Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
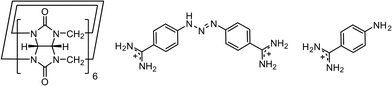
Host–guest complexes of cucurbit[6]uril with the trypanocide drug diminazene and its degradation product 4-aminobenzamidine†
Oksana
Danylyuk
*,
Helena
Butkiewicz
and
Volodymyr
Sashuk
Institute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland. E-mail: odanylyuk@ichf.edu.pl
First published on 11th April 2016
Abstract
Here, we describe the cocrystallization of the trypanocide drug diminazene with the macrocyclic host cucurbit[6]uril (CB6). When the acid-contaminated CB6 was used, diminazene and the product of its hydrolysis cocrystallized with CB6. The use of an acid-free macrocycle afforded a host–guest complex where diminazene guest molecules are complexed exo to the host cavity.
Introduction
African trypanosomiasis is a parasitic disease of humans and animals that plagues sub-Saharan Africa and is transmitted by tsetse flies. The infection is known as sleeping sickness in humans and is lethal if left untreated. However, the choice of chemotherapeutic agents is very limited, and approved drugs have many side effects.Diminazene (berenil) is used in the treatment of domestic animals and is quite effective against trypanosomiasis with a short treatment period and low cost.1 Diminazene formulated as a diminazene aceturate salt is registered as a veterinary drug but is not currently approved for use in humans. Diminazene is a bis-benzamidine compound composed of two benzamidine moieties linked with a triazene group (Fig. 1). The anti-trypanosomial activity of diminazene is due to its binding to the parasitic kinetoplast DNA via hydrogen bonds.2 The drug is administered by intramuscular injection, and its main side effect is a burning sensation at the injection site. It can potentially be administered orally; however, the drug molecule undergoes acid-catalyzed decomposition in the human gastrointestinal tract. Specifically, the triazene linker is susceptible to hydrolysis with formation of 4-aminobenzamidine and diazonium salt.3 The limitations of diminazene could potentially be overcome by developing coated formulations, for example nanoparticles4 or host–guest complexes.5
Here, we would like to present our results on the cocrystallization attempts for the drug diminazene with the macrocyclic host cucurbit[6]uril (CB6) (Fig. 1). Such complexation through co-crystallization can improve the solubility, bioavailability and stability of pharmaceutically active molecules.6 Cucurbit[n]urils are pumpkin-shaped supramolecular hosts which have attracted much attention owing to their excellent ability to bind various inorganic, organic and biological molecules and ions both in solution and in the solid state.7 The highly polar carbonyl rims of CB6 seem to be good candidates for multi-point interactions with two amidinium groups of diminazene. Besides favorable cation–dipole interactions between the cationic guest and the host,8 the CB6 carbonyl groups can potentially serve as acceptors for numerous hydrogen bonds in the amidinium groups of diminazene. The initial experiments on the co-crystallization of the drug with CB6 revealed that diminazene is susceptible to decomposition even in the presence of the macrocyclic host. The hydrolysis of the drug is induced by the remnants of the crystallization acid in CB6. The cleavage reaction is additionally promoted by CB6 through electrostatic enhancement of diminazene basicity.9 As a result, crystallization of the ternary complex of CB6 with diminazene and its degradation product 4-aminobenzamidine was observed. Interestingly, the crystals were unstable and slowly dissolved in the mother solution, which was followed by the crystallization of a new phase that turned out to be the host–guest complex of CB6 and 4-aminobenzamidine with simultaneous coordination of magnesium ions (magnesium chloride was used to improve the solubility of CB6). When calcium chloride was used instead of magnesium chloride, direct crystallization of a similar host–guest complex with coordinated calcium ions took place. It should be mentioned that the effect of metal ions on the cocrystallization pathway of host–guest complexes was already observed in the CB6–isoprenaline system.10 We wondered how the CB6–diminazene system would behave during cocrystallization in the total absence of the acid. To achieve this, extensive purification of CB6 from the crystallization acid remnants was performed. The cocrystallization of diminazene with the acid-free CB6 afforded a desirable host–guest complex but with the drug positioned exo with respect to the host macrocyclic cavity. In the following, we describe the cocrystallization and crystal structures of all four host–guest complexes in detail.
Results and discussion
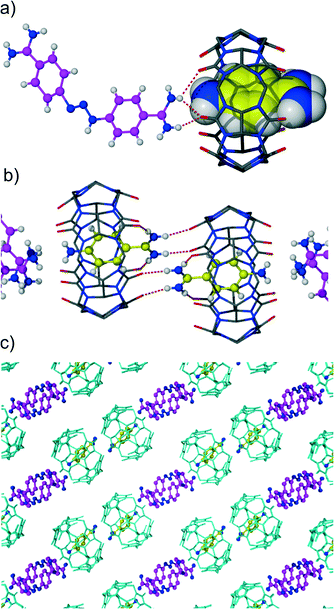
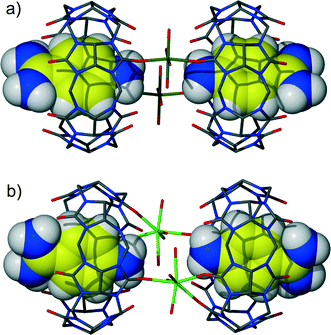
The cocrystallization of diminazene aceturate and CB6 from aqueous solution in the presence of magnesium chloride afforded yellow crystals. We also noticed a colour change of the mother solution from initially yellow (the colour of the diminazene solution) to red within several hours. X-ray analysis revealed the yellow crystals to be the mixed host–guest complex 1 between CB6, diminazene and 4-aminobenzamidine, which appears to be the product of diminazene degradation (Fig. 2a). 4-Aminobenzamidine nicely fits the macrocyclic host with its aromatic core situated within the host cavity, while the amidinium and amino groups are arranged at two CB6 portals. The guest amidinium group interacts with the carbonyl groups of the host via cation–dipole interactions and hydrogen bonding. The shortest N–H⋯O distances between nitrogen atoms of the guest and carbonyl oxygen atoms of the host are 2.86 and 2.91 Å. The amidinium group partially protrudes from the host cavity and additionally interacts with the symmetry-generated CB6 macrocycle (N–H⋯O distances: 2.85 and 2.89 Å) (Fig. 2b). Diminazene was modeled as disordered over two positions that differ in the rotation angle of the benzamidine moiety around the triazene linker. Diminazene is complexed outside the macrocyclic cavity, interacting with two CB6 molecules via both amidinium groups. Such a host–guest interaction mode leads to hydrogen bonded chains that consist of alternating diminazene dications and two CB6 inclusion complexes with 4-aminobenzamidinium (Fig. 2c). The complex is highly hydrated with disordered water molecules and chloride anions occupying wavy channels between the hydrogen bonded chains of the host–guest entities.Interestingly, we have observed that after several days at room temperature, new crystals of red colour appeared in the crystallization vial. X-ray analysis determined this new phase to be an inclusion complex of CB6 with 4-aminobenzamide but with simultaneous coordination of magnesium ions to a CB6 rim. We also noticed the absence of diminazene in the new crystals. Obviously, the crystallization does not prevent further acidic degradation of diminazene. The overall process should proceed through dissolution of the initial crystals of the CB6 mixed complex rather than decomposition of diminazene in the solution and subsequent crystallization of the new CB6 inclusion complex with 4-aminobenzamide. It should be mentioned that a similar solution-mediated transformation from one crystalline phase into another is known for several CB6 complexes.10,11 The asymmetric unit of new complex 2 comprises one CB6 molecule, one 4-aminobenzamidinium included into host cavity, one magnesium cation, water molecules and chloride anions. The inclusion mode of 4-aminobenzamidine is similar to that described in complex 1. The magnesium ion is coordinated to a CB6 rim from the side of an amino group of benzamidine. The coordination sphere of magnesium consists of four aqua ligands and two oxygen atoms of symmetry-related CB6 molecules. That is, the coordination dimer is formed where two CB6 molecules are linked by two coordinated magnesium ions (Fig. 3a). The distance between the carbonyl rims of two neighboring CB6 molecules of 4.2 Å is quite short. The direct coordination of such small ions to two CB6 molecules has been previously suggested to be unstable12 and was observed by us in the metastable phase of a CB6 tubular coordination complex with magnesium.13
When calcium chloride is used instead of magnesium chloride for increasing the CB6 solubility in water, direct crystallization of the calcium coordinated CB6 inclusion complex 3 with 4-aminobenzamide occurs. The complex is quite similar to the previous one with magnesium (Fig. 3b). However, the asymmetric unit of the calcium complex comprises two crystallographically independent CB6 molecules with 4-aminobenzamidinium guests included. The coordination dimers are also formed where each calcium ion is coordinated to two carbonyl oxygen atoms from one CB6 and one oxygen atom from another symmetry-generated macrocycle. The coordination sphere of the metal ion is complemented by four water molecules.
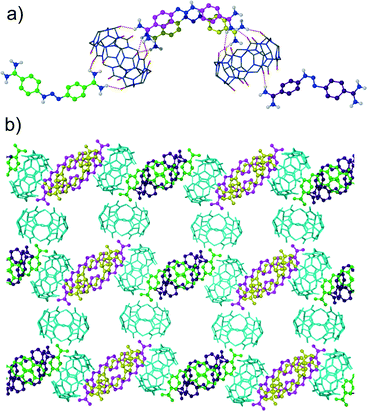
It is known that cucurbituril macrocycles usually are contaminated with acid used in their synthesis. Taking into account the acid-induced cleavage of the triazene group leading to the decomposition of diminazene, we wanted to investigate the complexation between the CB6 host and the diminazene guest in neutral pH. Therefore, the cucurbituril was purified from the acid remnants by excessive washing with Millipore water under sonication. The crystallization experiment using the acid-free CB6 resulted in yellow crystals of the host–guest complex 4 between CB6 and diminazene. No colour change in the mother solution was observed in this case. The inclusion of diminazene into the host macrocyclic cavity might be expected since 4-aminobenzamide (structurally similar to half of diminazene) forms an inclusion complex with CB6. Indeed, the encapsulation of diminazene by CB6 in the solution was observed in the NMR experiments.9 However, X-ray analysis revealed that the complex formed between CB6 and diminazene in the solid state is of the exclusion type. Interestingly, the unit cell is quite large, with the asymmetric unit comprising four crystallographically non-equivalent diminazene molecules and two CB6 molecules and being highly hydrated (Fig. 4a). The chloride counter-anions seem to be badly disordered (probably also in substitutional disorder with water molecules), and it is not possible to locate all of them. The cavities of the CB6 molecules are filled with water molecules. Each diminazene interacts with two CB6 molecules via cation–dipole and hydrogen bonding interactions. The interactions are realized between the amidinium groups of diminazene and the carbonyl oxygen atoms of CB6, leading to an assembly where diminazene bridges two CB6 macrocycles. The packing of the complex is also characterized by large water channels running along the a-axis (Fig. 4b).
Experimental
Materials and methods
Diminazene aceturate was purchased from Sigma-Aldrich. CB6 was synthesized according to a literature procedure [A. Day et al., J. Org. Chem., 2001, 66, 8094].Prior to its use in the crystallization of complex 4, CB6 was purified from the acid remnants by excessive washing with Millipore water (18.2 MΩ·cm). In brief, acid-contaminated CB6 was mixed with water and then sonicated. The obtained slurry was centrifuged, and the aqueous solution was discarded. The purification procedure was repeated until the indicator paper showed a neutral pH of the supernatant. Afterwards, the CB6 powder was sonicated and rinsed with copious amounts of water and then dried under vacuum (0.1 mbar) at 100 °C for 6 hours. Elemental analysis showed that chloride ions were not present in the sample.
Crystallography
Crystal data for 1: C57H77Cl3N34O35, Mr = 1902.9, yellow, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 12.6658(6), b = 15.8879(7), c = 22.143(1) Å, α = 89.449(4), β = 93.013(4), γ = 107.919(4)°, V = 4233.8(3) Å3, Z = 2, ρcalc = 1.49 g cm−3, μ(CuKα) = 1.91 mm−1, θmax = 71.5°, 31
, a = 12.6658(6), b = 15.8879(7), c = 22.143(1) Å, α = 89.449(4), β = 93.013(4), γ = 107.919(4)°, V = 4233.8(3) Å3, Z = 2, ρcalc = 1.49 g cm−3, μ(CuKα) = 1.91 mm−1, θmax = 71.5°, 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 990 reflections measured, 16
990 reflections measured, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 156 unique, 1535 parameters, R = 0.105, wR = 0.289 (R = 0.121, wR = 0.312 for all data), GooF = 1.18. CCDC 1450574.
156 unique, 1535 parameters, R = 0.105, wR = 0.289 (R = 0.121, wR = 0.312 for all data), GooF = 1.18. CCDC 1450574.
Crystal data for 2: C43H70Cl3MgN27O27, Mr = 1527.9, red, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 12.528(1), b = 15.355(1), c = 17.297(2) Å, α = 107.352(8), β = 100.180(9), γ = 95.791(8)°, V = 3083.7(5) Å3, Z = 2, ρcalc = 1.65 g cm−3, μ(CuKα) = 2.41 mm−1, θmax = 61.6°, 16
, a = 12.528(1), b = 15.355(1), c = 17.297(2) Å, α = 107.352(8), β = 100.180(9), γ = 95.791(8)°, V = 3083.7(5) Å3, Z = 2, ρcalc = 1.65 g cm−3, μ(CuKα) = 2.41 mm−1, θmax = 61.6°, 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 815 reflections measured, 9348 unique, 1000 parameters, R = 0.081, wR = 0.211 (R = 106, wR = 0.238 for all data), GooF = 0.92. CCDC 1450575.
815 reflections measured, 9348 unique, 1000 parameters, R = 0.081, wR = 0.211 (R = 106, wR = 0.238 for all data), GooF = 0.92. CCDC 1450575.
Crystal data for 3: C86H92Cl5.5Ca2N54O81.5, Mr = 3461.3, yellow, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 15.8539(3), b = 19.7194(3), c = 26.8892(5) Å, α = 98.389(2), β = 94.251(2), γ = 109.497(2)°, V = 7771.2(3) Å3, Z = 2, ρcalc = 1.48 g cm−3, μ(CuKα) = 2.51 mm−1, θmax = 72.1°, 55
, a = 15.8539(3), b = 19.7194(3), c = 26.8892(5) Å, α = 98.389(2), β = 94.251(2), γ = 109.497(2)°, V = 7771.2(3) Å3, Z = 2, ρcalc = 1.48 g cm−3, μ(CuKα) = 2.51 mm−1, θmax = 72.1°, 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 356 reflections measured, 29
356 reflections measured, 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 924 unique, 2202 parameters, R = 0.102, wR = 0.288 (R = 114, wR = 0.288 for all data), GooF = 1.12. CCDC 1450576.
924 unique, 2202 parameters, R = 0.102, wR = 0.288 (R = 114, wR = 0.288 for all data), GooF = 1.12. CCDC 1450576.
Crystal data for 4: C128H140Cl2N76O80, Mr = 4095.1, yellow, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 15.649(1), b = 20.975(2), c = 35.178(1) Å, α = 100.397(5), β = 99.817(4), γ = 103.379(6)°, V = 10
, a = 15.649(1), b = 20.975(2), c = 35.178(1) Å, α = 100.397(5), β = 99.817(4), γ = 103.379(6)°, V = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 774(1) Å3, Z = 2, ρcalc = 1.26 g cm−3, μ(CuKα) = 1.174 mm−1, θmax = 56.6°, 55
774(1) Å3, Z = 2, ρcalc = 1.26 g cm−3, μ(CuKα) = 1.174 mm−1, θmax = 56.6°, 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 046 reflections measured, 27
046 reflections measured, 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 483 unique, 2719 parameters, R = 0.157, wR = 0.375 (R = 0.261, wR = 0.439 for all data), GooF = 1.11. CCDC 1450577.
483 unique, 2719 parameters, R = 0.157, wR = 0.375 (R = 0.261, wR = 0.439 for all data), GooF = 1.11. CCDC 1450577.
In complex 1, the diminazene molecule was modeled as disordered over 2 positions using PART instructions (site occupancy factors were refined to 0.58 and 0.42). The ‘soft’ similarity restraints (SIMU and DELU) on the displacement parameters were applied.
In complexes 1, 3 and 4, some water molecules and chloride anions (especially those located in the large channels between the host–guest entities) were found to be disordered. The site occupancy factors of a few of the disordered water molecules were fixed to 0.5 or 0.25 to obtain reasonable displacement parameters. Where possible, the hydrogen atoms of ordered water molecules were located from a difference map and refined using the DFIX command to restrain the O–H distances. The hydrogen atoms of the disordered water molecules were not located during the refinement.
Conclusions
To conclude, cocrystallization of the trypanocide diminazene with the macrocyclic host cucurbit[6]uril does not prevent the acid-catalyzed hydrolysis of the drug. CB6 forms inclusion host–guest complexes with 4-aminobenzamidine generated in situ upon decomposition of diminazene. Diminazene does not penetrate the macrocyclic cavity of CB6; instead, extensive hydrogen bonding is realized between the amidinium groups of the drug and the cucurbituril portals in the solid state. Further studies are necessary to find out if the inclusion of diminazene into the host macrocyclic cavity is possible. To achieve this, variation of cocrystallization conditions and screening of other macrocyclic hosts will be carried out in our laboratory.Acknowledgements
The project was supported by the Polish Ministry of Science and Higher Education (Iuventus Plus grant NrIP2012008272).References
- A. S. Peregrine and M. Mamman, Acta Trop., 1993, 54, 185 CrossRef CAS PubMed.
- D. S. Pilch, M. A. Kirolos, X. Liu, G. E. Plum and K. J. Breslauer, Biochemistry, 1995, 34, 9962 CrossRef CAS PubMed.
- M. Campbell, R. J. Prankerd, A. S. Davie and W. N. Charman, J. Pharm. Pharmacol., 2004, 56, 1327 CrossRef CAS PubMed.
- M. Kroubi, S. Daulouede, H. Karembe, Y. Jallouli, M. Howsam, D. Mossalayi, P. Vincendeau and D. Betbeder, Nanotechnology, 2010, 21, 505102 CrossRef PubMed.
- T. Xu, A. V.-Z. Asadi and M. Barra, Int. J. Chem. Kinet., 2010, 42, 567 CrossRef CAS.
- W.-J. Ma, J.-M. Chen, L. Jiang, J. Yao and T.-B. Lu, Mol. Pharmaceutics, 2013, 10, 4698 CrossRef CAS PubMed; J. A. Plumb, B. Venugopal, R. Oun, N. A. Gomez-Roman, Y. Kawazoe, N. S. Venkataramanan and N. J. Wheate, Metallomics, 2012, 4, 561 RSC; F. J. McInnes, N. G. Anthony, A. R. Kennedy and N. J. Wheate, Org. Biomol. Chem., 2010, 8, 765 Search PubMed.
- X.-L. Ni, X. Xiao, H. Cong, L.-L. Liang, K. Cheng, X.-J. Cheng, N.-N. Ji, Q.-J. Zhu, S.-F. Xue and Z. Tao, Chem. Soc. Rev., 2013, 42, 9480 RSC; X.-L. Ni, X. Xiao, H. Cong, Q.-J. Zhu, S.-F. Xue and Z. Tao, Acc. Chem. Res., 2014, 47, 1386 CrossRef CAS PubMed; K. I. Assaf and W. M. Nau, Chem. Soc. Rev., 2015, 44, 394 RSC; S. J. Barrow, S. Kasera, M. J. Rowland, J. del Barrio and O. A. Scherman, Chem. Rev., 2015, 115, 12320 CrossRef PubMed.
- J. W. Lee, S. Samal, N. Selvapalam, H.-J. Kim and K. Kim, Acc. Chem. Res., 2003, 36, 621 CrossRef CAS PubMed; X. Sun, B. Li, Q. Zhou, H. Zhang, G. Cheng and X. Zhou, Cryst. Growth Des., 2008, 8, 2970 Search PubMed.
- V. Sashuk, H. Butkiewicz, M. Fialkowski and O. Danylyuk, Chem. Commun., 2016, 52, 4191 RSC.
- O. Danylyuk, V. P. Fedin and V. Sashuk, CrystEngComm, 2013, 15, 7414 RSC.
- O. Danylyuk, V. P. Fedin and V. Sashuk, Chem. Commun., 2013, 49, 1859 RSC.
- W.-J. Chen, D.-H. Yu, X. Xiao, Y.-Q. Zhang, Q.-J. Zhu, S.-F. Xue, Z. Tao and G. Wei, Inorg. Chem., 2011, 50, 6956 CrossRef CAS PubMed.
- O. Danylyuk and V. P. Fedin, CrystEngComm, 2014, 16, 3699 RSC.
Footnote |
| † CCDC 1450574–1450577. X-ray crystallographic files in CIF format. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ce00257a |
| This journal is © The Royal Society of Chemistry 2016 |