Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Inclusion complexes of Cethyl-2-methylresorcinarene and pyridine N-oxides: breaking the C–I⋯−O–N+ halogen bond by host–guest complexation†
Rakesh
Puttreddy
a,
Ngong Kodiah
Beyeh
*ab and
Kari
Rissanen
*a
aDepartment of Chemistry, University of Jyvaskyla, Nanoscience Center, P.O. Box 35, FI-40014, Finland. E-mail: kari.t.rissanen@jyu.fi; Tel: +358 50 562 3721
bDepartment of Applied Physics, Aalto University School of Science, P.O. Box 11100, FI-00076, Finland. Fax: +358 9 855 4019; Tel: +358 9 47001
First published on 23rd December 2015
Abstract
C
ethyl-2-Methylresorcinarene forms host–guest complexes with aromatic N-oxides through multiple intra- and intermolecular hydrogen bonds and C–H⋯π interactions. The host shows conformational flexibility to accommodate 3-methylpyridine N-oxide, while retaining a crown conformation for 2-methyl- and 4-methoxypyridine N-oxides highlighting the substituent effect of the guest. N-Methylmorpholine N-oxide, a 6-membered ring aliphatic N-oxide with a methyl at the N-oxide nitrogen, is bound by the equatorial −N–CH3 group located deep in the cavity. 2-Iodopyridine N-oxide is the only guest that manifests intermolecular N–O⋯I–C halogen bond interactions, which are broken down by the host resulting in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 pseudocapsular complex stabilized by additional C–I⋯π interactions between the two 2-iodopyridine N-oxides located in two adjacent hosts. These host–guest complexes were analyzed in the solid state by single crystal X-ray crystallography and in solution by 1H NMR spectroscopy.
2 pseudocapsular complex stabilized by additional C–I⋯π interactions between the two 2-iodopyridine N-oxides located in two adjacent hosts. These host–guest complexes were analyzed in the solid state by single crystal X-ray crystallography and in solution by 1H NMR spectroscopy.
Introduction
Resorcinarenes represent a unique family of host compounds, which are extensively studied in host–guest chemistry due to their π-rich electron cavity in the C4v conformation.1 In the C4v conformation, the bowl shaped cavity of resorcinarenes accommodates a wide range of guest molecules via non-covalent interactions such as cation⋯π, C–H⋯π and π⋯π interactions depending upon the size and charge distribution of the guest molecules.2 Besides lattice stabilization, the phenolic groups participate in hydrogen bonds (HBs) with appropriate guest molecules during complexation.2 As a result, the construction of hydrogen bonded supramolecular networks utilizing resorcinarenes as the key components has been studied with alcohols,3 sugars,4 steroids,5 as well as heterocyclic five- and six-membered ring compounds6 as guest molecules.Pyridine N-oxides (PyNOs) are widely recognized as synthetic intermediates for the functionalization of pyridine rings in organic synthesis.7 This is due to the specific electronic nature of the +N–O− group which makes the aromatic ring electron deficient and thus a very interesting guest molecule with electron-rich host systems.8 Alternatively, the structural and electronic properties of these N-oxide compounds with the polar +N–O− group make them excellent hydrogen bond acceptors.9 In spite of the increasing number of reports on PyNO complexes with calixarenes8a,10 and cavitands,8b,11 reports on the host–guest chemistry of N-oxides and resorcinarenes are very rare.8c,12 The reports on host–guest complexes between Cethyl-2-methylresorcinarene and aromatic N-oxides have highlighted the importance of π⋯π and C–H⋯π interactions with the PyNOs located inside the Cethyl-2-methylresorcinarene cavity.8c These observations prompted us to further probe Cethyl-2-methylresorcinarene as a reaction vessel to control the coordination sphere of copper(II) in the multicomponent reactions of PyNO copper(II) complexes.12 The π⋯π, C–H⋯π and HB interactions held the PyNOs in the Cethyl-2-methylresorcinarene cavity thus controlling the geometry around copper(II).12 The π-rich nature of Cethyl-2-methylresorcinarene and the π-deficient nature of the PyNO guests with multiple HB interaction sites make them a perfect pair for host–guest complexation. Thus, exploring a range of structurally and electronically different aromatic N-oxides with subtle changes in their structure and the ability to template supramolecular host–guest complexes with π-rich host compounds will give a crystal engineering tool to study the intermolecular interactions involved. Despite the numerous literature reports on resorcinarenes, only a handful of crystal structures13 containing Cethyl-2-methylresorcinarene (1) can be found in the Cambridge Crystallographic Database (CSD). Furthermore, there has been no systematic study on host–guest complexes between PyNOs as guests and any member of the resorcinarene family as the host.
In the present study, we explore five different host–guest systems utilizing Cethyl-2-methylresorcinarene (1) as the host and five structurally and electronically different PyNOs as guests (Fig. 1). Cethyl-2-Methylresorcinarene (1) adopts the C4v crown conformation in the solid state.3a,8c,12 Initially, we used 2-methylpyridine N-oxide (2MePyNO) and 3-methylpyridine N-oxide (3MePyNO) to get insight into the effect of substituents on the structure of the host–guest complexes. 4-Methoxypyridine N-oxide (4MeOPyNO) was utilized to study the electronic influence of oxygen atom as a para-substituent. N-Methylmorpholine N-oxide (NMO) was also used as a guest to study the influence of the methyl group at the +N–O− group within an alicyclic ring system.
As the electron-deficient aromatic ring system in PyNOs will polarize the iodine atom in ortho-iodopyridine N-oxide (2-IPyNO) and thus induce possible halogen bonding,14 it was selected as the fifth and multifunctional guest. Based on previous reports, the halogen bond (XB) between the donor part (the iodine atom) and the XB acceptor part (the N-oxide oxygen) of 2-IPyNO was envisaged.15 Based on earlier studies on halo-PyNOs,15 the self-complementary XB between the XB donor part (the iodine atom) and the XB acceptor part (the N-oxide oxygen) of 2-IPyNO was envisaged. The hypothesis was to probe if in the complex between 2-IPyNO and Cethyl-2-methylresorcinarene the resorcinarene host would be able to break the moderately strong dimeric C–I⋯−O–N+ XB by forming a stronger 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 2-IPyNO:1 complex. The obtained host–guest complexes were analysed in the solid state by single crystal X-ray diffraction and in solution by 1H NMR spectroscopy.
1 2-IPyNO:1 complex. The obtained host–guest complexes were analysed in the solid state by single crystal X-ray diffraction and in solution by 1H NMR spectroscopy.
Results and discussion
X-ray crystallography
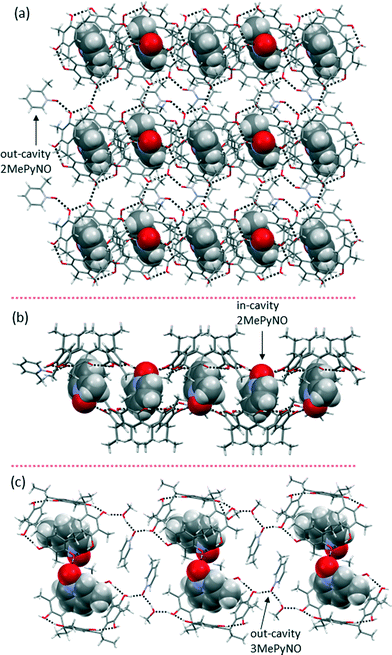
Complexes 2MePyNO@1 and 3MePyNO@1 crystallized (see the ESI† for experimental procedures) in the monoclinic space group P21/n and in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , respectively. In both cases, there are two molecules of N-oxides in the asymmetric unit, one sitting inside the cavity and the other outside the cavity. In complex 2MePyNO@1, one 2MePyNO sits inside the cavity with the N–O group pointing up, and is a bifurcated HB acceptor for two host –OH groups [d(O–H⋯O), 2.679(3) Å and 2.674(3) Å;
, respectively. In both cases, there are two molecules of N-oxides in the asymmetric unit, one sitting inside the cavity and the other outside the cavity. In complex 2MePyNO@1, one 2MePyNO sits inside the cavity with the N–O group pointing up, and is a bifurcated HB acceptor for two host –OH groups [d(O–H⋯O), 2.679(3) Å and 2.674(3) Å; ![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) O–H⋯O, 159° and 166°], as shown in Fig. 2b. The exo-cavity 2MePyNO directly interacts with the –OH group of host 1 [d(O–H⋯O), 2.551(3) Å;
O–H⋯O, 159° and 166°], as shown in Fig. 2b. The exo-cavity 2MePyNO directly interacts with the –OH group of host 1 [d(O–H⋯O), 2.551(3) Å; ![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) O–H⋯O, 160°] via a monodentate hydrogen bond (Fig. 2a). All the O⋯O distances are below the sum of the van der Waals radii of oxygen atoms, and clearly the monodentate HB interaction is stronger than the bidentate HB. Along the a-direction, the –OH groups of host 1 form HB by (O–H)host⋯(O–H)host interactions to give a 2-D polymeric sheet-like structure with the exo-cavity 2MePyNO being a passive spectator, as shown in Fig. 2a. 3MePyNO@1 forms a complex 2-D HB network with the in-cavity 3MePyNO being monodentate and directly hydrogen bonded to the host –OH group [d(O–H⋯O) 2.659(7) Å;
O–H⋯O, 160°] via a monodentate hydrogen bond (Fig. 2a). All the O⋯O distances are below the sum of the van der Waals radii of oxygen atoms, and clearly the monodentate HB interaction is stronger than the bidentate HB. Along the a-direction, the –OH groups of host 1 form HB by (O–H)host⋯(O–H)host interactions to give a 2-D polymeric sheet-like structure with the exo-cavity 2MePyNO being a passive spectator, as shown in Fig. 2a. 3MePyNO@1 forms a complex 2-D HB network with the in-cavity 3MePyNO being monodentate and directly hydrogen bonded to the host –OH group [d(O–H⋯O) 2.659(7) Å; ![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) O–H⋯O, 171°]. The exo-cavity 3MePyNO together with the methanol molecule connects 3MePyNO@1 units by (O–H)host⋯(O–H)CH3OH⋯Opy⋯(H–O)host interactions, as shown in Fig. 2c.
O–H⋯O, 171°]. The exo-cavity 3MePyNO together with the methanol molecule connects 3MePyNO@1 units by (O–H)host⋯(O–H)CH3OH⋯Opy⋯(H–O)host interactions, as shown in Fig. 2c.
C
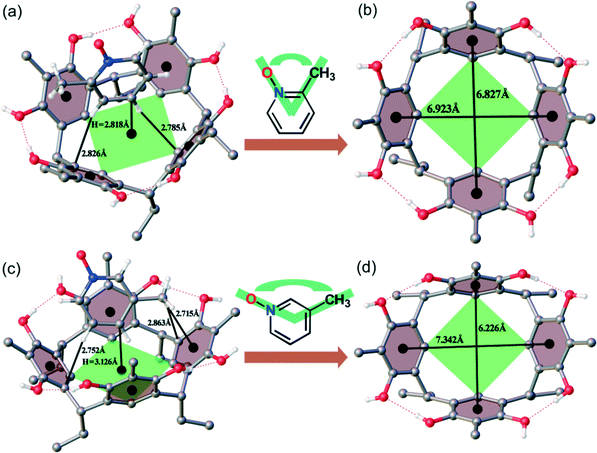
ethyl-2-Methylresorcinarene (1) exhibits remarkable conformational flexibility due to the positioning of the methyl substituents in 2MePyNO and 3MePyNO, thus resulting in varied C–H⋯π interactions.16 The para- and meta-protons of 2MePyNO (Fig. 3a) show C–H⋯π interactions at distances of ca. 2.785 Å [![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) C–H⋯C, 149°] and 2.826 Å [
C–H⋯C, 149°] and 2.826 Å [![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) C–H⋯C, 163°], respectively. On the other hand, in complex 3MePyNO@1, the meta- and –CH3 hydrogens of 3MePyNO show C–H⋯π interactions at distances of ca. 2.752 Å [
C–H⋯C, 163°], respectively. On the other hand, in complex 3MePyNO@1, the meta- and –CH3 hydrogens of 3MePyNO show C–H⋯π interactions at distances of ca. 2.752 Å [![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) C–H⋯C, 160°] and 2.863 Å [
C–H⋯C, 160°] and 2.863 Å [![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) C–H⋯C, 162°], respectively. As shown in Fig. 3c, the hydrogen of the –CH3 group to the centroid of the aromatic ring has the shortest contact at distances of ca. 2.715 Å [
C–H⋯C, 162°], respectively. As shown in Fig. 3c, the hydrogen of the –CH3 group to the centroid of the aromatic ring has the shortest contact at distances of ca. 2.715 Å [![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) C–H⋯π(centroid), 147°]. Guests with substituents close to the N–O group sit deep in the cavity. As a result, 2MePyNO sits at a height of 2.818 Å, while 3MePyNO at distances of 3.126 Å from the centroids of the lower rim carbon atoms of host 1. Furthermore, 2MePyNO with an approximately 60° angle between the N–O and methyl groups sits inside the host cavity without deformation resulting in near similar centroid-to-centroid [6.923 Å and 6.827 Å] distances between opposite aromatic rings (Fig. 3c). However, 3MePyNO with an approximately 120° angle caused significant changes in the host centroid-to-centroid distances between opposite aromatic rings [7.342 Å and 6.226 Å], as shown in Fig. 3d.
C–H⋯π(centroid), 147°]. Guests with substituents close to the N–O group sit deep in the cavity. As a result, 2MePyNO sits at a height of 2.818 Å, while 3MePyNO at distances of 3.126 Å from the centroids of the lower rim carbon atoms of host 1. Furthermore, 2MePyNO with an approximately 60° angle between the N–O and methyl groups sits inside the host cavity without deformation resulting in near similar centroid-to-centroid [6.923 Å and 6.827 Å] distances between opposite aromatic rings (Fig. 3c). However, 3MePyNO with an approximately 120° angle caused significant changes in the host centroid-to-centroid distances between opposite aromatic rings [7.342 Å and 6.226 Å], as shown in Fig. 3d.
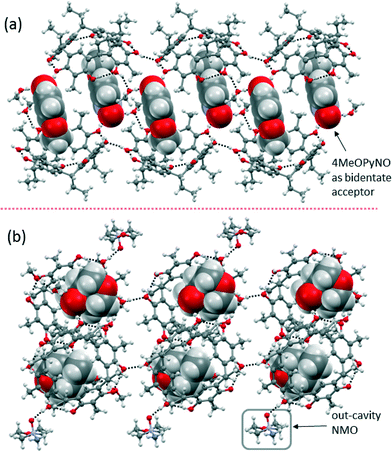
Complex 4MeOPyNO@1 forms a 2-D polymeric sheet structure with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest ratio. In 4MeOPyNO@1, the N–O group of 4MeOPyNO is pointing up and is bidentate with N–O⋯(O–H)host and N–O⋯(O–H)CH3OH interactions at distances of 2.650(3) Å [
1 host–guest ratio. In 4MeOPyNO@1, the N–O group of 4MeOPyNO is pointing up and is bidentate with N–O⋯(O–H)host and N–O⋯(O–H)CH3OH interactions at distances of 2.650(3) Å [![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) O–H⋯O, 158°] and 2.580(3) Å [
O–H⋯O, 158°] and 2.580(3) Å [![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) O–H⋯O, 175°], respectively (Fig. 4a). The extent of the HB interaction of 4MeOPyNO and 2MePyNO with the host –OH groups are very similar. Unlike 2MePyNO@1 and 3MePyNO@1, no C–H⋯π(host) interactions between the aromatic ring protons of 4MeOPyNO and Cethyl-2-methylresorcinarene (1) are observed. Moreover, the in-cavity –OCH3 group also assists the 3D crystal packing by weak O⋯H–C interactions with the adjacent Cethyl chain of the host.
O–H⋯O, 175°], respectively (Fig. 4a). The extent of the HB interaction of 4MeOPyNO and 2MePyNO with the host –OH groups are very similar. Unlike 2MePyNO@1 and 3MePyNO@1, no C–H⋯π(host) interactions between the aromatic ring protons of 4MeOPyNO and Cethyl-2-methylresorcinarene (1) are observed. Moreover, the in-cavity –OCH3 group also assists the 3D crystal packing by weak O⋯H–C interactions with the adjacent Cethyl chain of the host.
Complex NMO@1 contains in-cavity and exo-cavity NMO molecules. The in-cavity N–O group and the host –OH group are connected by two methanol molecules while the exo-cavity NMO directly HB to the –OH group of host 1 (Fig. 4b). The difference in host cavity distortions depends on the guest height situated in the cavity, viz. 3.077 Å for 4MeOPyNO and 2.920 Å for NMO. In both cases, the guest molecules are situated to the corner of the host, stabilized via C–H⋯π interactions. In 4MeOPyNO@1, the –OCH3 group and the host aromatic ring are stabilized by C–H⋯π interactions at distances ranging between 2.919 Å and 3.129 Å, of which C–H⋯centroid was observed to have the shortest contact with a distance of 2.681 Å (Fig. S4a†). In NMO@1, the in-cavity NMO interacts with the host aromatic ring through C–H⋯π at distances of 2.937 Å and 3.221 Å (Fig. S4b†). The N–O group appears to be the reason for the presence of the in-cavity –N–CH3 group, which makes NMO a unique guest molecule from other N-oxides.
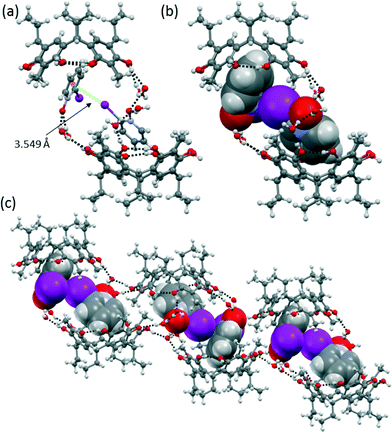
Complex 2-IPyNO@1 reveals a pseudo-capsular arrangement as shown in Fig. 5. The asymmetric unit contains two Cethyl-2-methylresorcinarenes, each accommodating 2-IPyNO molecules together with six exo-cavity water molecules. The N–O groups of in-cavity 2-IPyNO acts as bidentate and tridentate (Fig. S5†) HB acceptors for the exo-cavity water and adjacent host molecules in stabilizing the pseudo-capsular arrangements by O–H⋯O interactions. One of the iodines in the cavity of one host interacts with the phenyl ring of the 2-IPyNO located in the cavity of the second host through intermolecular C–I⋯π contacts at distances of 3.549 Å [![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) C–I⋯π (centroid), 161° and
C–I⋯π (centroid), 161° and ![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) C–I⋯π (plane), 155°]. The height of the capsule, defined as the distance between the centroids of the lower rim carbons, is 13.479 Å (Fig. S6†),8c in which the two 2-IPyNO molecules are accommodated at heights of 2.720 Å and 3.153 Å. The hosts adopt a distorted crown conformation with centroid-to-centroid distances of 6.790/6.960 Å and 6.816/6.961 Å (Fig. S7†). The height and orientation of the 2-IPyNO molecules increase the number of C–H⋯π interactions with the host aromatic ring. The 2-IPyNO parallel to the host aromatic ring and situated at a height of 3.153 Å is stabilized by two C–H⋯π interactions, while the non-parallel 2-IPyNO situated deep in the cavity at a height of 2.720 Å is stabilized uniquely by three C–H⋯π interactions (Fig. S7†).
C–I⋯π (plane), 155°]. The height of the capsule, defined as the distance between the centroids of the lower rim carbons, is 13.479 Å (Fig. S6†),8c in which the two 2-IPyNO molecules are accommodated at heights of 2.720 Å and 3.153 Å. The hosts adopt a distorted crown conformation with centroid-to-centroid distances of 6.790/6.960 Å and 6.816/6.961 Å (Fig. S7†). The height and orientation of the 2-IPyNO molecules increase the number of C–H⋯π interactions with the host aromatic ring. The 2-IPyNO parallel to the host aromatic ring and situated at a height of 3.153 Å is stabilized by two C–H⋯π interactions, while the non-parallel 2-IPyNO situated deep in the cavity at a height of 2.720 Å is stabilized uniquely by three C–H⋯π interactions (Fig. S7†).
The orientation of the guest aromatic ring deep in the host cavity with the N–O group pointing up has been a primary prerequisite to encapsulate PyNOs by Cethyl-2-methylresorcinarene (1). As a consequence, the guest molecules become responsive to these interactions. As such, the self-assembly process can be controlled with respect to the guest interactions. To illustrate this, 2-IPyNO was crystallised under similar solvent conditions to compare the nature of guest interactions in the absence of Cethyl-2-methylresorcinarene (1). The crystal structure of 2-IPyNO (Fig. 6) displays a classical intermolecular N–O⋯I–C XB at distances of 2.791 Å with an XB ratio (RXB = dXB/(Xvdw + Bvdw)) of 0.791.14
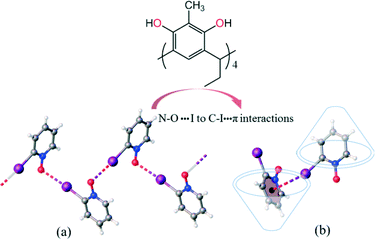 | ||
| Fig. 6 (a) 1-D polymeric N–O⋯I interaction based XB complex of 2-IPyNO, and (b) confined C–I⋯π interactions between 2-IPyNO by Cethyl-2-methylresorcinarene (1) inside complex 2-IPyNO@1. | ||
The type and strength of the electron withdrawing group attached to the aromatic ring and its influence on the hybridization of the aromatic ring affect the polarization of the halogen atom (usually Br or I) acting either as an electron donor or acceptor. Although iodine has weak interactions with ortho-carbons [d(C–I⋯C), 3.716 Å; ![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) C–I⋯C, 141.30°] and nitrogen [d(C–I⋯N), 3.649 Å;
C–I⋯C, 141.30°] and nitrogen [d(C–I⋯N), 3.649 Å; ![[rectangle open, vertical]](https://www.rsc.org/images/entities/char_25af.gif) C–I⋯N, 141.28°] of the pyridine N-oxide ring, the centroid of the aromatic ring is influenced by the shortest contact with a distance of 3.549 Å. Thus, the iodine substituent clearly demonstrates the presence of weak C–I⋯π halogen type interactions which are enhanced inside the pseudo capsular arrangement.
C–I⋯N, 141.28°] of the pyridine N-oxide ring, the centroid of the aromatic ring is influenced by the shortest contact with a distance of 3.549 Å. Thus, the iodine substituent clearly demonstrates the presence of weak C–I⋯π halogen type interactions which are enhanced inside the pseudo capsular arrangement.
A CCDC search was carried out to survey the type and nature of molecules involved during intermolecular C–I⋯aromatic ring interactions. The first search was limited to any 2-substituted iodo-aromatic compounds and their non-bonded interactions with an adjacent aromatic ring. In total, 22 hits were found,13d,17 and of all the structures, one of those reported constitutes the shortest distance of 3.321 Å.17o A search for perfluorinated iodobenzene related C–I⋯π interactions revealed zero hits. Consequently, individual surveys were carried out on compounds that have neutral aprotic electron-withdrawing groups (–F, –Cl, –NO2, –CN, –CF3, –CCl3 and –COCl) and electron donating groups (alkyl and –NR2) at the 2-substituted position of iodo-aromatic compounds. Only the chloro substituent retrieved one hit, which has a C–I⋯π distance of 3.537 Å.18
NMR analyses
Solution studies between Cethyl-2-methylresorcinarene (1) as the host and 2MePyNO, 3MePyNO, 4MeOPyNO, NMO, and 2-IPyNO as the guests were conducted via1H NMR experiments in CD3OD at room temperature. In the experiments, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixtures of the host and guests were prepared; the 1H NMR spectra were measured and the results were compared with the free host (6.6 mM) and free guests (6.6 mM).
2 mixtures of the host and guests were prepared; the 1H NMR spectra were measured and the results were compared with the free host (6.6 mM) and free guests (6.6 mM).
Significant complexation-induced shielding of the guest proton resonances was observed in all cases. The shielding effects of the aromatic rings of the bowl-shaped host cavity upon addition of the guest are responsible for this upfield shift and clearly point to a fast guest exchange on the NMR time scale. Taking the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
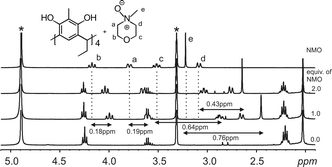
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture between Cethyl-2-methylresorcinarene (1) and NMO as an example (Fig. 7), the methyl group protons (e) are the most shielded (0.76 ppm). These shift changes clearly confirm the orientation of the guest in the host cavity. The large shift change for the methyl protons suggests that the protons are situated deep in the cavity of the host. This is analogous to the X-ray structure (Fig. 4b).
1 mixture between Cethyl-2-methylresorcinarene (1) and NMO as an example (Fig. 7), the methyl group protons (e) are the most shielded (0.76 ppm). These shift changes clearly confirm the orientation of the guest in the host cavity. The large shift change for the methyl protons suggests that the protons are situated deep in the cavity of the host. This is analogous to the X-ray structure (Fig. 4b).
Analyses of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
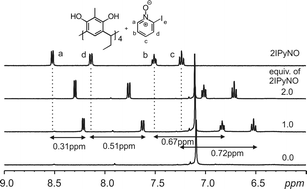
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture between Cethyl-2-methylresorcinarene (1) and 2-IPyNO (Fig. 8) reveal the aromatic protons (b, c) to be the most shielded (0.67–0.72 ppm) with the proton next to the –NO group the least shielded (0.31 ppm). This supports the orientation of the guest within the host cavity as seen from the X-ray structures (Fig. 5 and 6). The analyses of the 1H NMR results between the host and the other guests (2MePyNO, 3MePyNO and 4MeOPyNO) also confirm the orientation of the guests in the host cavity (Fig. S1–S3†) and support the structures observed from solid state analyses (Fig. 2 and 4). Additionally, the flexibility of the host when accommodating the guest is observed from the small changes in the host upper rim methyl groups and the aromatic protons upon complex formation. This again supports the observation from solid-state studies.
1 mixture between Cethyl-2-methylresorcinarene (1) and 2-IPyNO (Fig. 8) reveal the aromatic protons (b, c) to be the most shielded (0.67–0.72 ppm) with the proton next to the –NO group the least shielded (0.31 ppm). This supports the orientation of the guest within the host cavity as seen from the X-ray structures (Fig. 5 and 6). The analyses of the 1H NMR results between the host and the other guests (2MePyNO, 3MePyNO and 4MeOPyNO) also confirm the orientation of the guests in the host cavity (Fig. S1–S3†) and support the structures observed from solid state analyses (Fig. 2 and 4). Additionally, the flexibility of the host when accommodating the guest is observed from the small changes in the host upper rim methyl groups and the aromatic protons upon complex formation. This again supports the observation from solid-state studies.
Conclusions
Five host–guest complexes between Cethyl-2-methylresorcinarene (1) and five different N-oxides, three aromatic (2MePyNO, 3MePyNO, and 4MeOPyNO), one aliphatic (NMO) and 2-iodopyridine N-oxide (2-IPyNO), were obtained and analysed in the solid state and in solution via single crystal X-ray diffraction studies and 1H NMR analyses, respectively. The conformational flexibility of the host was observed when ortho- and meta-methylated guest molecules were utilized. All the aromatic guests were located in the cavity such that the N–O groups point upwards (out of the cavity). However, the aliphatic analogue NMO reveals the equatorial –N–CH3 group to be located deep in the cavity of the host, which greatly changes the cavity size and conformation of the host. The 1-D polymeric halogen bonded complex was disrupted in the presence of the host, resulting in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest pseudo capsular complex when 2-iodopyridine N-oxide (2-IPyNO) was used. Extra C–I⋯π interactions between the two 2-IPyNO located in two different hosts help to glue the two capsule halves. In all the complexes, the binding of the N-oxides proceeds through multiple intra- and intermolecular hydrogen bonds, –C–H⋯π, π⋯π and –C–I⋯π interactions. The solution analyses through 1H NMR measurements clearly support the structures observed in the solid state. Aromatic and aliphatic N-oxides are proving to be suitable guest compounds for the resorcinarene cavity when in the C4v conformation. N-Oxides have huge potential with numerous applications as ligands in organo-metallic chemistry. Their ability to interact with resorcinarenes implies that they can be utilized in tandem to tune and construct functional assemblies.
2 host–guest pseudo capsular complex when 2-iodopyridine N-oxide (2-IPyNO) was used. Extra C–I⋯π interactions between the two 2-IPyNO located in two different hosts help to glue the two capsule halves. In all the complexes, the binding of the N-oxides proceeds through multiple intra- and intermolecular hydrogen bonds, –C–H⋯π, π⋯π and –C–I⋯π interactions. The solution analyses through 1H NMR measurements clearly support the structures observed in the solid state. Aromatic and aliphatic N-oxides are proving to be suitable guest compounds for the resorcinarene cavity when in the C4v conformation. N-Oxides have huge potential with numerous applications as ligands in organo-metallic chemistry. Their ability to interact with resorcinarenes implies that they can be utilized in tandem to tune and construct functional assemblies.
Acknowledgements
The Academy of Finland (K. R.: grant no. 265328 and 263256; N. K. B.: grant no. 258653), the University of Jyvaskyla and Aalto University are gratefully acknowledged for financial support.Notes and references
- (a) P. Timmerman, W. Verboom and D. N. Reinhoudt, Tetrahedron, 1996, 52, 2663–2704 CrossRef CAS; (b) V. Böhmer, Angew. Chem., Int. Ed. Engl., 1995, 34, 713–745 CrossRef; (c) K. Rissanen, Angew. Chem., Int. Ed., 2005, 44, 3652–3654 CrossRef CAS PubMed.
- (a) J. L. Atwood and A. Szumna, Chem. Commun., 2003, 940–941 RSC; (b) J. L. Atwood, L. J. Barbour and A. Jerga, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4837–4841 CrossRef CAS PubMed; (c) L. R. MacGillivray and J. L. Atwood, Chem. Commun., 1999, 181–182 RSC; (d) A. Shivanyuk, E. F. Paulus, K. Rissanen, E. Kolehmainen and V. Böhmer, Chem. – Eur. J., 2001, 7, 1944–1951 CrossRef CAS PubMed; (e) N. K. Beyeh, M. Kogej, A. Åhman, K. Rissanen and C. A. Schalley, Angew. Chem., Int. Ed., 2006, 45, 5214–5218 CrossRef CAS PubMed.
- (a) N. K. Beyeh, D. P. Weimann, L. Kaufmann, C. A. Schalley and K. Rissanen, Chem. – Eur. J., 2012, 18, 5552–5557 CrossRef CAS PubMed; (b) L. Avram, Y. Cohen and J. Rebek Jr., Chem. Commun., 2011, 47, 5368–5375 RSC; (c) I. A. Koshets, Z. I. Kazantseva, A. E. Belyaev and V. I. Kalchenko, Sens. Actuators, B, 2009, 140, 104–108 CrossRef CAS; (d) O. Ugono and K. T. Holman, Chem. Commun., 2006, 2144–2146 RSC; (e) O. D. Fox, J. F.-Y. Leung, J. M. Hunter, N. K. Dalley and R. G. Harrison, Inorg. Chem., 2000, 39, 783–790 CrossRef CAS PubMed.
- (a) E. Kalenius, T. Kekäläinen, R. Neitola, K. Beyeh, K. Rissanen and P. Vainiotalo, Chem. – Eur. J., 2008, 14, 5220–5228 CrossRef CAS PubMed; (b) M. He, R. J. Johnson, J. O. Escobedo, P. A. Beck, K. K. Kim, N. N. S. Luce, C. J. Davis, P. T. Lewis, F. R. Fronczek, B. J. Melancon, A. A. Mrse, W. D. Treleaven and R. M. Strongin, J. Am. Chem. Soc., 2002, 124, 5000–5009 CrossRef CAS PubMed; (c) T. Evan-Salem, I. Baruch, L. Avram, Y. Cohen, L. C. Palmer and J. Rebek, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 12296–12300 CrossRef CAS PubMed; (d) T. Rhlalou, M. Ferhat, M. A. Frouji, D. Langevin, M. Métayer and J.-F. Verchère, J. Membr. Sci., 2000, 168, 63–73 CrossRef CAS.
- (a) A. Shivanyuk and J. Rebek, Chem. Commun., 2001, 2374–2375 RSC; (b) J. D. Faull and V. K. Gupta, Thin Solid Films, 2003, 440, 129–137 CrossRef CAS; (c) J. D. Faull and V. K. Gupta, Langmuir, 2002, 18, 6584–6592 CrossRef CAS.
- (a) M. Nissinen, E. Wegelius, D. Falábu and K. Rissanen, CrystEngComm, 2000, 2, 151 RSC; (b) M. Nissinen and K. Rissanen, Supramol. Chem., 2003, 15, 581–590 CrossRef CAS.
- (a) A. E. V. Gorden, J. Xu, K. N. Raymond and P. Durbin, Chem. Rev., 2003, 103, 4207–4282 CrossRef CAS PubMed; (b) R. R. Schrock, Chem. Rev., 2002, 102, 145–180 CrossRef CAS PubMed; (c) J. A. Pool, B. L. Scott and J. L. Kiplinger, J. Am. Chem. Soc., 2005, 127, 1338–1339 CrossRef CAS PubMed.
- (a) G. Zheng, Y.-Y. Li, H.-D. Guo, S.-Y. Song and H.-J. Zhang, Chem. Commun., 2008, 4918–4920 RSC; (b) L. Adriaenssens and P. Ballester, Chem. Soc. Rev., 2013, 42, 3261–3277 RSC; (c) N. K. Beyeh, R. Puttreddy and K. Rissanen, RSC Adv., 2015, 5, 30222–30226 RSC.
- (a) N. J. Babu, L. S. Reddy and A. Nangia, Mol. Pharmaceutics, 2007, 4, 417–434 CrossRef CAS PubMed; (b) N. R. Goud, N. J. Babu and A. Nangia, Cryst. Growth Des., 2011, 11, 1930–1939 CrossRef CAS; (c) S. G. Bodige, M. A. Zottola, S. E. McKay and S. C. Blackstock, Cryst. Eng., 1998, 1, 243–253 CrossRef CAS; (d) M. Muthuraman, R. Masse, J.-F. Nicoud and G. R. Desiraju, Chem. Mater., 2001, 13, 1473–1479 CrossRef CAS.
- (a) B. Verdejo, G. Gil-Ramírez and P. Ballester, J. Am. Chem. Soc., 2009, 131, 3178–3179 CrossRef CAS PubMed; (b) G. Zheng, W. Fan, S. Song, H. Guo and H. Zhang, J. Solid State Chem., 2010, 183, 1457–1463 CrossRef CAS; (c) G. W. Orr, L. J. Barbour and J. L. Atwood, Science, 1999, 285, 1049–1052 CrossRef CAS PubMed; (d) B.-T. Zhao, H. Wang, H.-Y. Zhang and Y. Liu, J. Mol. Struct., 2005, 740, 101–105 CrossRef CAS; (e) K. Xiong, F. Jiang, M. Wu, Y. Gai, Q. Chen, S. Zhang, J. Ma, D. Han and M. Hong, J. Solid State Chem., 2012, 192, 215–220 CrossRef CAS; (f) J. L. Atwood, G. W. Orr and K. D. Robinson, Supramol. Chem., 1994, 3, 89–91 CrossRef CAS.
- A. Galán, E. C. Escudero-Adán, A. Frontera and P. Ballester, J. Org. Chem., 2014, 79, 5545–5557 CrossRef PubMed.
- N. K. Beyeh and R. Puttreddy, Dalton Trans., 2015, 44, 9881–9886 RSC.
- (a) N. K. Beyeh, A. Valkonen and K. Rissanen, CrystEngComm, 2014, 16, 3758–3764 RSC; (b) K. Aoki, T. Nagae, R. Matsubara and I. Fujisawa, Chem. Lett., 2004, 33, 1264–1265 CrossRef CAS; (c) I. Fujisawa, D. Takeuchi, R. Kato, K. Murayama and K. Aoki, Bull. Chem. Soc. Jpn., 2011, 84, 1133–1135 CrossRef CAS; (d) K. Aoki, T. Nagae, S. Yamaguchi and I. Fujisawa, Bull. Chem. Soc. Jpn., 2005, 78, 2066–2068 CrossRef CAS.
- (a) G. R. Desiraju, P. S. Ho, L. Kloo, A. C. Legon, R. Marquardt, P. Metrangolo, P. Politzer, G. Resnati and K. Rissanen, Pure Appl. Chem., 2013, 85, 1711 CrossRef CAS; (b) P. Metrangolo, G. Resnati, T. Pilati and S. Biella, Halogen Bonding: Fundamentals and Applications, Springer, 2008 Search PubMed; (c) R. Wilcken, M. O. Zimmermann, A. Lange, A. C. Joerger and F. M. Boeckler, J. Med. Chem., 2013, 56, 1363–1388 CrossRef CAS PubMed; (d) K. Rissanen, CrystEngComm, 2008, 10, 1107–1113 RSC; (e) C. B. Aakeröy, M. Baldrighi, J. Desper, P. Metrangolo and G. Resnati, Chem. – Eur. J., 2013, 19, 16240–16247 CrossRef PubMed; (f) F. Meyer and P. Dubois, CrystEngComm, 2013, 15, 3058–3071 RSC; (g) R. W. Troff, T. Mäkelä, F. Topić, A. Valkonen, K. Raatikainen and K. Rissanen, Eur. J. Org. Chem., 2013, 2013, 1617–1637 CrossRef CAS.
- (a) M. T. Messina, P. Metrangolo, W. Panzeri, T. Pilati and G. Resnati, Tetrahedron, 2001, 57, 8543–8550 CrossRef CAS; (b) C. B. Aakeroy, T. K. Wijethunga, J. Benton and J. Desper, Chem. Commun., 2015, 51, 2425–2428 RSC; (c) C. B. Aakeroy, T. K. Wijethunga and J. Desper, CrystEngComm, 2014, 16, 28–31 RSC.
- (a) C. Janiak, J. Chem. Soc., Dalton Trans., 2000, 3885–3896 RSC; (b) G. R. Desiraju, Acc. Chem. Res., 2002, 35, 565–573 CrossRef CAS PubMed.
- (a) M. Barceló-Oliver, A. Terrón, A. García-Raso, J. J. Fiol, E. Molins and C. Miravitlles, J. Inorg. Biochem., 2004, 98, 1703–1711 CrossRef PubMed; (b) M. Livendahl, C. Goehry, F. Maseras and A. M. Echavarren, Chem. Commun., 2014, 50, 1533–1536 RSC; (c) M. G. D. Leed, N. Wolkow, D. M. Pham, C. L. Daniel, J. L. Dunaief and K. J. Franz, J. Inorg. Biochem., 2011, 105, 1161–1172 CrossRef CAS PubMed; (d) M. Casimiro, L. Roces, S. García-Granda, M. J. Iglesias and F. López Ortiz, Org. Lett., 2013, 15, 2378–2381 CrossRef CAS PubMed; (e) O. Knop, T. S. Cameron, P. K. Bakshi, A. Linden and S. P. Roe, Can. J. Chem., 1994, 72, 1870–1881 CrossRef CAS; (f) M. N. Arshad, M. N. Tahir, I. U. Khan, W. A. Siddiqui and M. Shafiq, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2009, 65, o230 CAS; (g) D. Das and G. R. Desiraju, Chem. – Asian J., 2006, 1, 231–244 CrossRef CAS PubMed; (h) S. Renganayaki, E. Subramanian, S. Shanmuga Sundara Raj and H.-K. Fun, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1999, 55, 1525–1526 Search PubMed; (i) J. Powell, A. Lough and T. Saeed, J. Chem. Soc. Dalton Trans., 1997, 4137–4138 RSC; (j) R. Trokowski, S. Akine and T. Nabeshima, Chem. – Eur. J., 2011, 17, 14420–14428 CrossRef CAS PubMed; (k) B. V. Meprathu, M. W. Justik and J. D. Protasiewicz, Tetrahedron Lett., 2005, 46, 5187–5190 CrossRef CAS; (l) C. V. Ramana, Y. Goriya, K. A. Durugkar, S. Chatterjee, S. Krishnaswamy and R. G. Gonnade, CrystEngComm, 2013, 15, 5283–5300 RSC; (m) B. K. Saha and A. Nangia, Heteroat. Chem., 2007, 18, 185–194 CrossRef CAS; (n) Ö. Aydin, N. Çaylak Delibacs, H. Necefouglu and T. Hökelek, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2012, 68, m601–m602 Search PubMed; (o) P. P. Kapadia, D. C. Swenson and F. C. Pigge, Cryst. Growth Des., 2012, 12, 698–706 CrossRef CAS; (p) S. K. Nayak, M. K. Reddy, D. Chopra and T. N. Guru Row, CrystEngComm, 2012, 14, 200–210 RSC; (q) M. Barceló-Oliver, A. García-Raso, A. Terrón, E. Molins, M. J. Prieto, V. Moreno, J. Martínez, V. Lladó, I. López, A. Gutiérrez and P. V. Escribá, J. Inorg. Biochem., 2007, 101, 649–659 CrossRef PubMed; (r) K. Goubitz, E. J. Sonneveld and H. Schenk, Z. Kristallogr. - Cryst. Mater., 2001, 216, 176 CAS.
- C. R. Hubbard, V. L. Himes, A. D. Mighell and S. W. Page, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 2819–2821 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Crystal structures and crystallographic data. CCDC numbers 1407237–1407242. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ce02354h |
| This journal is © The Royal Society of Chemistry 2016 |