Open Access Article
Open Access ArticleSingle-crystal to single-crystal conformational polymorphic transformation in tolbutamide at 313 K. Relation to other polymorphic transformations in tolbutamide and chlorpropamide†
T. N.
Drebushchak
*ab,
V. A.
Drebushchak
bc,
N. A.
Pankrushina
d and
E. V.
Boldyreva
a
aInstitute of Solid State Chemistry and Mechanochemistry SB RAS, Kutateladze, 18, Novosibirsk, 630128, Russia. E-mail: tanya@xray.nsu.ru
bNovosibirsk State University, Pirogova, 2, Novosibirsk, 630090, Russia
cV. S. Sobolev Institute of Geology and Mineralogy SB RAS, Pr. Ak. Koptyuga, 3, Novosibirsk, 630090, Russia
dN. N. Vorozhtsov Novosibirsk Institute of Organic Chemistry SB RAS, Pr. Ak. Lavrentieva, 9, Novosibirsk, 630090, Russia
First published on 15th June 2016
Abstract
Three polymorphs of tolbutamide (IL, II, and III) were studied using single-crystal X-ray diffraction, from 100 K to room temperature (forms II and III) and to 350 K (form I), and differential scanning calorimetry. The reversible transformation, IL ⇔ IH, was found to be of the single-crystal to single-crystal type and the structure of the high-temperature form (IH) was solved and refined. The structure of IH differs from that of IL only in the conformation of the molecule, with molecular arrangements being practically unchanged (isostructural conformational transformation). The transition takes place at 313 K with no sign of hysteresis. The volume change, ΔV/V, across the reversible transformation IL ⇔ IH was calculated and compared to those for the other conformational transformations. Two types of conformational polymorphic transformations (irreversible reconstructive and reversible isostructural) in tolbutamide and chlorpropamide were compared.
Introduction
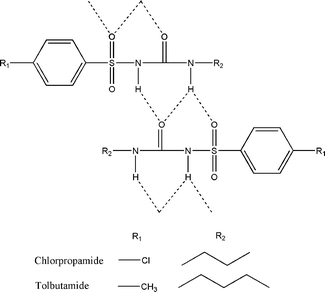
Conformational polymorphism is common among organic crystals.1–3 Polymorphic transformations can be accompanied by a change in the geometry of molecules. If the two conformations correspond to two different local minima on the gas potential energy surface (gas-PES), one refers to this as conformational change; if the two conformations correspond to the same local minimum, this is termed conformational adjustment.1 One can consider two types of conformational polymorphic transformations (accompanied by a conformational change, not conformational adjustment): (1) transformations involving changes in molecular conformations only, with the packing of the molecules preserved (isostructural conformational transformations) and (2) transformations involving changes in both the conformations and packing of molecules, including, in particular, reconstruction of a hydrogen-bond network (reconstructive conformational transformations). A transformation of the first type can proceed without fragmentation of a single crystal (a single-crystal to single-crystal transition). Two sulphonylurea derivatives used in the treatment of type 2 diabetes mellitus, tolbutamide (N-[(butylamino)carbonyl]-4-methylbenzenesulfonamide) and chlorpropamide (4-chloro-N-(propylaminocarbonyl)benzenesulfonamide), provide examples of rich conformational polymorphism (molecular conformations are different in each of their polymorphs).4–18 All known polymorphs of tolbutamide and chlorpropamide contain identical hydrogen-bond motifs, forming infinite chains of molecules of alternating orientation (Scheme 1). Bonded via their central parts, the head and tail regions of the molecules remain flexible. It is therefore not surprising that polymorphs differ mainly in the conformation of the alkyl tail and in the orientation of the benzene ring head.On heating from room temperature, all polymorphs of the two sulphonylureas transform into their respective high-temperature forms (denoted ε for chlorpropamide8 and IH for tolbutamide14,15), each of which is stable up to their melting points. The crystal structure of ε-chlorpropamide has been known for a number of years.7 This high-temperature form, once obtained, can also be preserved at ambient temperature.6,7 The crystal structure of IH tolbutamide has remained undocumented to date (CSD version 5.36).20 The high-temperature polymorph of tolbutamide, contrary to that of chlorpropamide, has never been observed at room temperature. The IL ⇔ IH transformation in tolbutamide is reversible, with the transition temperature close to 40 °C.14,15
Polymorphs of chlorpropamide behave differently during temperature variations. On cooling, β-chlorpropamide undergoes two reversible phase transitions.10 The second phase transition (βII ⇔ βIII) is related to a change in the conformation of the alkyl tail in 1/4 of the molecules across the transition point. If cooled below room temperature, ε-chlorpropamide transforms into a different polymorph, ε′, which differs only in the conformation of the molecule from the parent ε-chlorpropamide crystal structure.9 The α-, γ-, and δ-polymorphs do not undergo phase transitions on cooling.9,19 Form III of tolbutamide transforms reversibly into the form denoted as III2 between 100 and 150 K and is of the single-crystal to single-crystal type.17 The translation symmetry changes, since the conformation in every third molecule changes during the course of the transformation. The crystal structure of form III2 was also reported as being “a high Z′ variation of form III”.16 This polymorph was claimed to be the result of special crystallization conditions, although its crystal structure was also solved originally at 100 K, i.e. below the phase transition point, and only after that – at ambient temperature.
The purpose of this work was to undertake a structural investigation of the three polymorphs of tolbutamide (I, II, and III) over a wide temperature range in order to fill in the gaps in the available structural data. We aimed to solve the crystal structure of the IH high-temperature form of tolbutamide, in order to clarify the nature of this reversible transformation in tolbutamide, as well as to compare the different types of conformational polymorphic transformations on cooling and heating in tolbutamide and chlorpropamide.
Experimental
Single crystals of three tolbutamide polymorphs were grown from solutions of tolbutamide (SIGMA) in different solvents: form I from ethyl acetate, form II from acetonitrile, and form III from a mixture of MeOH–H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The mixtures of three polymorphs often crystallised concomitantly. Single crystals suitable for X-ray diffraction were selected from the crystallized batches under a microscope.
1). The mixtures of three polymorphs often crystallised concomitantly. Single crystals suitable for X-ray diffraction were selected from the crystallized batches under a microscope.
Single-crystal X-ray diffraction was carried out using an Oxford Diffraction Gemini R Ultra diffractometer with a Ruby CCD detector (MoKα radiation). A low-temperature Oxford Instruments Cryojet HT device was used for varying the temperature. CrysAlis software was used for data collection and processing.21 For polymorph IL, unit-cell parameters were determined at temperatures from 100 to 350 K, with temperature steps ranging from 10 to 25 K. Data for structure solution and refinement were collected at 100, 200, 295, and 330 K. Crystal structures were solved by direct methods using SHELXS,22 and refined on F2 with all data using SHELXL23 with anisotropic thermal parameters for all non-hydrogen atoms. Hydrogen atoms were either located in the difference maps or were positioned geometrically and refined with a riding model. Additional restraints were imposed on the distances, and the anisotropic displacement parameters for the alkyl tails of tolbutamide molecules in the structures were refined at 330 K, above the phase transition. At this temperature, the alkyl tails are disordered over two positions (Fig. 1). For polymorph I, all measurements were carried out with the same single crystal for all temperatures; this includes both the IL and IH forms. Selected crystallographic parameters, data collection and refinement details are summarized in Table 1.
| Form IL | Form IH | Form II | |||
|---|---|---|---|---|---|
| Formula | C12H18N2O3S | C12H18N2O3S | C12H18N2O3S | ||
| Formula weight | 270.34 | 270.34 | 270.34 | ||
| Crystal system | Orthorhombic | Orthorhombic | Monoclinic | ||
| Space group | Pna21 | Pna21 | Pc | ||
| T/K | 100 | 200 | 295 | 330 | 100 |
| a/Å | 19.4331(3) | 19.7525(3) | 20.2133(6) | 20.7500(15) | 9.0256(2) |
| b/Å | 7.8010(1) | 7.7998(1) | 7.8233(2) | 7.9160(7) | 17.1443(5) |
| c/Å | 9.0277(1) | 9.0583(1) | 9.0717(2) | 9.0580(5) | 17.7904(5) |
| β/° | 90 | 90 | 90 | 90 | 94.268(3) |
| V/Å3 | 1368.58(3) | 1395.57(3) | 1434.55(6) | 1487.8(2) | 2745.21(13) |
| Z | 4 | 4 | 4 | 4 | 8 |
| D c/g cm−3 | 1.312 | 1.287 | 1.252 | 1.207 | 1.308 |
| μ (Mo Kα)/mm−1 | 0.239 | 0.234 | 0.228 | 0.220 | 0.238 |
| θ range/° | 3.35–36.32 | 3.05–26.37 | 3.29–26.35 | 1.96–26.35 | 3.10–26.37 |
| Measured reflections | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 528 528 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 224 224 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 312 312 |
5834 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 260 |
| Independent reflections | 6600 | 2857 | 2924 | 2781 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 214 214 |
| Reflections with I > 2σ(I) | 6147 | 2741 | 2725 | 1609 | 8469 |
| R int | 0.036 | 0.024 | 0.027 | 0.032 | 0.099 |
| R[F2 > 2σ(F2)] | 0.028 | 0.027 | 0.040 | 0.057 | 0.063 |
| wR(F2) | 0.072 | 0.072 | 0.115 | 0.182 | 0.109 |
Additionally, for the tolbutamide polymorphs, II and III, the data have been collected in the temperature range from ambient to 100 K, with unit cell parameters refined with a step size of 25 K. Data for tolbutamide III at three temperature points (100, 150, and 295 K) were reported previously17 and agreed with the results in the present work. The crystal structure of tolbutamide II at 295 K was refined under the assumption that one in four molecules in the asymmetric unit had a disordered alkyl tail, and thus agreed well with that reported in,15 where it was refined at 153 K. We have also refined the crystal structure of tolbutamide II at 100 K. A model with disorder suggested the occupancy of the second orientation of the alkyl tail below 20%, without a significant improvement in the value of the R-factor. Since the second orientation was also rather close to the main one (conformer adjustment at the most), we have given preference to a model without disorder over two selected positions, but with high values of atomic displacement parameters in general, which is common for many structures with alkyl tails (see Table 1). The unit cell parameters for polymorphs I, II, and III for all temperatures are reported in Table S1 of the ESI.†
The X-ray powder diffraction patterns were recorded on a STOE STADI-MP diffractometer (CuKα1 radiation, curved Ge monochromator, transmission/Debye–Scherrer mode) with a high temperature attachment for capillaries.
The programs WinGX,24 ORTEP3 for Windows,25 WinXPOW,26 and Mercury27 were used for visualization and analysis.
DSC measurements of the IL ⇔ IH transformation were carried out using a DSC-204 Netzsch. Standard aluminum crucibles (25 μL), covered with a lid (but not sealed), were used and a flow of high-purity Ar (26 mL min−1) was employed. Measurements were carried out in a single experiment, cycling heating and cooling at decreasing heating rates of 3, 2, 1, 0.5, and 0.2 K min−1. The sample (1.6 mg powder) was taken from a commercial batch of tolbutamide (SIGMA) without further purification or recrystallization. According to the X-ray powder diffraction pattern, this was the pure tolbutamide form, IL.
Results and discussion
Phase transformation IL ⇔ IH, crystal structure of form IH
The unit cell parameters and space group for form IH at 330 K (Table 1) agree well with those reported by Hasegawa et al. at 363 K,14 if thermal expansion is accounted for. In,14 the crystal structure of IH has been claimed to be solved from X-ray powder diffraction data. Unfortunately, the crystallographic information file and atomic coordinates were not reported by Hasegawa et al.14 and are missing from the Cambridge Structural Database.20 We have for the first time observed a single-crystal to single-crystal transition from IL to IH and have solved the crystal structure of the latter based on single-crystal diffraction data.According to our data, IL and IH differ in the conformation of tolbutamide molecules. Ordered in IL, the tolbutamide alkyl tail becomes disordered across the IL ⇒ IH transformation, split over two positions. The torsion angle, C9–C10–C11–C12, changes by 120° for one position and by 75° for the other. Three of the four alkyl tail atoms attract particular attention when considering this transition. The thermal ellipsoids of C10 and C12 increase significantly in size, while that of C11 splits into two ellipsoids with occupancies of 0.59(3) and 0.41(3). Despite this disordering, the space group remains unchanged across the IL ⇒ IH transformation, and both the packing and hydrogen-bond framework remain largely unchanged. Only a slight displacement of neighboring molecules can be detected (Fig. 2).
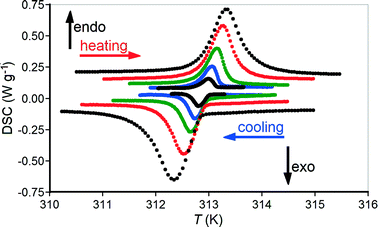
Thermal studies of the IL ⇔ IH transition at different heating/cooling rates (Fig. 3) show endothermic peaks on heating and exothermic peaks on cooling. Usually, the onset temperature on heating is greater than that on cooling because of the thermal inertia in the measuring system. The greater the heating/cooling rate, the greater the thermal inertia and the difference between the onset points, with the onset temperature on cooling always being below the onset temperature on heating. Here, we have a completely different case when the onset temperature of 312.75 K on heating is less than that of 313.0 K on cooling with the smallest heating/cooling rates. Such a case is rather rare and can be explained in terms of randomly distributed transition points among particles in the powder sample; different particles (microcrystals) transform from one polymorph to another at different temperatures. The integral thermal effect of a transformation in a powder appears as a normal distribution in accordance with the central limit theorem. This effect has been discussed for the thermal character of the irreversible γ → α transformation in glycine over a temperature range of approximately 430 to 445 K (Fig. 1 in ref. 28). The temperature interval of 0.25 K between the onset temperatures on heating and cooling for the reversible IL ⇔ IH transformation in tolbutamide I powder is extremely narrow and indicates a mechanism without nucleation. Hasegawa et al.14 reported the transition point at 311 K. Their DSC experiments were carried out at a heating/cooling rate of 10 K min−1, which is too high for accurate measurements of transition points. Furthermore, no information regarding the accuracy of the temperature calibration was reported.14
Temperature-dependence of cell volume
The volume per molecule for the three polymorphs of tolbutamide as a function of temperature is shown in Fig. 4. It becomes evident that the low-temperature phase in tolbutamide III2 (III2 in ref. 17 and the “high Z′ crystal structure of form III” in ref. 16) is the result of a polymorphic transformation occurring between 125 and 150 K. The data in Fig. 4 allowed calculation of the difference in volume (corrected for thermal expansion) across polymorphic transformations, IL ⇔ IH and III2 ⇔ III, in tolbutamide (Table 2). For irreversible transformations, II ⇒ IH , III ⇒ IH, and IV ⇒ IH, in tolbutamide, the increase in the normalized volume (ΔV/V) was calculated as the difference between the volumes of the initial (II, III, and IV) and resulting (IH) polymorphs. The corresponding values for chlorpropamide polymorphs are listed in Table 2 for comparison, with our data borrowed from.5–10 The error in the ΔV/V estimate is less than ±0.2% for all data. There is no obvious difference in the ΔV/V changes between the isostructural and reconstructive transformations in either tolbutamide or chlorpropamide. The smallest volume change has been observed for the transformations that are accompanied by a change in the conformations of only a part of the molecules (βII ⇔ βIII transition in chlorpropamide and III2 ⇔ III in tolbutamide). Still, the isostructural transformations in tolbutamide differ in nature from those in chlorpropamide. Transformations IL ⇔ IH and III2 ⇔ III in tolbutamide are accompanied by disordering of the alkyl tail atoms, in which the low-temperature form is ordered and the high-temperature one is disordered. In chlorpropamide, no disordering of the alkyl tails has been observed, either in the high-temperature or in the low-temperature forms.| ΔV/V × 100% | Type of transformation | T tr/K | Comment | Ref. | |
|---|---|---|---|---|---|
| Tolbutamide | |||||
| IL ⇔ IH | 2.49(8) | Isostructural conformational | 313 | Fixed point, DSC | 15, this work |
| III2 ⇔ III | 0.59(7) | Isostructural conformational | Between 125 and 150 | Fixed point, X-ray | 17 |
| II ⇒ IH | 3.48(8) | Reconstructive conformational | Between 363 and 373 | Kinetic, X-ray | This work |
| III ⇒ IH | 2.13(8) | Reconstructive conformational | Between 353 and 373 | Kinetic, X-ray | This work |
| 379 | DSC | 15 | |||
| IV ⇒ IH | 2.74(9) | Reconstructive conformational | 361 | DSC | 15 |
| Chlorpropamide | |||||
| ε′ ⇔ ε | 4.95(9) | Isostructural conformational | 215 | Fixed point, DSC | This work |
| βII ⇔ βIII | 0.22(7) | Isostructural conformational | Between 125 and 150 | Fixed point, X-ray | 10 |
| α ⇒ ε | 5.45(6) | Reconstructive conformational | 393 | Kinetic, DSC | 8 |
| β ⇒ ε | 1.83(6) | Reconstructive conformational | 398 | Kinetic, DSC | 8 |
| γ ⇒ ε | 2.98(9) | Reconstructive conformational | 390 | Kinetic, DSC | 8 |
| δ ⇒ ε | 5.80(6) | Reconstructive conformational | 393 | Kinetic, DSC | 8 |
Tolbutamide II does not show any polymorphic transformations across the temperature range 100 to 300 K. Its normalized volume above 250 K is the smallest (and correspondingly its density is the largest) among all tolbutamide polymorphs.
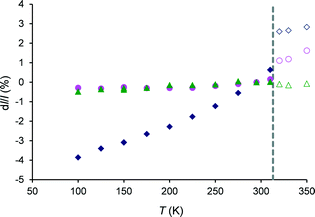
Anisotropy of thermal expansion in tolbutamide I
The relative changes in the unit cell parameters versus temperature are shown for tolbutamide IL and IH in Fig. 5. Both polymorphs are orthorhombic and the principal axes of the strain ellipsoid coincide with the crystallographic axes. The thermal expansion character can be clearly defined by two regions: (1) conventional linear thermal expansion and (2) a singular change. The latter is observed at the transition point, while the remainder of the thermal expansion is characterized as (1). The largest conventional thermal expansion is observed along axis a, which is perhaps not surprising as this does not contain hydrogen bonds (Table 1, Fig. 2). The singular change at the transition point is also observed along axis a (Fig. 5). Singular thermal expansion along other axes (b and c) (at the transition point) is negligible. At the transition point, parameter b increases by over 1%, while parameter c decreases slightly. The hydrogen-bond chains are directed exactly along the c axis (Fig. 2).The temperature dependences of the donor-acceptor distances across hydrogen bonds are shown in Fig. 6. The IL ⇔ IH phase transition is accompanied by very interesting changes in the hierarchy of the bonds. The strongest and the shortest hydrogen bond, N1–H1⋯O3, remains unchanged, but the bifurcated hydrogen bonds, N2–H2⋯O2 and N2–H2⋯O3, change. The distance N2⋯O3 increases and becomes longer than the distance N2⋯O2 in the high-temperature form IH, while in the ambient-temperature form (IL) the distance N2⋯O2 was longer. Similar changes in the hydrogen bonds were seen in α-chlorpropamide across its transformation to the high-pressure polymorph.11
Isostructural conformational and single-crystal-to-single-crystal transformations
We use the term ‘isostructural’ in this paper, as defined by Coles et al.,29 to describe phase transitions without a major change in molecular packing, with particular emphasis on their relation to single-crystal to single-crystal phase transitions. For many molecular crystals, reversible phase transitions that do not break single crystals have been reported.29–33 In this paper, we limit discussion to a comparison of the isostructural transformations in tolbutamide with those in chlorpropamide (Table 2). Transformations IL ⇔ IH and III2 ⇔ III in tolbutamide are indicated in Table 2 as being isostructural and conformational in nature. None of the hydrogen bonds are broken across these transformations; they are reversible, and single crystals preserve their shape and transparency. The transformation ε′ ⇔ ε in chlorpropamide9 is similar to the IL ⇔ IH transformation in tolbutamide (both transformations are accompanied by a simultaneous change in the conformation of the alkyl tails of all molecules). The main difference between these two cases is quantitative, the volume change being 2.5% for tolbutamide and 5.0%, twice as large, for chlorpropamide. This difference leads to a qualitative difference in the behavior of single crystals: a single crystal of ε-chlorpropamide is destroyed after several heating/cooling cycles across the ε′ ⇔ ε transformation, whereas that of tolbutamide is preserved. When discussing the preservation of single crystals over the course of a phase transition, it is important to remember that the size of the crystal may be of crucial importance. For example, a glycine–glutaric acid co-crystal of ∼0.4 mm is preserved across a polymorphic transformation between 200 and 225 K with ΔV/V ≈ 2.7%, and the two phases can be studied by single-crystal X-ray diffraction, but a larger (∼3 mm) crystal used for DSC measurements breaks across this same transition.30The III2 ⇔ III transformation in tolbutamide is similar to the βII ⇔ βIII transition in chlorpropamide10 (the two transformations are accompanied by a change in only part of the molecules). The main difference between these two cases is in the number of molecules changing their conformations. In tolbutamide, the alkyl tail turns in every third molecule, giving Z′ = 3 in the low-temperature polymorph. In chlorpropamide, the conformation changes in every fourth molecule, resulting in Z′ = 4. As a direct result of these conformational changes, one of the unit cell parameters increases a whole number of times. Nevertheless, we also consider this type of transformation to be isostructural (to describe the INTERmolecular region, as opposed to the molecules themselves) because, while the changes in the formal mathematical description of symmetry are significant, they are accompanied by negligible changes in the packing of molecules.
Isostructural conformational transformations versus reconstructive transformations
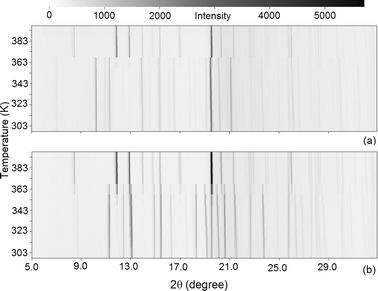
The tolbutamide and chlorpropamide isostructural conformational transformations are also reversible at the macroscopic level. A single crystal can undergo a phase transition (or a series of phase transitions) with variations in T and P conditions, but will be exactly the same after the initial conditions are restored. In contrast, the initial single crystal is immediately lost (irreversibly) upon any reconstructive transformation, even if its crystal structure can be restored in the reverse transition. In many cases, after such a transformation, the initial phase (even as a powder) cannot be restored on returning to the initial conditions. For example, after determining the crystal structure of form IH, we have proved (using in situ X-ray powder diffraction in a capillary on heating), that forms II and III of tolbutamide transform on heating into IH (II ⇒ IH and III ⇒ IH), without any trace of the starting form in the high-temperature form (Fig. 7). On cooling, the high-temperature form transforms into IL. These transformations (II ⇒ IH and III ⇒ IH) are thus irreversible.The rate of a reconstructive transformation is often limited by nucleation. After a nucleus of the new phase reaches a critical size, it begins growing irreversibly. Comparative analysis of the initial and post-transformation structures shows that the reconstructive transformation cannot proceed without breaking bonds. For example, the infinite chains in all polymorphs of both chlorpropamide and tolbutamide, except tolbutamide II, are directed along a well-defined direction. In tolbutamide II, the chains that are formed by two of four symmetry-independent molecules (Ch1 and Ch2) run perpendicularly to the tapes formed by the remaining two symmetry independent molecules (Ch3 and Ch4).15 Because of this, even if the hydrogen-bond motifs (Scheme 1) are identical in all tolbutamide polymorphs, the II ⇒ IH transformation cannot occur except via nucleation and subsequent recrystallization. The same is valid for chlorpropamide, where transformations α ⇒ ε and γ ⇒ ε are not only accompanied by a change in space group, but also by a change in the symmetry of every second chain. Nucleation in reconstructive transformations is sensitive to thermal prehistory. In DSC measurements, the start of a reconstructive polymorphic transformation depends on the heating rate.34 It is obvious that no hysteresis occurs, as there is no reverse transformation. Reconstructive transformations have no well-defined transition temperature and can occur at any temperature beyond the limits of polymorphic stability. For example, the isothermal kinetics of the III ⇒ IH transformation in tolbutamide were studied at 65, 70, 75, and 80 °C in.35 In isostructural transformations, the DSC peaks are reversible. Sometimes they are sharp peaks with hysteresis like that in a conventional first-order phase transition,30 and sometimes they appear as a triangular peak typical of a second-order phase transition (see βII ⇔ β10). As for the IL ⇔ IH transformation (Fig. 3), it surely resembles a first-order phase transition but, surprisingly, without hysteresis.
Comparing the polymorphs of tolbutamide and chlorpropamide
There are a number of common features between the polymorphic transformations of tolbutamide and chlorpropamide. For both compounds, there exists only one high-temperature polymorph, into which other forms transform before melting. However, each case is characterized by its peculiar features.Of the five polymorphs of tolbutamide (IL, II, III, IV, V), three (II, III and IV) transform into form IH on heating below its melting point of 128 °C. Form V is unstable at room temperature and transforms into IL.16 Depending on the heating conditions, form II can melt at its own melting point of 117 °C, subsequently crystallizing exothermally to tolbutamide IH, which then melts at 128 °C,15 but it can also transform into IHvia a solid-state transition (see Fig. 7). Form IH is the most stable polymorph of tolbutamide at high temperatures. However, form IL is less stable than form III below 40 °C,36 transforming to form III in aqueous solution.
Of the five polymorphs of chlorpropamide (α, β, γ, δ, ε), four (α, β, γ, and δ) transform into form ε on heating. The latter melts at 128 °C. For α and β, the temperature of their transformation into ε depends on the heating rate and can overlap with the melting point of the product. In such cases, the endothermic start of the process is followed by an exothermic stage, subsequently resuming its endothermic behaviour. The ε form is the most stable polymorph of chlorpropamide at high temperatures. However, it is less stable than the α form below room temperature, and the ε ⇒ α transformation occurs on storage at ambient conditions. This transformation was also observed on cryogrinding.37
The most stable high-temperature polymorphs of both tolbutamide (IH) and chlorpropamide (ε) undergo reversible conformational isostructural transformations into their low-temperature metastable daughter forms (IL and ε′, respectively), albeit at different temperatures: 313 K for IL ⇔ IH (see Fig. 3) and ∼215 K for ε′ ⇔ ε (see Fig. 8).
 | ||
| Fig. 8 DSC results for reversible ε′ ⇔ ε transformation in chlorpropamide; the first (red) and second (blue) runs with the same powder sample (6.9 mg, 6 K min−1). | ||
Conclusions
Structural investigation of the three polymorphs of tolbutamide (I, II, and III) over a wide temperature range (from 100 to 300 K for forms II and III and from 100 to 350 K for form I) allowed, for the first time, determination of the crystal structure of the high-temperature form, IH, and calculation of the volume change ΔV/V for two isostructural transformations, IL ⇔ IH and III2 ⇔ III. The reversible transformation, IL ⇔ IH, was found to be of the single-crystal to single-crystal type. The structure of IH differs from that of IL only in the conformation of the molecules. The exact temperature range of the IL ⇔ IH transition in commercial powder was measured using DSC and was found to be extremely narrow (between 312.8 K at heating and 313.0 K at cooling). The temperature hysteresis was found to be less than the dispersion of the transition points for individual particles in the powder sample.Comparison between polymorphic transformations in tolbutamide and chlorpropamide reveals much similarity. All transformations accompanied by a conformational change can be categorized into two types, isostructural and reconstructive. Reconstructive transformations are irreversible, affecting both the packing of molecules and molecular conformations. Isostructural transformations are also reversible at the macroscopic level and mainly affect the conformations of molecules, leaving the hydrogen bonds unchanged. Single-crystal to single-crystal transformations are isostructural in nature.
Acknowledgements
The study was supported by Project No. V.44.3.4 of the Siberian Branch of the Russian Academy of Sciences. VAD acknowledges that his work was supported by state assignment project No. 0330-2016-004.References
- A. J. Cruz-Cabeza and J. Bernstein, Chem. Rev., 2014, 114, 2170 CrossRef PubMed.
- A. Nangia, Acc. Chem. Res., 2008, 41, 595 CrossRef PubMed.
- J.-P. Brog, C.-L. Chanez, A. Crochet and K. M. Fromm, RSC Adv., 2013, 3, 16905 RSC.
- C. H. Koo, S. I. Cho and Y. H. Yeon, Arch. Pharmacal Res., 1980, 3, 37 CrossRef CAS.
- T. N. Drebushchak, N. V. Chukanov and E. V. Boldyreva, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2006, 62, o4393 CrossRef CAS.
- T. N. Drebushchak, N. V. Chukanov and E. V. Boldyreva, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2007, 63, o355 CrossRef CAS PubMed.
- T. N. Drebushchak, N. V. Chukanov and E. V. Boldyreva, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2008, 64, o623 CrossRef CAS PubMed.
- V. A. Drebushchak, T. N. Drebushchak, N. V. Chukanov and E. V. Boldyreva, J. Therm. Anal. Calorim., 2008, 93, 343 CrossRef CAS.
- T. N. Drebushchak, Y. A. Chesalov and E. V. Boldyreva, Acta Crystallogr., Sect. B: Struct. Sci., 2009, 65, 770 CrossRef CAS PubMed.
- T. N. Drebushchak, V. A. Drebushchak and E. V. Boldyreva, Acta Crystallogr., Sect. B: Struct. Sci., 2011, 67, 163 CrossRef CAS PubMed.
- Yu. V. Seryotkin, T. N. Drebushchak and E. V. Boldyreva, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2013, 69, 77 CrossRef CAS.
- K. A. Nirmala and D. S. Sake Gowda, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1981, 37, 1597 CrossRef.
- J. D. Donaldson, J. R. Leary, S. D. Ross, M. J. K. Thomas and C. H. Smith, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1981, 37, 2245 CrossRef.
- G. Hasegawa, T. Komasaka, R. Bando, Y. Yoshihashi, E. Yonemochi, K. Fujii, H. Uekusa and K. Terada, Int. J. Pharm., 2009, 369, 12 CrossRef CAS PubMed.
- S. Thirunahari, S. Aitipamula, P. S. Chow and R. B. H. Tan, J. Pharm. Sci., 2010, 99, 2975 CrossRef CAS PubMed.
- N. K. Nath and A. Nangia, CrystEngComm, 2011, 13, 47 RSC.
- T. N. Drebushchak, N. A. Pankrushina and E. V. Boldyreva, Dokl. Phys. Chem., 2011, 437, 61 CrossRef CAS.
- T. N. Drebushchak, N. A. Pankrushina, A. N. Mikheev and M. K. A. Thumm, CrystEngComm, 2013, 15, 3582 RSC.
- T. N. Drebushchak and E. V. Boldyreva. Our unpublished data on cooling of γ-, and δ-polymorphs of chlorpropamide (100–298 K).
- F. H. Allen, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 380 CrossRef.
- Agilent, CrysAlisPro, 2010, Agilent Technologies Ltd, Yarnton, England Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- G. M. Sheldrick, SHELXL2014, University of Göttingen, Germany Search PubMed.
- L. J. Farrugia, J. Appl. Crystallogr., 2012, 45, 849 CrossRef CAS.
- L. J. Farrugia, J. Appl. Crystallogr., 1997, 30, 565 CrossRef CAS.
- STOE & Cie GmbH, WinXPOW, 2011, STOE & Cie GmbH, Darmstadt, Germany Search PubMed.
- C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. van de Streek and P. A. Wood, J. Appl. Crystallogr., 2008, 41, 466 CrossRef CAS.
- E. V. Boldyreva, V. A. Drebushchak, T. N. Drebushchak, I. E. Paukov, Y. A. Kovalevskaya and E. S. Shutova, J. Therm. Anal. Calorim., 2003, 73, 419 CrossRef CAS.
- S. J. Coles, T. L. Threlfall and G. J. Tizzard, Cryst. Growth Des., 2014, 14, 1623 Search PubMed.
- B. A. Zakharov, E. A. Losev, B. A. Kolesov, V. A. Drebushchak and E. V. Boldyreva, Acta Crystallogr., Sect. B: Struct. Sci., 2012, 68, 287 CrossRef CAS PubMed.
- E. J. Chan, A. D. Rae and T. R. Welberry, Acta Crystallogr., Sect. B: Struct. Sci., 2009, 65, 509 CrossRef CAS PubMed.
- C. H. Gorbitz, J. Phys. Chem. B, 2011, 115, 2447 CrossRef CAS PubMed.
- C. H. Gorbitz, F. Alebachew and V. Petricek, J. Phys. Chem. B, 2012, 116, 10715 CrossRef CAS PubMed.
- V. A. Drebushchak, T. N. Drebushchak and E. V. Boldyreva, J. Therm. Anal. Calorim., 2013, 113, 419 CrossRef CAS.
- T. Umeda, N. Ohnishi, T. Yokoyama, T. Kuroda, Y. Kita, K. Kuroda, E. Tatsumi and Y. Matsuda, Chem. Pharm. Bull., 1985, 33, 2073 CrossRef CAS PubMed.
- E. L. Rowe and B. D. Anderson, J. Pharm. Sci., 1984, 73, 1673 CrossRef CAS PubMed.
- T. N. Drebushchak, A. A. Ogienko and E. V. Boldyreva, CrystEngComm, 2011, 13, 4405 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The unit cell parameters for polymorphs I, II, and III of tolbutamide (Table 1S). CCDC 1469769–1469773. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ce00764c |
| This journal is © The Royal Society of Chemistry 2016 |