Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fast and efficient synthesis of a host guest system: a mechanochemical approach†
Manuel
Wilke
ab,
Maria
Klimakow
ab,
Klaus
Rademann
b and
Franziska
Emmerling
*a
aBAM Federal Institute for Materials Research and Testing, Richard-Willstaetter-Strasse 11, 12489 Berlin, Germany. E-mail: franziska.emmerling@bam.de; Fax: +49 30 8104 1137; Tel: +49 30 8104 1133
bDepartment of Chemistry, Humboldt-Universität zu Berlin, Brook-Taylor-Strasse 2, 12489 Berlin, Germany
First published on 11th January 2016
Abstract
An unusually fast and effective synthesis procedure for a host guest system consisting of a metal organic framework (MOF) and a polyoxometalate (POM) is described. The material was synthesised mechanochemically and the evolution of the structure was monitored ex and in situ using synchrotron X-ray diffraction (XRD).
Polyoxometalates (POMs) are metal oxygen cluster anions formed by early transition metals, foremost vanadium, molybdenum, and tungsten.1 Their structural variety and unusual electronic properties lead to applications in different fields such as analytical chemistry, catalysis, biochemistry, and medicine.2–5 To increase the field of applications attempts were made to stabilise and immobilise these clusters. One possibility is the encapsulation in porous coordination polymers (PCPs).6 Zubieta and co-workers described the first host guest system, where [Mo8O26]6−-cluster were encapsulated between Cu- and Ni-bipyridine chains.7 Numerous structures have been characterised where the POM guests are arranged between PCP chains and layers.8 Liu et al. synthesised a series of compounds (denoted NENU-n),9 consisting of Keggin-type POMs as guest species and a Cu–BTC (BTC = 1,3,5-benzenetricarboxylate) framework (HKUST-110) as host. As an example, the authors studied the catalytic properties of [Cu12(BTC)8][H3PW12O40] (NENU-3a) for the hydrolysis of organic esters. The catalytic activity of NENU-3a was four times higher than for the free heteropoly acid. Furthermore NENU-3a has a high selectivity for small molecules. The structures were synthesised by heating the Keggin compound, Cu(NO3)2·2H2O, 1,3,5-benzenetricarboxylic acid H3BTC, and tetramethylammonium hydroxide ((CH3)4NOH) at 180 °C in a Teflon-lined autoclave for 24 h (yield = 60–80%).9 The addition of (CH3)4NOH was necessary for deprotonation and charge compensation.
For industrial applications, it is necessary to produce catalysts in a facile and efficient way. In this context, mechanochemical syntheses have gained increasing popularity and are already used in industrial processes.11,12 Mechanosyntheses are easy to handle and produce only small amounts of waste.11,13–16
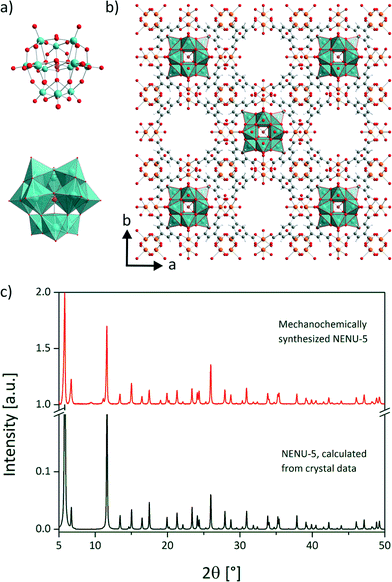
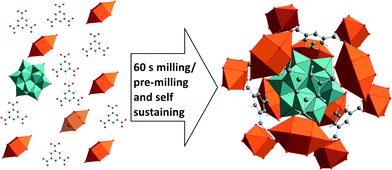
Here, we present a fast and complete mechanochemical synthesis of the host guest supra-molecule NENU-5 in a three component reaction.17 In this structure, Keggin ions [PMo12O40]6− (Fig. 1a) are encapsulated in the HKUST-1 framework. The mechanochemical synthesis of HKUST-1 was described earlier.18–21
A vibration ball mill (Pulverisette 23, FRITSCH GmbH, Germany) was used equipped with a 10 mL stainless steel vessel and two stainless steel balls (10 mm, 4 g). In the first step 229.5 mg (0.12 mmol) of the heteropoly acid H3PMo12O40·1.5H2O and 296.8 mg (1.49 mmol) copper(II) acetate monohydrate were neat milled at 30 Hz for 5 min. The heteropoly acid was synthesised according to literature.22 To the gained homogeneous mixture 208.3 mg (0.99 mmol) of H3BTC and 400 mL of ethanol were added. The reaction mixture was milled at 50 Hz for 15 min. Copper(II) acetate monohydrate (cryst. extra pure, Merck), 1,3,5-benzenetricarboxylic acid (98%, Alfa Aesar) and ethanol (absolute, Merck) were purchased commercially and were used without further purification. The identification of the product was accomplished by powder XRD on a D8 diffractometer (Cu Kα1 radiation, Bruker AXS, Karlsruhe, Germany). The comparison of the obtained sample with the simulated diffraction pattern for NENU-5 proves the purity of the compound (Fig. 1c). Unlike previously reported, the use of (CH3)4NOH was not necessary for the successful synthesis. Further details are given in the ESI.† The structure of the host guest supra-molecule is depicted in Fig. 1b. Half of the pores of the framework are filled with the Keggin-type POM.
To gain insights in the underlying formation mechanism, the reaction progress was investigated by XRD. First experiments were analyzed ex situ. Every 30 s the milling experiment was stopped and a small amount of the sample was drawn for XRD measurements. Fig. 2a shows the resulting diffraction patterns during the synthesis of NENU-5. These measurements include a pre-milling step of copper(II) acetate monohydrate with the POM. The pre-milling was performed at 30 Hz for 5 min. After the addition of ethanol and H3BTC the mixture was milled at 50 Hz. The first pattern was detected after the pre-milling. The reflections of copper(II) acetate monohydrate and the POM can be assigned. The next diffraction pattern (t = 0 s) shows the reaction mixture after the addition of ethanol and H3BTC before the milling was started. The pattern is already dominated by the product reflections and the reaction is almost completed. 30 s later a weak peak at 2θ = 12.8° indicates that copper(II) acetate monohydrate is still present in a very small ratio. The reaction is completed after 60 s. In the following, the diffraction patterns show no further changes. The interval between sample drawing and measurement was 1.5 h.
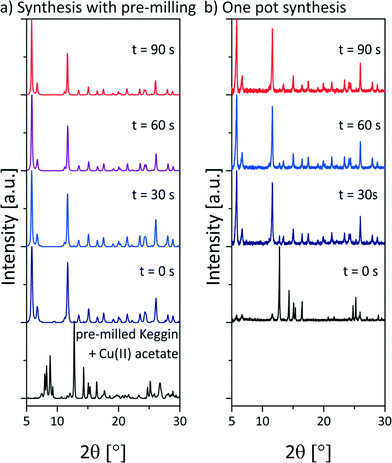 | ||
| Fig. 2 Diffraction patterns of a) the ex situ investigation of the synthesis of NENU-5 including a pre-milling step of copper(II) acetate monohydrate and the POM. The measurements were performed at the μSpot-Beamline at Bessy II (Helmholtz Centre Berlin for Materials and Energy, Germany23). b) Diffraction patterns of the ex situ investigation of the one pot synthesis of NENU-5. The diffraction patterns were measured on a D8 diffractometer (Bruker AXS, Karlsruhe, Germany); pre-milled Keggin + Cu(II) acetate = diffraction pattern of the mixture of copper(II) acetate monohydrate and H3PMo12O40·1.5H2O after 5 min milling time at 30 Hz. The time given above the curves refers to the milling time at 50 Hz after the addition of the remaining compounds. | ||
The data indicate that the strong tendency of NENU-5 to self-assemble promotes a self-sustaining reaction. The short milling period of only two of three reactants is sufficient to trigger the reaction. The importance of the pre-milling step is evident from the ex situ investigation of the one pot synthesis (Fig. 2b). The reaction was conducted at 50 Hz. The diffraction pattern before the milling was collected 2 h after the sample drawing (see Fig. 2b below (black)). Two very weak reflections at 5.8° and 6.7° indicate the formation of NENU-5 in a very small ratio. 90 s of milling time are required until the reaction is completed.
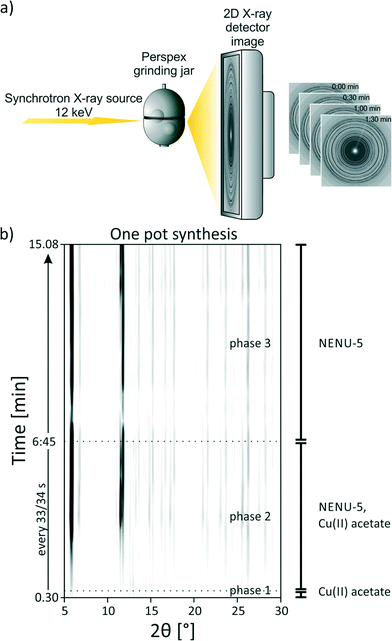
The milling reactions were further investigated in situ. The in situ monitoring of mechanochemical reactions with X-ray diffraction is a recently developed and promising method.24–27 The setup used here was described earlier.26 A schematic representation of the used setup is shown in Fig. 3a. In brief, the respective compounds and two stainless steel balls (10 mm, 4 g) were ground together in a custom-made 10 mL Perspex vessel. The reactions were investigated in situ using synchrotron XRD (μSpot-Beamline at Bessy II, Helmholtz Centre Berlin for Materials and Energy, Germany). The measurement time was 30 s with a delay time of 3–4 s. Masses were used as described for the synthesis of NENU-5.
Four different synthesis pathways were analysed comprising two principles: either two reactants were pre-milled first and the third reactant was added later (reactions 1–3) or all reactants were milled together in a one pot synthesis (reaction 4). For reactions 1–3 two reactants were pre-milled at 30 Hz for 5 min. Afterwards 300 μL of ethanol and the third starting material were added. This reaction mixture was then milled for 25 min at 30 Hz. For the in situ investigation of reaction 4 all three starting materials and ethanol were milled together at 30 Hz for 25 min. The lower frequency was chosen to decrease the reaction time for a better detection of the intermediates.
The 2D plots of the in situ investigations of the reactions 1–3 (with pre-milling) are shown in Fig. S1–S3.† For all pre-milling steps no reaction was observed during dry milling. Only reflections of the starting materials can be detected. The POM was detected in its hydrated form with thirteen water molecules in the crystal structure. After the addition of ethanol and the third compound reflections of the POM vanished in reaction 1–3. The reactions 1–3 result in the formation of NENU-5 after 30 s of milling. During these reactions the reflections of starting materials or side products vanish at different times. In reaction 1 (pre-milling step of copper(II) acetate monohydrate and the POM), the conversion is completed after 8 min. At this point the formation of the product can be deduced from several sharp and intense reflections. The intensity increases in the next minutes. In reaction 2 (pre-milling step of copper(II) acetate monohydrate and H3BTC) reflections of the framework HKUST-1 appear after addition of ethanol and before the second milling was started. The weak intensity of the reflections implicate that only a small amount of HKUST-1 is formed. The compound is stable for 60 s. Afterwards, only reflections of NENU-5 can be detected. It is unlikely that the Keggin ions fill the pores of an existing HKUST-1 framework, since the channels between the pores are too small for the Keggin ions. In milling experiments starting from powdery HKUST-1, the Keggin-type POM, and ethanol (LAG conditions) showed no conversion. For the three component reaction, we can therefore assume that in the vicinity of NENU-5 the temporarily formed HKUST-1 is destroyed and rebuilt as NENU-5. The reflections of copper(II) acetate monohydrate disappear after 6 min of milling time in reaction 2. The ones of HKUST-1 vanish 30 s later. Only a few peaks of NENU-5 with low intensity can be detected at this point. The crystallinity starts to increase after 13 min of milling. In reaction 3 (pre-milling step of H3BTC and the POM) reflections of the copper starting material with low intensity persist until 11:45 min. After 8 min all reflections of NENU-5 are already detectable with a high intensity.
The analysis of reaction 1–3 indicate that the pre-milling of copper(II) acetate monohydrate is the most effective reaction regarding the speed of product formation. The reflections of copper(II) acetate monohydrate always vanish at last, comparing all starting materials. These reflections were detected for the longest time (6:45 min after the restart) in reaction 3 where copper(II) acetate monohydrate is not pre-milled. Reaction 2, where copper(II) acetate monohydrate and H3BTC were pre-milled, is the fastest, based on the detection of starting materials. All reflections of the starting materials vanished 60 s after the restart. Only a few reflections of the final product are detectable at this time. Reaction 1, where copper(II) acetate monohydrate is pre-milled with the POM shows the shortest interval until a high number of intensive reflections of the final product are reached. The diffraction pattern after the addition of ethanol and H3BTC but before the milling shows no reflections of the product contrary to the respective ex situ investigation. The waiting time is obviously necessary for the self-sustaining mechanism. These results are in accordance with previous described mechanochemical self-sustaining reactions.28
Fig. 3b shows the 2D plot of the in situ investigation of the one pot synthesis (reaction 4), where all starting materials and ethanol were milled together at once. In the first measurement after 30 s only reflections of copper(II) acetate monohydrate appear (phase 1). Other starting materials cannot be observed. This is similar to reactions 1–3 after the addition of ethanol. After 60 s reflections belonging to NENU-5 appear (start of phase 2). The intensity of the reflections of copper(II) acetate monohydrate decreases and vanishes completely after 6:30 min. Our findings show that the three component reaction can be performed as one pot synthesis. The pre-milling is not necessary for the formation of the final product, but increases the reaction rate after the addition of the third compound. Based on our results we conclude that the compound shows a strong tendency to self-assembly. The Keggin ions act as template and the metal–organic framework is formed around the ions (Fig. 4).
Summarizing, we report an unexpected fast mechanochemical synthesis of a host guest system consisting of a Cu–BTC MOF and Keggin ions. The pronounced tendency of the system to self-assemble was revealed based on in situ synchrotron XRD data. Ex situ studies show that the reaction is self sustaining after the dry pre-milling of two compounds of the three starting materials.
Acknowledgements
We gratefully acknowledge financial support by the Deutsche Forschungsgemeinschaft through SPP 1415 (crystalline non-equilibrium phases and polymorphs): Ra 494/15-2, and Em 198/3-2.References
- M. T. Pope and A. Mueller, Polyoxometalate chemistry: from topology via self-assembly to applications, Kluwer Academic Publishers, Dordrecht, 2001 Search PubMed.
- M. T. Pope and A. Mueller, Angew. Chem., 1991, 103, 56–70 CrossRef CAS.
- X. Lopez, J. J. Carbo, C. Bo and J. M. Poblet, Chem. Soc. Rev., 2012, 41, 7537–7571 RSC.
- H. Stephan, M. Kubeil, F. Emmerling and C. E. Muller, Eur. J. Inorg. Chem., 2013, 1585–1594 CrossRef CAS.
- M. Genovese and L. Kerynl, Curr. Opin. Solid State Mater. Sci., 2015, 19, 126–137 CrossRef CAS.
- S. R. Batten, N. R. Champness, X. M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Ohrstrom, M. O'Keeffe, M. P. Suh and J. Reedijk, Pure Appl. Chem., 2013, 85, 1715–1724 CrossRef CAS.
- D. Hagrman, C. Zubeita, D. J. Rose, J. Zubieta and R. C. Haushalter, Angew. Chem., Int. Ed. Engl., 1997, 36, 873–876 CrossRef CAS.
- R. M. Yu, X. F. Kuang, X. Y. Wu, C. Z. Lu and J. P. Donahue, Coord. Chem. Rev., 2009, 253, 2872–2890 CrossRef CAS.
- C. Y. Sun, S. X. Liu, D. D. Liang, K. Z. Shao, Y. H. Ren and Z. M. Su, J. Am. Chem. Soc., 2009, 131, 1883–1888 CrossRef CAS PubMed.
- S. S. Y. Chui, S. M. F. Lo, J. P. H. Charmant, A. G. Orpen and I. D. Williams, Science, 1999, 283, 1148–1150 CrossRef CAS PubMed.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friscic, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- S. Domingos, V. Andre, S. Quaresma, I. C. B. Martins, M. F. M. da Piedade and M. T. Duarte, J. Pharm. Pharmacol., 2015, 67, 830–846 CrossRef CAS PubMed.
- T. Friscic, Chem. Soc. Rev., 2012, 41, 3493–3510 RSC.
- E. Boldyreva, Chem. Soc. Rev., 2013, 42, 7719–7738 RSC.
- L. Tröbs, M. Wilke, W. Szczerba, U. Reinholz and F. Emmerling, CrystEngComm, 2014, 16, 5560–5565 RSC.
- D. Prochowicz, K. Sokolowski, I. Justyniak, A. Kornowicz, D. Fairen-Jimenez, T. Friscic and J. Lewinski, Chem. Commun., 2015, 51, 4032–4035 RSC.
- W. B. Yuan, T. Friscic, D. Apperley and S. L. James, Angew. Chem., Int. Ed., 2010, 49, 3916–3919 CrossRef CAS PubMed.
- M. Klimakow, P. Klobes, A. F. Thünemann, K. Rademann and F. Emmerling, Chem. Mater., 2010, 22, 5216–5221 CrossRef CAS.
- W. Yuan, A. L. Garay, A. Pichon, R. Clowes, C. D. Wood, A. I. Cooper and S. L. James, CrystEngComm, 2010, 12, 4063–4065 RSC.
- M. Klimakow, P. Klobes, K. Rademann and F. Emmerling, Microporous Mesoporous Mater., 2012, 154, 113–118 CrossRef CAS.
- D. Crawford, J. Casaban, R. Haydon, N. Giri, T. McNally and S. L. James, Chem. Sci., 2015, 6, 1645–1649 RSC.
- V. Sasca, M. Stefanescu and A. Popa, J. Therm. Anal. Calorim., 1999, 56, 569–578 CrossRef CAS.
- O. Paris, C. H. Li, S. Siegel, G. Weseloh, F. Emmerling, H. Riesemeier, A. Erko and P. Fratzl, J. Appl. Crystallogr., 2007, 40, S466–S470 CrossRef CAS.
- T. Friscic, I. Halasz, P. J. Beldon, A. M. Belenguer, F. Adams, S. A. J. Kimber, V. Honkimaki and R. E. Dinnebier, Nat. Chem., 2013, 5, 66–73 CrossRef CAS PubMed.
- I. Halasz, A. Puskaric, S. A. J. Kimber, P. J. Beldon, A. M. Belenguer, F. Adams, V. Honkimaki, R. E. Dinnebier, B. Patel, W. Jones, V. Strukil and T. Friscic, Angew. Chem., Int. Ed., 2013, 52, 11538–11541 CrossRef CAS PubMed.
- L. Batzdorf, F. Fischer, M. Wilke, K. J. Wenzel and F. Emmerling, Angew. Chem., Int. Ed., 2015, 54, 1799–1802 CrossRef CAS PubMed.
- F. Fischer, G. Scholz, L. Batzdorf, M. Wilke and F. Emmerling, CrystEngComm, 2015, 17, 824–829 RSC.
- A. Pichon, A. Lazuen-Garay and S. L. James, CrystEngComm, 2006, 8, 211–214 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: In situ data for experiments including a pre-milling step (Fig. S1–S3), calculated diffraction patterns of all compounds detected during the reaction (Fig. S4). See DOI: 10.1039/c5ce01868d |
| This journal is © The Royal Society of Chemistry 2016 |