani+ cation showed an orientation disorder of the Ani+ cation with 180° inversion along the a-axis (Fig. 2). The Ani+ cation was sandwiched between two tst-DCH[18]crown-6 molecules. Each tst-DCH[18]crown-6 molecule formed hydrogen bonds with the disordered ammonium moiety. Six O atoms of the tst-DCH[18]crown-6 approached the ammonium moiety of Ani+ at distances of 2.8–3.1 Å, which are the typical N–H⋯O hydrogen bond distances (Fig. S1†).16
, respectively. In 1, a 1d columnar assembly along the a-axis was constructed by the alternative stack of the Ani+ cation and the tst-DCH[18]crown-6 molecule. Four [Ni(dmit)2]− anions surrounded the Ani+ cation, filling the spaces between the 1d supramolecular cations (Fig. 1b). Ani+ and the tst-DCH[18]crown-6 formed a supramolecular cation through hydrogen bonding in the [–(Ani+)-(tst-DCH[18]crown-6)–]n column (Fig. 1a). The Ani+ cation showed an orientation disorder of the Ani+ cation with 180° inversion along the a-axis (Fig. 2). The Ani+ cation was sandwiched between two tst-DCH[18]crown-6 molecules. Each tst-DCH[18]crown-6 molecule formed hydrogen bonds with the disordered ammonium moiety. Six O atoms of the tst-DCH[18]crown-6 approached the ammonium moiety of Ani+ at distances of 2.8–3.1 Å, which are the typical N–H⋯O hydrogen bond distances (Fig. S1†).16
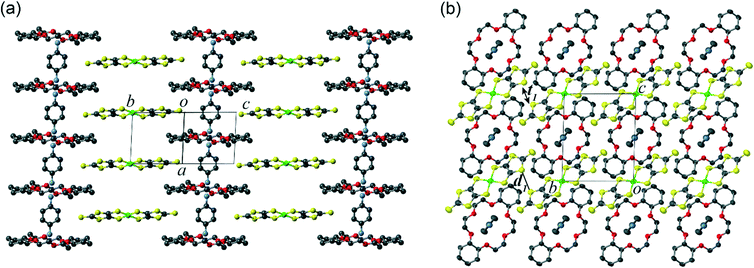 | ||
| Fig. 1 Crystal structure of 1 at 173 K viewed along (a) (b+c)-axis and (b) a-axis (d1 = 3.4322(7) Å, t1 = −6.6 meV). | ||
The tst-DCH[18]crown-6 molecule had a highly planar conformation, where all non-hydrogen atoms were located within 0.579 Å from the mean plane. The conformation showed a keen difference with DB[18]crown-6, in which the farthest non-hydrogen atom of DB[18]crown-6 was located 1.336 Å away from the molecular mean plane.8 The resulting V-shaped conformation enabled the CH–π interaction between the benzene rings in the (Ani+)(DB[18]crown-6)[Ni(dmit)2]− crystal to form a 2d layer structure. In the (Ani+)(csc-DHC[18]crown-6)[Ni(dmit)2]− crystal, the csc-DHC[18]crown-6 molecule had a distorted conformation because of the axial substitution of the cyclohexane ring. The farthest non-hydrogen atom was located 1.336 Å away from the molecular mean plane.8
Because of the planarity of tst-DCH[18]crown-6, the Ani+ cation can form hydrogen bonds from the upper and lower sides of the molecule. The molecular inversion centre of the tst-DCH[18]crown-6, which belongs to the C2h point group, was identical with the inversion centre of the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group of the crystal. As a result, a crystallographically equivalent environment was achieved on both sides of the tst-DCH[18]crown-6 molecule. The DB[18]crown-6 molecule, which belongs to the D2h point group, has a mirror symmetry plane parallel to the molecular plane and an inversion centre. However, the DB[18]crown-6 molecule formed a V-shaped conformation forming intermolecular CH–π interaction in the (Ani+)(DB[18]crown-6)[Ni(dmit)2]− crystal. Thus, the inversion centre of the C2/c space group did not locate in the DB[18]crown-6 molecule, which resulted in unidirectional hydrogen bonding. As for csc-DHC[18]crown-6, which belongs to the C2v point group, no inversion centre and mirror plane parallel to the molecular plane exist. Therefore, the csc-DHC[18]crown-6 could form only unidirectional hydrogen bonding.
space group of the crystal. As a result, a crystallographically equivalent environment was achieved on both sides of the tst-DCH[18]crown-6 molecule. The DB[18]crown-6 molecule, which belongs to the D2h point group, has a mirror symmetry plane parallel to the molecular plane and an inversion centre. However, the DB[18]crown-6 molecule formed a V-shaped conformation forming intermolecular CH–π interaction in the (Ani+)(DB[18]crown-6)[Ni(dmit)2]− crystal. Thus, the inversion centre of the C2/c space group did not locate in the DB[18]crown-6 molecule, which resulted in unidirectional hydrogen bonding. As for csc-DHC[18]crown-6, which belongs to the C2v point group, no inversion centre and mirror plane parallel to the molecular plane exist. Therefore, the csc-DHC[18]crown-6 could form only unidirectional hydrogen bonding.
The planarity of tst-DCH[18]crown-6 and its ability for bidirectional hydrogen bonding should be the driving force for the construction of a 1d supramolecular cation column. Since the composition of Ani+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tst-DCH[18]crown-6 in 1 was 1
tst-DCH[18]crown-6 in 1 was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Ani+ was disordered in the crystal with the C–N bond facing in the +a and −a directions. The Ani+ and tst-DCH[18]crown-6 molecules were stacked alternately to form a 1d supramolecular column along the a-axis. The absence of large intermolecular interactions between the tst-DCH[18]crown-6 molecules was also a key to forming a 1d column. Intermolecular interactions such as the CH–π interaction of DB[18]crown-6 could induce a V-shaped conformation, which can be the driving force to forming 2d layers of supramolecular cations. The tst-DCH[18]crown-6 had no particular site for relatively strong interaction with the adjacent crown ether molecules. The bidirectional hydrogen bonding mainly contributed to controlling the arrangement of the supramolecular cations. In addition, the large cyclohexane ring had an important role in stabilizing the columnar structure. The non-substituted [18]crown-6 has the same inversion symmetry and planarity as the tst-DCH[18]crown-6. However, [18]crown-6 can take various arrangements in the crystal. Indeed, multiple polymorphs of the (Ani+)([18]crown-6)[Ni(dmit)2]− crystal have already been reported.10 A relatively large cyclohexane substituent of tst-DCH[18]crown-6 prevented packing arrangements other than a columnar structure.
1, Ani+ was disordered in the crystal with the C–N bond facing in the +a and −a directions. The Ani+ and tst-DCH[18]crown-6 molecules were stacked alternately to form a 1d supramolecular column along the a-axis. The absence of large intermolecular interactions between the tst-DCH[18]crown-6 molecules was also a key to forming a 1d column. Intermolecular interactions such as the CH–π interaction of DB[18]crown-6 could induce a V-shaped conformation, which can be the driving force to forming 2d layers of supramolecular cations. The tst-DCH[18]crown-6 had no particular site for relatively strong interaction with the adjacent crown ether molecules. The bidirectional hydrogen bonding mainly contributed to controlling the arrangement of the supramolecular cations. In addition, the large cyclohexane ring had an important role in stabilizing the columnar structure. The non-substituted [18]crown-6 has the same inversion symmetry and planarity as the tst-DCH[18]crown-6. However, [18]crown-6 can take various arrangements in the crystal. Indeed, multiple polymorphs of the (Ani+)([18]crown-6)[Ni(dmit)2]− crystal have already been reported.10 A relatively large cyclohexane substituent of tst-DCH[18]crown-6 prevented packing arrangements other than a columnar structure.
Crystal structure of 2
A 1d supramolecular cation column was formed in 2 as well as in 1. The crystal system and the space group were triclinic and P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , respectively. The m-FAni+ and the tst-DCH[18]crown-6 were stacked alternately, forming a [–(m-FAni+)-(tst-DCH[18]crown-6)–]n columnar structure (Fig. 3a). There were three crystallographically independent tst-DCH[18]crown-6 molecules, all of which had planar conformations (Fig. 4, represented as crowns i, ii and iii). All non-hydrogen atoms of crown ethers i, ii and iii were located within 0.465, 0.647 and 0.681 Å from their molecular mean plane, respectively.
, respectively. The m-FAni+ and the tst-DCH[18]crown-6 were stacked alternately, forming a [–(m-FAni+)-(tst-DCH[18]crown-6)–]n columnar structure (Fig. 3a). There were three crystallographically independent tst-DCH[18]crown-6 molecules, all of which had planar conformations (Fig. 4, represented as crowns i, ii and iii). All non-hydrogen atoms of crown ethers i, ii and iii were located within 0.465, 0.647 and 0.681 Å from their molecular mean plane, respectively.
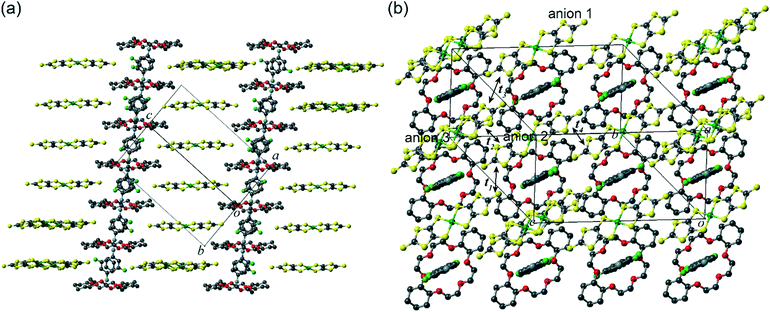 | ||
Fig. 3 Crystal structure of 2 at 173 K viewed along (a) (a+b)-axis and (b) [1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 2] direction (t1 = −2.91 meV, t2 = 14.28 meV, t3 = 0.12 meV, t4 = −0.04 meV). 2] direction (t1 = −2.91 meV, t2 = 14.28 meV, t3 = 0.12 meV, t4 = −0.04 meV). | ||
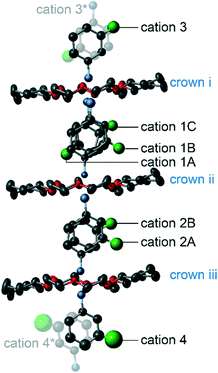 | ||
| Fig. 4 Molecular arrangement of the crystallographically independent supramolecular cation column unit in 2. | ||
As the m-FAni+ cation had an F atom, complicated disorders based on the directions of the ammonium moiety and the F atom were observed. There were four crystallographically independent cation sites in 2 (Fig. 4, represented as cations 1, 2, 3 and 4). The m-FAni+ cations and the tst-DCH[18]crown-6 molecules were stacked alternately, forming a supramolecular cation unit of (3–i–1–ii–2–iii–4). In the cation 1 site, that was sandwiched by crowns i and ii, the m-FAni+ cation was disordered at three positions (1A, 1B and 1C) with the occupancy factors 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The cations 1A and 1B formed hydrogen bonds with crown i, whereas cation 1C formed hydrogen bonds with crown ii. As a result, cation 1C was oriented at an angle of 180° with respect to 1A and 1B in the columnar direction. Moreover, the F atom of cations 1A and 1B was oriented at an angle of 180° with respect to the rotation around the C–N bond. At the cation 2 site, sandwiched by crowns i, ii and iii, the m-FAni+ cation was disordered between two positions (2A and 2B) with the occupancy factor 1
2. The cations 1A and 1B formed hydrogen bonds with crown i, whereas cation 1C formed hydrogen bonds with crown ii. As a result, cation 1C was oriented at an angle of 180° with respect to 1A and 1B in the columnar direction. Moreover, the F atom of cations 1A and 1B was oriented at an angle of 180° with respect to the rotation around the C–N bond. At the cation 2 site, sandwiched by crowns i, ii and iii, the m-FAni+ cation was disordered between two positions (2A and 2B) with the occupancy factor 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The ammonium moiety of 2A and 2B formed hydrogen bonds with crowns ii and iii, respectively. Cation 3 and 4 were located on the inversion centres and were disordered with respect to the inversion with the occupancy factor of 50%. Thus, the 1d column is represented as [–3–i–1–ii–2–iii–4–iii–2–ii–1–i–]n. There were three crystallographically independent [Ni(dmit)2]− anions (Fig. 3 and S2,† represented as anions 1, 2 and 3). Four [Ni(dmit)2]− anions surrounded the m-FAni+ cation, filling the spaces between the 1d supramolecular cations, as in the case of 1.
1. The ammonium moiety of 2A and 2B formed hydrogen bonds with crowns ii and iii, respectively. Cation 3 and 4 were located on the inversion centres and were disordered with respect to the inversion with the occupancy factor of 50%. Thus, the 1d column is represented as [–3–i–1–ii–2–iii–4–iii–2–ii–1–i–]n. There were three crystallographically independent [Ni(dmit)2]− anions (Fig. 3 and S2,† represented as anions 1, 2 and 3). Four [Ni(dmit)2]− anions surrounded the m-FAni+ cation, filling the spaces between the 1d supramolecular cations, as in the case of 1.
As the tst-DCH[18]crown-6 molecule had the planar conformation, the steric hindrance between m-FAni+ and tst-DCH[18]crown-6 was small and the m-FAni+ cation could have a variety of arrangements that affected those of anions. The [Ni(dmit)2]− anion 3, surrounding m-FAni+, had a disorder with displacement along the molecular plane at a distance of 2.495(5) Å. Because the F atoms of cations 1B, 1C, 2A and 2B had the same direction, a dipole moment perpendicular to the columnar direction was induced. However, inversion of the dipole moment by molecular rotation was restricted because of the steric hindrance between the m-FAni+ cation and the neighbouring [Ni(dmit)2]− anion. The rotational potential of the m-FAni+ cation in 2, evaluated from the crystal structure and ab initio calculations, indicated much higher potential barrier (Fig. S5†) than that in (m-FAni+)(DB[18]crown-6)[Ni(dmit)2]−.
Magnetic properties
In both 1 and 2, the [Ni(dmit)2]− anions had side-by-side contacts, forming 2d layers perpendicular to the supramolecular columnar direction (Fig. 1b and 3b). In 1, the S–S distance represented as d1 (3.4322(7) Å) was shorter than the sum of the van der Waals radii (3.7 Å). The transfer integral between the lowest unoccupied molecular orbitals (LUMOs) of the [Ni(dmit)2]− anions was t1 = −6.6 meV, indicating an intermolecular interaction along the b-axis. In 2, the S–S distances represented as d1–d5 in Fig. S2† were shorter than 3.7 Å. The transfer integrals between the [Ni(dmit)2]− anions were t1 = −2.91, t2 = 14.28, t3 = 0.12 and t4 = −0.04 meV, indicating an intermolecular interaction in the molecular plane. The crystals 1 and 2 showed antiferromagnetic properties of the Curie–Weiss type. The Curie constants (C) and the Weiss temperatures (θ) were C = 0.46 emu mol−1, θ = −0.18 K in 1 and C = 0.33 emu mol−1, θ = −0.73 K in 2, respectively (Fig. S4†).Conclusions
In this study, we synthesized (Ani+)(tst-DCH[18]crown-6)[Ni(dmit)2]− (1), (m-FAni+)(tst-DCH[18]crown-6)[Ni(dmit)2]− (2) (Ani+ = anilinium+, m-FAni+ = m-fluoroanilinium+, tst-DCH = trans-syn-trans-dicyclohexano) and examined the supramolecular structure induced by the planar tst-DCH[18]crown-6 molecule. The tst-DCH[18]crown-6 molecule had a highly planar conformation in the crystals. The tst-DCH[18]crown-6 drove the columnar arrangements with the arylammonium cations, because of the bidirectional hydrogen bonding ability of tst-DCH[18]crown-6. Further investigation is in progress on the proton transfer through the crown ether using the hydrogen bonding ability of tst-DCH[18]crown-6 to amine and ammonium groups from both the upper and lower sides of the molecular plane. The proton transfer may cause ferroelectricity as in the cases of croconic acid, phenazine/chloranil and diazabicyclo[2]octane.17–19Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research from The Ministry of Education, Culture, Sports, Science and Technology (MEXT) of JAPAN and the Japan Society for the Promotion of Science (JSPS) Core-to-Core Program, A. Advanced Research Network.Notes and references
- J.-M. Lehn, Angew. Chem., Int. Ed. Engl., 1988, 27, 89 CrossRef.
- J.-M. Lehn, Supramolecular Chemistry, Weinheim, VCH, 1995 Search PubMed.
- (a) S. Erbas-Cakmak, D. A. Leigh, C. T. McTernan and A. L. Nussbaumer, Chem. Rev., 2015, 115, 10081 CrossRef CAS PubMed; (b) T. R. Kelly, H. De Silva and R. A. Silva, Nature, 1999, 401, 150 CrossRef CAS PubMed; (c) D. A. Leigh, J. K. Y. Wong, F. Dehez and F. Zerbetto, Nature, 2003, 424, 174 CrossRef CAS PubMed; (d) N. Koumura, R. W. Zijlstra, R. A. van Delden, N. Harada and B. L. Feringa, Nature, 1999, 401, 152 CrossRef CAS PubMed.
- (a) L. Stryer, Biochemistry, Freeman, New York, 1995 Search PubMed; (b) M. Schliwa and G. Woehlke, Nature, 2003, 422, 759 CrossRef CAS PubMed; (c) Y. Ishii, M. Nishiyama and T. Yanagida, Curr. Protein Pept. Sci., 2004, 5, 81 CrossRef CAS PubMed; (d) M. Yoshida, E. Muneyuki and T. Hisabori, Nat. Rev. Mol. Cell Biol., 2001, 2, 669 CrossRef CAS PubMed; (e) H. Noji, R. Yasuda, M. Yoshida and K. Kinosita, Nature, 1997, 386, 299 CrossRef CAS PubMed.
- C. J. Pedersen, J. Am. Chem. Soc., 1967, 89, 7017 CrossRef CAS.
- (a) C. Lemouchi, C. S. Vogelsberg, L. Zorina, S. Simonov, P. Batail, S. Brown and M. A. Garcia-Garibay, J. Am. Chem. Soc., 2011, 133, 6371 CrossRef CAS PubMed; (b) A. Coskun, M. Banaszak, R. D. Astumian, J. F. Stoddart and B. A. Grzybowski, Chem. Soc. Rev., 2012, 41, 19 RSC; (c) G. J. E. Davidson and S. J. Loeb, Angew. Chem., Int. Ed., 2003, 42, 74 CrossRef CAS; (d) D. J. Hoffart and S. J. Loeb, Angew. Chem., Int. Ed., 2005, 44, 901 CrossRef CAS PubMed; (e) V. N. Vukotic, K. J. Harris, K. Zhu, R. W. Schurko and S. J. Loeb, Nat. Chem., 2012, 4, 456 CrossRef CAS PubMed; (f) K. Zhu, V. N. Vukotic, C. A. O'Keefe, R. W. Schurko and S. J. Loeb, J. Am. Chem. Soc., 2014, 136, 7403 CrossRef CAS PubMed; (g) J. D. Badjic, V. Balzani, A. Credi, S. Silvi and J. F. Stoddart, Science, 2004, 303, 1845 CrossRef CAS PubMed; (h) S. Shinkai, T. Nakaji, T. Ogawa, K. Shigematsu and O. Manabe, J. Am. Chem. Soc., 1981, 103, 111 CrossRef CAS.
- T. Akutagawa, H. Koshinaka, D. Sato, S. Takeda, S. Noro, H. Takahashi, R. Kumai, Y. Tokura and T. Nakamura, Nat. Mater., 2009, 8, 342 CrossRef CAS PubMed.
- T. Akutagawa, D. Sato, H. Koshinaka, M. Aonuma, S. Noro, S. Takeda and T. Nakamura, Inorg. Chem., 2008, 47, 5951 CrossRef CAS PubMed.
- Z. Liu, K. Kubo, S. Noro, T. Akutagawa and T. Nakamura, Cryst. Growth Des., 2014, 14, 537 CAS.
- S. Nishihara, T. Akutagawa, T. Hasegawa, S. Fujiyama, T. Nakamura and T. Nakamura, J. Solid State Chem., 2002, 168, 661 CrossRef CAS.
- K. Yamato, R. A. Bartsch, M. L. Dietz and R. D. Rogers, Tetrahedron Lett., 2002, 43, 2153 CrossRef CAS.
- G. Steimecke, H.-J. Sieler, R. Kirmse and E. Hoyer, Phosphorous Sulfur Relat. Elem., 1979, 7, 49 CrossRef CAS.
- (a) M. C. Burla, R. Caliandro, M. Camalli, B. Carrozzini, G. L. Cascarano, L. De Caro, C. Giacovazzo, G. Polidori and R. Spagna, J. Appl. Crystallogr., 2005, 38, 381 CrossRef CAS; (b) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed; (c) C. Kabuto, S. Akine and E. Kwon, Nippon Kessho Gakkaishi, 2009, 51, 218 CrossRef; (d) Crystal Structure: Single crystal structure analysis software, Ver. 4.1, Rigaku Corporation and Molecular Structure Corporation Search PubMed.
- T. Mori, A. Kobayashi, Y. Sasaki, H. Kobayashi, G. Saito and H. Inokuchi, Bull. Chem. Soc. Jpn., 1984, 57, 627 CrossRef CAS.
- (a) T. A. Albright, P. Hofmann and R. Hoffmann, J. Am. Chem. Soc., 1977, 99, 7546 CrossRef CAS; (b) K. A. Jørgensen, R. A. Wheeler and R. Hoffmann, J. Am. Chem. Soc., 1987, 109, 3240 CrossRef; (c) D. A. Keszler and R. Hoffmann, J. Am. Chem. Soc., 1987, 109, 118 CrossRef CAS.
- (a) G. A. Jeffrey, An Introduction to Hydrogen Bonding, ed. D. G. Truhla, Oxford University Press, New York, 1997 Search PubMed; (b) G. C. Pimentel and A. L. McClellan, The Hydrogen Bond, Freemann, San Francisco, 1960 Search PubMed; (c) W. C. Hamilton and J. A. Ibers, Hydrogen Bonding in Solids, ed. R. Breslow, Benjamin Inc, New York, 1968 Search PubMed.
- S. Horiuchi, Y. Tokunaga, G. Giovannetti, S. Picozzi, H. Itoh, R. Shimano, R. Kumai and Y. Tokura, Nature, 2010, 463, 789–792 CrossRef CAS PubMed.
- (a) S. Horiuchi, F. Ishii, R. Kumai, Y. Okimoto, H. Tachibana, N. Nagaosa and Y. Tokura, Nat. Mater., 2005, 4, 163 CrossRef CAS PubMed; (b) R. Kumai, S. Horiuchi, H. Sagayama, T.-H. Arima, M. Watanabe, Y. Noda and Y. Tokura, J. Am. Chem. Soc., 2007, 129, 12920 CrossRef CAS PubMed; (c) S. Horiuchi, R. Kumai and Y. Tokura, J. Am. Chem. Soc., 2005, 127, 5010 CrossRef CAS PubMed.
- (a) M. Szafrański and A. Katrusiak, Chem. Phys. Lett., 2000, 318, 427 CrossRef; (b) M. Szafrański and A. Katrusiak, J. Phys. Chem. B, 2004, 108, 15709 CrossRef; (c) M. Szafrański and A. Katrusiak, J. Phys. Chem. B, 2008, 112, 6779 CrossRef PubMed; (d) A. Katrusiak and M. Szafrański, Phys. Rev. Lett., 1999, 82, 576 CrossRef CAS; (e) M. Szafrański, A. Katrusiak and G. McIntyre, Phys. Rev. Lett., 2002, 89, 215507 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Crystallographic data, crystal structure, magnetic properties and CIF files of crystals 1 and 2. CCDC 1473486–1473487. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ce00980h |
| This journal is © The Royal Society of Chemistry 2016 |