Open Access Article
Open Access ArticleSingle crystals that spontaneously spawn other single crystals: a ternary and a binary adduct of thiourea and 2,5-dimethylpyrazine†‡
Christina
Taouss
,
Cindy
Döring
,
Peter G.
Jones
*,
Lukas
Pinkert
and
Mark
Strey
Institute of Inorganic and Analytical Chemistry, Technical University of Braunschweig, P.O. Box 3329, D-38023 Braunschweig, Germany. E-mail: p.jones@tu-bs.de; Fax: +49 531 5387; Tel: +49 531 5382
First published on 9th February 2016
Abstract
Liquid diffusion of n-pentane into a solution of thiourea in 2,5-dimethylpyrazine led to a crystalline 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 adduct (1), in which corrugated thiourea layers are crosslinked with pyrazines. Attempts to obtain adducts with other stoichiometries, by crystallizing thiourea from a mixture of 2,5-dimethylpyrazine and methanol, formed the ternary 1
3 adduct (1), in which corrugated thiourea layers are crosslinked with pyrazines. Attempts to obtain adducts with other stoichiometries, by crystallizing thiourea from a mixture of 2,5-dimethylpyrazine and methanol, formed the ternary 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct 2 instead. Adduct 2 displays a layer structure in which parallel thiourea ribbons are linked on the one side by pyrazines and on the other side by methanol and pyrazines, leading to repeating crosslink sequences (⋯thiourea⋯methanol⋯pyrazine⋯methanol⋯thiourea⋯pyrazine⋯); the ribbons within a layer are thus unequally spaced. In inert oil, individual single crystals of 2 spontaneously convert to several smaller crystals, some single, of 1. The process may be regarded as a single-crystal to single-crystal transformation, although not in the usual sense. The 4
1 adduct 2 instead. Adduct 2 displays a layer structure in which parallel thiourea ribbons are linked on the one side by pyrazines and on the other side by methanol and pyrazines, leading to repeating crosslink sequences (⋯thiourea⋯methanol⋯pyrazine⋯methanol⋯thiourea⋯pyrazine⋯); the ribbons within a layer are thus unequally spaced. In inert oil, individual single crystals of 2 spontaneously convert to several smaller crystals, some single, of 1. The process may be regarded as a single-crystal to single-crystal transformation, although not in the usual sense. The 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 adduct (3) of thiourea with 2-methylpyrazine is isotypic to 1. In all three structures, the preponderant secondary interactions are classical hydrogen bonds.
3 adduct (3) of thiourea with 2-methylpyrazine is isotypic to 1. In all three structures, the preponderant secondary interactions are classical hydrogen bonds.
Introduction
We are interested in the crystalline adducts of urea and thiourea (and their simple derivatives), component A, with organic liquids, component B; the latter may be termed “solvents”, because component A must dissolve in them before adduct crystals can form, usually after overlayering a suitable precipitant such as pentane.1–3 In such adducts, the residues are linked by classical, and in some cases also by “weak”, hydrogen bonds. An important class of such adducts is formed from urea (A) with aza-aromatics such as pyridine and related compounds (B), which form either 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 2
1 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (urea
1 (urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solvent) adducts; the former adducts generally display a urea ribbon substructure consisting of linked R22(8) rings, with molecules of B peripherally attached (Fig. 1),3–7 whereas the latter adducts display a corrugated layer of urea molecules, out of which individual molecules project and are linked to the molecules of B above and below the layer.3 It is not easy to direct the stoichiometry of the adducts; we have generally attempted to maximise the content of B by using neat liquid B as a reaction medium, without resorting to a second liquid such as methanol as a conventional solvent. In principle, an adduct of a different composition (a higher A
solvent) adducts; the former adducts generally display a urea ribbon substructure consisting of linked R22(8) rings, with molecules of B peripherally attached (Fig. 1),3–7 whereas the latter adducts display a corrugated layer of urea molecules, out of which individual molecules project and are linked to the molecules of B above and below the layer.3 It is not easy to direct the stoichiometry of the adducts; we have generally attempted to maximise the content of B by using neat liquid B as a reaction medium, without resorting to a second liquid such as methanol as a conventional solvent. In principle, an adduct of a different composition (a higher A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B ratio) might be obtained upon reducing the effective concentration of component B, by dissolving A in a mixture of B and another solvent C.
B ratio) might be obtained upon reducing the effective concentration of component B, by dissolving A in a mixture of B and another solvent C.
 | ||
Fig. 1 Packing diagram of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct between urea and 2,6-lutidine (“urea ribbon substructure”), showing two ribbons running horizontally, with the attached lutidines occupying the space between the ribbons. Classical and “weak” hydrogen bonds are drawn as thick or thin dashed lines respectively. Adapted slightly from ref. 3. 1 adduct between urea and 2,6-lutidine (“urea ribbon substructure”), showing two ribbons running horizontally, with the attached lutidines occupying the space between the ribbons. Classical and “weak” hydrogen bonds are drawn as thick or thin dashed lines respectively. Adapted slightly from ref. 3. | ||
Adducts of urea generally display simpler packing patterns than those of thiourea because the acceptor properties of the urea oxygen atom are more rigid; it generally accepts two hydrogen bonds from D–H donors that effectively lie parallel to the urea molecular plane, so that the packing tends to involve linear or planar groupings. The thiourea sulfur atom, in contrast, often accepts more than two hydrogen bonds, and the D–H vectors can subtend large angles to the thiourea molecular plane; thus the packing patterns are often three-dimensional and complex.8,9
We have now extended our studies to the investigation of adducts between urea or thiourea as component A and various liquid pyrazines as component B, and present here our results for thiourea and 2,5-dimethylpyrazine. Methanol was used as the additional solvent C.
Experimental
Preparation of the adducts
Binary adduct 1: 100 mg of thiourea was dissolved in 2 mL of 2,5-dimethylpyrazine. The solution was distributed over several small ignition tubes and overlayered with n-pentane. Crystals in the form of colourless blocks were obtained; these crystals lost transparency when exposed to air, presumably via loss of the volatile component. X-ray structure determination of adduct 1 (see below) revealed the composition to be 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (thiourea
3 (thiourea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2,5-dimethylpyrazine) (Scheme 1).
2,5-dimethylpyrazine) (Scheme 1).
Ternary adduct 2: 54 mg of thiourea was dissolved in a mixture of 1.1 mL 2,5-dimethylpyrazine and 0.9 mL methanol. The solution was distributed over several small ignition tubes and overlayered with n-heptane. Crystals in the form of large (2 mm) colourless laths were obtained.
Structure determination of the laths 2 was made difficult by the fact that they fragmented badly upon cutting. Initial attempts led to crystals of poor quality, for which nonetheless a cell could be determined and was clearly different from that of 1. To our surprise, a crystal of good optical quality was then found and proved to have the same cell as that of 1. A more thorough investigation under the microscope showed that the laths were slowly disappearing, with the concomitant formation of new crystals of 1, some of them being single crystals (Fig. 2). The structure corresponding to the laths was then successfully determined by waiting for a small section to become isolated from the main crystal via formation of 1, then mounting this fragment rapidly. Frustratingly, the crystals also proved to be sensitive to very low temperatures (whereby they shattered and were irretrievably lost), but could be successfully measured at 170 K, revealing the presence of the ternary 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct 2 between thiourea, 2,5-dimethylpyrazine and methanol (Scheme 1). The sample on the microscope slide had been immersed in a protective layer of inert oil, so the transformation from 2 to 1 presumably occurred via gradual loss of methanol, redissolution of the adduct and then crystallization of 1 as the proportion of 2,5-dimethylpyrazine in the solvent drop increased.
1 adduct 2 between thiourea, 2,5-dimethylpyrazine and methanol (Scheme 1). The sample on the microscope slide had been immersed in a protective layer of inert oil, so the transformation from 2 to 1 presumably occurred via gradual loss of methanol, redissolution of the adduct and then crystallization of 1 as the proportion of 2,5-dimethylpyrazine in the solvent drop increased.
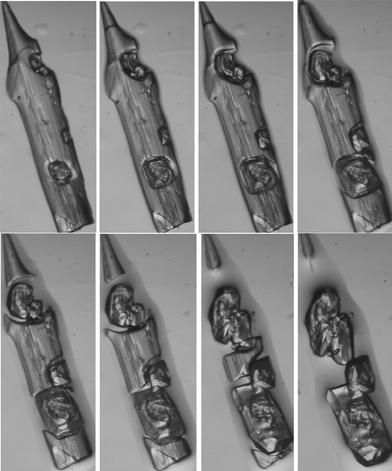 | ||
| Fig. 2 A lath-shaped crystal of ternary adduct 2 converts to several crystals of the binary adduct 1 at room temperature over a period of ca. 30 min. The length of the original crystal was ca. 2 mm. | ||
When the study was extended to the adducts of thiourea and 2-methylpyrazine, the 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 adduct 3 was obtained and was isotypic to 1; this structure has been deposited but is not discussed any further. A 3
3 adduct 3 was obtained and was isotypic to 1; this structure has been deposited but is not discussed any further. A 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adduct was also obtained, but this proved to be a known clathrate structure type in which the guest molecules are severely disordered.10
1 adduct was also obtained, but this proved to be a known clathrate structure type in which the guest molecules are severely disordered.10
X-ray crystallography
Details of the intensity measurements and refinements are given in Table S1.‡ Crystals were mounted in inert oil on glass fibres. Data for 1 were measured with an Oxford Diffraction Xcalibur E diffractometer using monochromated Mo Kα radiation, and, for 2, with an Oxford Diffraction Nova A diffractometer using mirror-focussed Cu Kα radiation;11 multi-scan absorption corrections were performed. The structures were solved with direct methods using SHELXS-97, and structure refinement was performed with full-matrix least-squares on F2 using SHELXL-97.12 NH and OH hydrogens were clearly identified in difference maps and refined freely (in some cases with distance restraints), other hydrogen atoms were refined using rigid methyl groups or a riding model. Molecular graphics were prepared with XP.13Results and discussion
Crystal structures of 1 and 2
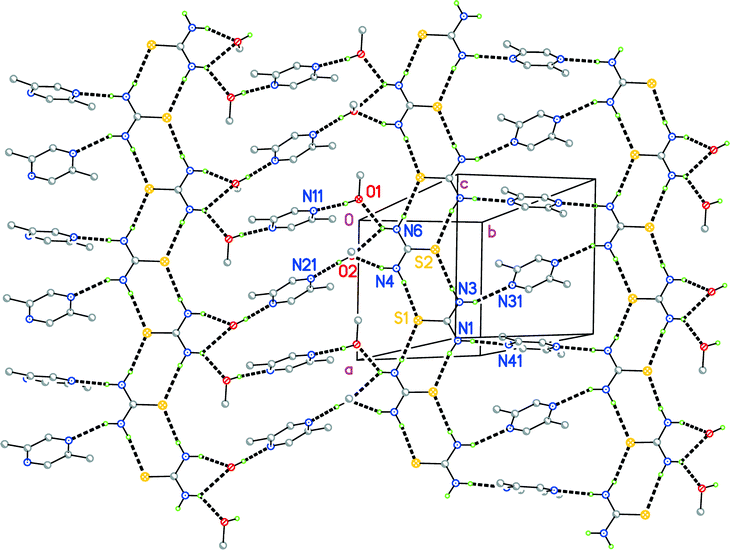
The structure of 2 is conceptually simpler and will be discussed first. The adduct crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , whereby the asymmetric unit consists of two thioureas (interplanar angle 15.63(4)°), two methanols and four half pyrazines; the latter are all extended to complete molecules via inversion symmetry (Fig. 3). It is clear that the two thioureas are topologically different; one is hydrogen-bonded directly to the pyrazines (N1⋯N41 and N3⋯N31), whereas the other is hydrogen-bonded to the two methanols (N4⋯O2 and the three-centre system N6⋯O1, O2), which are in turn hydrogen-bonded to the pyrazines (O1⋯N11 and O2⋯N21).
, whereby the asymmetric unit consists of two thioureas (interplanar angle 15.63(4)°), two methanols and four half pyrazines; the latter are all extended to complete molecules via inversion symmetry (Fig. 3). It is clear that the two thioureas are topologically different; one is hydrogen-bonded directly to the pyrazines (N1⋯N41 and N3⋯N31), whereas the other is hydrogen-bonded to the two methanols (N4⋯O2 and the three-centre system N6⋯O1, O2), which are in turn hydrogen-bonded to the pyrazines (O1⋯N11 and O2⋯N21).
The packing of 2 (Fig. 4) involves ten classical hydrogen bond systems (Table S2‡), counting the three-centre interaction as one system. Each sulfur atom accepts two hydrogen bonds. Despite the tendency of the thiourea sulfur atom to accept hydrogen bonds from any angle to its molecular plane, all four such hydrogen bonds in 2 do in fact subtend small angles to the relevant planes (Table S2‡). The thiourea molecules thereby form an approximately planar ribbon substructure, topologically identical to the urea ribbon shown in Fig. 1, parallel to the a axis. The crosslinks between the ribbons lead to repeating sequences (⋯thiourea⋯methanol⋯pyrazine⋯methanol⋯thiourea⋯pyrazine⋯), which run diagonally across Fig. 4. The unequal spacing between ribbons of a given layer is clearly recognisable and is attributable to the methanol spacers lying between N4, N6 and N11, N21. The extended structure is a layer parallel to (0![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 3).
3).
The 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 adduct 1 also crystallizes in P
3 adduct 1 also crystallizes in P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ; the asymmetric unit consists of two thioureas, one entire pyrazine and one half pyrazine (Fig. 5). The interplanar angle between the two thioureas is 89.15(4)°.
; the asymmetric unit consists of two thioureas, one entire pyrazine and one half pyrazine (Fig. 5). The interplanar angle between the two thioureas is 89.15(4)°.
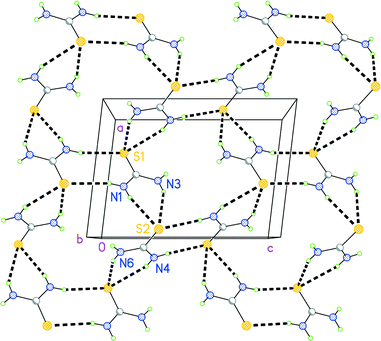
In the packing of 1, each of the eight potential hydrogen bond donors forms one hydrogen bond (Table S3‡). Each sulfur atom accepts three hydrogen bonds. It is not possible for all three D–H donor systems to lie close to the molecular plane of the corresponding acceptor; instead the simple donors (N1–H02 and N4–H06) subtend small angles to the relevant planes, whereas the bifurcated donor systems (N1–H01/N3–H03 and N4–H05/N6–H07) are approximately perpendicular to the relevant planes (Table S3‡). In this way, the thiourea molecules combine to form a corrugated layer structure parallel to the ac plane at y ≈ 0, 1, etc. (Fig. 6), from which the two remaining hydrogen bond donors project outwards.
The pyrazines occupy the spaces between the layers (Fig. 7) and act thereby as hydrogen bond acceptors (although N11 does not accept any classical hydrogen bonds). The hydrogen bond H04⋯N14 is terminal, whereas H08⋯N21 acts as a bridge between thiourea layers. Closer inspection shows that the pyrazines in fact form two “weak” hydrogen bonds (Table S3‡) and a probable π⋯π interaction (the intercentroid distance for neighbouring pyrazines based on N11 is 3.56 Å), but these are omitted from Fig. 7 for clarity.
We have also observed the same 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 stoichiometry for an adduct of thiourea with morpholine,2 but the packing is quite different from that of 1, consisting of an open framework of thiourea molecules forming channels that are occupied with ordered morpholines.
3 stoichiometry for an adduct of thiourea with morpholine,2 but the packing is quite different from that of 1, consisting of an open framework of thiourea molecules forming channels that are occupied with ordered morpholines.
There is no clear evidence for any major conservation of the packing features ongoing from the structure of 2 to that of 1; it seems that the molecules of thiourea and 2,5-dimethylpyrazine are completely redistributed (whereby some of the latter and all of the methanol is lost) during the process of redissolution and recrystallization. Consistent with this, a pool of liquid can be seen to form around the crystals (Fig. 2).
Single-crystal to single-crystal transformations, which may be reversible, are of course a phenomenon that has been widely studied, partly because of potential applications such as molecular switches. In general, the external habit of the crystal is retained. A recent special issue of CrystEngComm was devoted to the theme of single-crystal to single-crystal transformations,14 and the reader can refer to this for general literature references. Spontaneous transformations involving simple organic compounds, adducts or metal complexes appear to be much rarer, although we have observed the spontaneous transformation of one polymorph of cyano(3,5-lutidine)gold(I), consisting of large plates, into small blocks of another polymorph;15 unfortunately we were unable to take suitable photographs because of the small quantity of the available material. Such serendipitously observed processes, although they may be formally regarded as (one)-single-crystal to (several)-single-crystal transformations, do not fit well into the general theme of single-crystal to single-crystal transformations; the crystal habit is not retained, and it is not easy to correlate the packing patterns of the two structures in terms of robust synthons.
Conclusions
Under suitable conditions, individual single crystals of the ternary adduct thiourea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2,5-dimethylpyrazine
2,5-dimethylpyrazine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (1
methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) slowly convert to several crystals, some single, of the binary adduct thiourea
1) slowly convert to several crystals, some single, of the binary adduct thiourea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2,5-dimethylpyrazine (4
2,5-dimethylpyrazine (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3).
3).
Notes and references
- P. G. Jones, C. Taouss, N. Teschmit and L. Thomas, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2013, 69, 405–413 CAS.
- C. Taouss, L. Thomas and P. G. Jones, CrystEngComm, 2013, 15, 6829–6836 RSC.
- C. Taouss and P. G. Jones, CrystEngComm, 2014, 16, 5695–5704 RSC.
- J. D. Lee and S. C. Wallwork, Acta Crystallogr., 1965, 19, 311–313 CrossRef CAS.
- J. Ashurov, B. Ibragimov and S. Talipov, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2012, 68, o504 CAS.
- R. Custelcean, Chem. Commun., 2008, 295–307 RSC (review article).
- A second urea ribbon substructure, not directly relevant to this paper, consists of chains of R12(6) rings linked by bifurcated (N–H⋯)2O units, see ref. 6. An example is furnished by 1,3-dimethylurea: C. Näther, C. Döring, I. Jess, P. G. Jones and C. Taouss, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2013, 69, 70–76 Search PubMed.
- F. H. Allen, C. M. Bird, R. S. Rowland and P. R. Raithby, Acta Crystallogr., Sect. B: Struct. Sci., 1997, 53, 680–695 CrossRef.
- J. A. Platts, S. T. Howard and B. R. F. Bracke, J. Am. Chem. Soc., 1996, 118, 2726–2733 CrossRef CAS.
- See e.g. T. Maris, M. J. Henson, S. J. Heyes and K. Prout, Chem. Mater., 2001, 13, 2483–2492 CrossRef CAS.
- Agilent, CrysAlis PRO., Agilent Ltd., Yarnton, England, 2014 Search PubMed.
- (a) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed; (b) SHELXL-1997, a Program for refining Crystal Structures, G. M. Sheldrick, University of Göttingen, Germany, 1997 Search PubMed.
- Siemens XP, version 5.03. Siemens Analytical X–Ray Instruments, Madison, Wisconsin, U.S.A, 1994 Search PubMed.
- P. Naumov and P. K. Bharadwaj, CrystEngComm, 2015, 17, 8775 RSC.
- C. Döring and P. G. Jones, Z. Naturforsch., B: J. Chem. Sci., 2013, 68, 474–492 Search PubMed.
Footnotes |
| † This paper is dedicated to the memory of Prof. Armand Blaschette (d. 23.9.2015), a highly respected colleague who introduced me to the structural chemistry of urea derivatives. – P. G. J. |
| ‡ Electronic supplementary information (ESI) available. CCDC 1445109 (1), 1445110 (2) and 1445111 (3) contain the supplementary crystallographic data for this paper. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ce00100a |
| This journal is © The Royal Society of Chemistry 2016 |