Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: A narrow amide I vibrational band observed by sum frequency generation spectroscopy reveals highly ordered structures of a biofilm protein at the air/water interface
Zhuguang
Wang
a,
M. Daniela
Morales-Acosta
b,
Shanghao
Li
c,
Wei
Liu
a,
Tapan
Kanai
a,
Yuting
Liu
a,
Ya-Na
Chen
a,
Frederick J.
Walker
b,
Charles H.
Ahn
b,
Roger M.
Leblanc
c and
Elsa C. Y.
Yan
*a
aDepartment of Chemistry, Yale University, 225 Prospect Street, New Haven, Connecticut 06520, USA. E-mail: elsa.yan@yale.edu
bDepartment of Applied Physics, Yale University, 15 Prospect Street, New Haven, Connecticut 06520, USA
cDepartment of Chemistry, University of Miami, 1301 Memorial Drive, Coral Gables, Florida 33146, USA
First published on 4th August 2016
Abstract
Correction for ‘A narrow amide I vibrational band observed by sum frequency generation spectroscopy reveals highly ordered structures of a biofilm protein at the air/water interface’ by Zhuguang Wang et al., Chem. Commun., 2016, 52, 2956–2959.
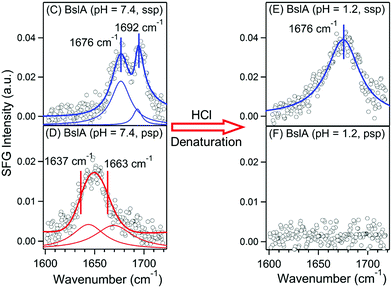
In follow-up studies by the authors, it was found that due to an inaccuracy in calibration of spectrometer, there should be a blue-shift of 7 cm−1 in the peak positions of the SFG spectra published in this article. The new peak positions allowed more straightforward spectral assignments of the reported SFG spectral based on the standard amide I frequencies of various protein secondary structures.1,2 The chiral SFG spectrum of BslA at pH = 7.4 (Fig. 1D) is now assigned to antiparallel β-sheet B2 mode (1637 cm−1) and β-turn (1663 cm−1). The peak assignments in the achiral spectra (Fig. 1C and E) remain unchanged: β-turn (1676 cm−1) and antiparallel β-sheet B1 mode (1692 cm−1). The recalibrated spectra (Fig. 1C–F) and fitting parameters are provided below. The conclusion of the studies reported in the article remains unaffected.
| Parameters |
Amide I (achiral-ssp)
Fig. 1C (pH 7.4) |
|
|---|---|---|
| χ NR (a.u.) | 0.020 ± 0.005 | |
| ω (cm−1) | 1675.5 ± 0.7 | 1692.3 ± 0.4 |
| A (a.u.) | 1.99 ± 0.24 | 0.53 ± 0.16 |
| Γ (a.u.) | 11.75 ± 1.29 | 5.25 ± 1.09 |
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- L. K. Tamm and S. A. Tatulian, Infrared Spectroscopy of Proteins and Peptides in Lipid Bilayers, Q. Rev. Biophys., 1997, 30, 365–429 CrossRef CAS PubMed.
- A. Barth and C. Zscherp, What Vibrations Tell Us About Proteins, Q. Rev. Biophys., 2002, 35, 369–430 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |