Supramolecular tryptophan-zipper forms a tripeptide as a regular proton transporter†
Abstract
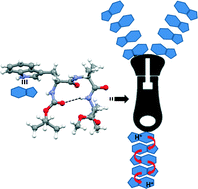
The diverse structure and assembly of two peptide analogues have been investigated. The tripeptide containing tryptophan, α-aminoisobutyric acid and valine self-assembles through various noncovalent interactions to form a tryptophan-zipper-like structure by intermolecular hydrogen bonding and π–π interactions. But the leucine analogue adopts a water-mediated hydrogen-bonded cage-like structure. X-ray crystallography shed some light on the structure of the tryptophan-zipper at the atomic level. Moreover, in U-tube experiments using pH gradient as the driving force across a liquid chloroform phase, the supramolecular tryptophan-zipper acts as a passive regular proton transporter.
- This article is part of the themed collection: 2016 New talent

 Please wait while we load your content...
Please wait while we load your content...