Highlights from Faraday Discussion: Chemistry in the urban atmosphere, United Kingdom, April 2016
Zongbo
Shi
*,
Mohammed Salim
Alam
and
Irina
Nikolova
School of Geography Earth and Environmental Sciences, The University of Birmingham, Birmingham, B15 2TT, UK. E-mail: Z.shi@bham.ac.uk
First published on 4th July 2016
The Great Smog of 1952 in London (see Fig. 1) was arguably the greatest air pollution event in the history of atmospheric science. This brought London to a virtual standstill and caused severe adverse health effects, prompting research into atmospheric science and the introduction of air quality legislation. Over 60 years later, on 6 April 2016, the Royal Society of Chemistry (RSC) welcomed over 200 atmospheric scientists from around the world to attend the 275th Faraday Discussion meeting titled ‘Chemistry in the Urban Atmosphere’. Scientists gathered to discuss current ‘hot topics’ in atmospheric science and air pollution, including the emissions of the oxides of nitrogen and particulate matter, which still pose a significant health threat in urban environments. | ||
| Fig. 1 Police using flames at Marble Arch to direct the traffic. The great smog was so thick people that could not see their feet. Photograph: Trinity Mirror/Alamy. | ||
Although this Faraday Discussion was the 6th meeting related to atmospheric chemistry and air pollution, it was the first meeting focused on the urban atmosphere. Previous meetings included:
• Chemical Reactions in the Atmosphere (1964, Edinburgh)
• Atmospheric Chemistry (1995, Norwich)
• Atmospheric Chemistry (2005, Cambridge)
• The Physical Chemistry of Aerosols (1960, Bristol)
• Tropospheric Aerosol – Formation, Transformation, Fate and Impacts (2013, Leeds)
The meeting was opened by the chair of the organising committee, Professor Roy Harrison (University of Birmingham), at the RSC library in Burlington House (Fig. 2). In an informative 10 minute introductory speech, he presented the scale of air pollution processes in the urban area (Fig. 3) and compared them to regional and global processes. He demonstrated that urban processes are usually associated with: (1) a high level of primary emissions, (2) strong concentration gradients – both horizontal and vertical, (3) mixing processes, (4) typically higher aerosol surface areas, and (5) a dominance of rapid chemical processes. This indeed provided the motivation for organising such a meeting, as the scale of the processes within the urban atmosphere are fundamentally different to those of the regional and global atmospheres.
 | ||
| Fig. 2 The venue of the Faraday Discussion meeting: the RSC library at Burlington House (photo by Zhe Tian). | ||
Opening lecture
Professor Xavier Querol (CSIC, Spain) introduced the opening lecture which was given by Professor Urs Baltensperger (Paul Scherrer Institute), who started his talk by presenting a similar figure to that shown by Professor Roy Harrison, and discussed some published literature to highlight the complexity of urban atmospheric chemistry (DOI: C6FD00065G). Drawing upon the recent Volkswagen scandal, Professor Baltensperger challenged the current NO2 emission inventory, and asked the ‘million dollar’ question: “is diesel bad and gasoline good?” He then discussed recent advances in secondary organic aerosols (SOAs), emphasizing the need to better understand the emissions of semi-volatile compounds from vehicles. Very interesting data highlighting the role of ammonium, amines, oxidized organics, and highly oxygenated multifunctional organic compounds in new particle formation were presented.1–3 He also showed that field data from Jungfraujoch were consistent with CLOUD chamber data,4 about which the audience were very intrigued. Professor Baltensperger then spoke of the development of source apportionment techniques, including multi-linear engine 2 (ME-2),5 as well as the toxicity of particles. The talk concluded that although there is a huge advancement in urban atmospheric chemistry, many questions still remain unanswered. The lecture was followed by a series of questions, concerning general patterns of air pollution in the urban atmosphere and the relevance of smog chamber studies in understanding atmospheric processes.Session 1: Chemical complexity of the urban atmosphere and its consequences
The first session, chaired by Dr André Prévôt (Paul Scherrer Institute), discussed the complexity of organic aerosols (OAs) in the urban atmosphere using a range of analytical measurement techniques, and featured talks by Professor Neil Donahue (Carnegie Mellon University), Professor Markus Kalberer (University of Cambridge) and Dr Mohammed Alam (University of Birmingham). The session was opened by Professor Donahue who presented single-particle measurements of phase partitioning between primary and secondary organic aerosols (DOI: C5FD00214A). The experiments exploited quantitative single-particle mass spectrometry and explored the extent that primary organic aerosol (POA) particle populations interact with each other or with secondary organic aerosols (SOAs) representative of background OA populations. Single particle measurements provide constraints on the interactions of chemically distinct aerosol populations, allowing the measurements of phase partitioning between POA and SOA systems. Professor Donahue discussed how mixed aerosol populations will converge via gas phase exchange of semi-volatile organic compounds (SVOCs), the timescale of which is dependent upon the volatility of the constituents. All OA populations, however, are not fully miscible; for example, polar SOAs and non-polar POAs can remain distinct for hours. Specific thresholds for miscibility remain unclear and merit further investigation.Professor Markus Kalberer presented the second talk, where the composition of fine particulate matter at urban background, road tunnel and industrial harbour sites was analysed using direct infusion, nano-electrospray ionisation ultrahigh resolution mass spectrometry (UHRMS) (DOI: C5FD00206K). Approximately one thousand oxidised organic compounds were separated but not unambiguously identified at a molecular level due to the complexity of OAs. Double bond equivalents, aromaticity equivalents and aromaticity index classification criteria were utilised to assess the fraction of aromatic components at the different sampling locations. A larger number of mono-aromatic, polycyclic aromatic hydrocarbon (PAH) and nitrogen-containing mono-aromatic compounds were identified in the tunnel samples, while a higher fraction of sulphur-containing compounds were found at the urban background site, suggesting the influence of an atmospheric ageing process or the presence of non-traffic related sources, or most probably both. Professor Kalberer pointed out that electrospray ionisation mass spectrometry is mostly sensitive to more polar organic compounds. Thus, UHRMS in combination with instrumentation that is capable of separating thousands of compounds yet is less sensitive to polar organic species, could lead to promising results in characterising the entire organic compositional complexity, comprehensively, in atmospheric particles at a molecular level. Such an approach that could be used in combination is the two-dimensional gas chromatography-mass spectrometry instrument which appropriately introduces the following talk.
Dr Mohammed Alam, in the final talk of the first session, presented the use of thermal desorption coupled to comprehensive two-dimensional gas chromatography time-of-flight mass spectrometry (TD-GC × GC-ToF-MS) for the characterisation of diesel engine exhaust particles (DOI: C5FD00185D). Although tens of thousands of compounds were chromatographically resolved, a few hundred were confidently identified and quantitative results for n-alkanes, n-alkylcyclohexanes and PAHs were presented in both the gas and particulate phases in the diesel exhaust. Results were discussed for measurements made with and without the diesel oxidation catalyst (DOC), and indicated limited efficiency for removing high molecular weight compounds, under the studied engine conditions. This stimulated much discussion of whether test bed activities should be developed into more real world type setups, as the efficiency of a DOC is largely dependent upon its age, running conditions (i.e. operating temperature, engine conditions etc.), exhaust gas composition and space velocity. The concentrations of C12 to C33n-alkanes measured in 13 different size fractions were also presented showing an increase in the non-volatile concentration with decreasing size fraction. Although the Kelvin effect may be an unlikely reason for this observation, a sampling artefact cannot be disregarded and warrants further research.
After a spot of tea, the second afternoon session included talks from Professor Alastair Lewis (University of York), Dr Rachel Dunmore (University of York) and Dr Timothy Wallington (Ford Motor Company, Michigan). Professor Lewis evaluated the performance of low cost electrochemical sensors for air pollution research (DOI: C5FD00201J). These low cost commercially available sensors for CO, Ox, SO2, NO, NO2, particulate matter (PM) and volatile organic compounds (VOCs) were evaluated in laboratory based experiments and in ambient air, and were cross referenced against well-established reference standard measurement instruments. Professor Lewis highlighted that the interferences from stable trace gases were generally small (in absolute terms) but the sensors could be prone to large artefact responses if a high ratio of a co-pollutant to the measured substance was present. Ozone sensors demonstrated good overall agreement with the reference measurements, once the sensor calibration values had been linked to the reference observation. Although NO and PM sensors possessed notable negative and positive biases compared to their reference counterparts, general trends in atmospheric pollution were recreated. NO2 and VOC sensors, however, did not follow the general trends of their reference measurements and were shown to be responding to different air pollution metrics that require better definition. This talk received much discussion throughout the meeting, and Professor Lewis concluded that these devices can at best be considered as a ‘work in progress’ rather than ‘state-of-the-art’. Much research and development is required to improve these low cost sensors that are widely publicised in the media and utilised by various amateurs, in order to produce reliable results.
Time resolved measurements of atmospheric ethanol during the winter and summer Clean Air for London (ClearfLo) campaigns in 2012 and the potential impacts of future fuel formulations (DOI: C5FD00190K) were presented in the second talk by Dr Rachel Dunmore. Mean mixing ratios of approximately 5 ppb and maximum levels in excess of 30 ppb of ethanol were observed, making it the most abundant VOC in London air. Significant levels of acetaldehyde were also observed where the correlation of both ethanol and acetaldehyde with other VOCs suggest that their primary emission source is likely to be combustion and evaporative emissions from the use of ethanol blended gasoline. The impact of road transport related ethanol emissions on secondary species was also presented by means of detailed chemical box modelling and global and nested regional scale chemical transport modelling. The significant levels of acetaldehyde observed could not be accounted for from ethanol oxidation using detailed chemical box modelling (incorporating the Master Chemical Mechanism, MCMv3.2), see Fig. 4. However, the model significantly under-predicts the total levels of acetaldehyde indicating missing primary emission source(s), which Dr Dunmore suggested may be related to traffic emissions. In order to match observed mixing ratios with GEOS-Chem model simulations, acetaldehyde emissions would need to be increased by a factor of 40. Various reasons as to why acetaldehyde is underestimated in current atmospheric inventories were discussed, which may include missing reactions within explicit chemical schemes leading to its production, an underestimation of ethanol emissions and thus its secondary photochemical formation, direct emissions from ethanol combustion and/or an underestimation of its emissions from gasoline combustion.
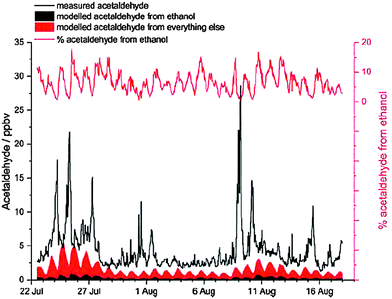 | ||
| Fig. 4 The measured acetaldehyde mixing ratios from the summer ClearfLo campaign and the simulated mixing ratios using MCMv3.2 (DOI: C5FD00190K). | ||
The final talk of the day was given by Dr Timothy Wallington, who discussed the impact of increasing biofuel content in fuels on vehicle emissions and urban air quality (DOI: C5FD00205B). The critical influence of engine calibration on the NOx emission was demonstrated by measuring the emissions from a Ford F-350 fuelled with either fossil diesel or biodiesel (butyl nonanoate) fuel. Using the current production chain engine calibration resulted in increased NOx emissions when using biodiesel fuel. However, when adjusting the engine calibration, reduced emissions of NOx were achieved. This signifies the importance of engine design and calibration for new vehicles, in order to utilise biodiesel fuel. Dr Wallington outlined that future increases in biofuel content may facilitate required emission reductions to improve urban air quality, providing that engine design and calibration for new vehicles are sufficiently optimised for specific ethanol blend levels.
The session was closed with an impressive series of 30 second “lightning” presentations, highlighting the selected posters that were due to be presented at the conference's two poster sessions. This intense rapid fire session allowed a large number of delegates to summarise their work clearly and concisely, and surprisingly within their time limits. This was followed by a gentle reminder from Professor Roy Harrison that delegates who had not yet read the remainder of the Faraday Discussion's papers, due to be presented over the next couple of days, were able to do so after the poster session, providing they did not visit the taproom on their free evening. The delegates were then provided with wine and refreshments whilst stimulating discussions continued during the extremely high quality poster session.
Session 2: Timescales of mixing and of chemistry
On Thursday morning, the delegates were ready to engage in Session 2 of the discussions, chaired by Dr Francis Pope (University of Birmingham), and including talks from Professor William Brune (Penn State University), Professor William Bloss (University of Birmingham), Dr Shengrui Tong (Institute of Chemistry, Chinese Academy of Sciences, Beijing) and Dr Saewung Kim (University of California, Irvine). The session was opened by Professor Brune who discussed ozone production chemistry in the presence of urban plumes (DOI: C5FD00204D). Concentrations of OH and HO2 radicals and OH reactivity from the CalNex_SJV study in Bakersfield CA in 2010 were presented and compared with simulations using a photochemical box model. Taking the known HO2 interference of the laser induced fluorescence (LIF) instrument into account, HO2 concentrations substantially exceeded those modelled in NOx-rich plumes. The observed HO2 and ozone production rates calculated from the measured HO2 decreased more slowly than the modelled HO2 and calculated ozone production rates as a function of NO, resulting in approximately 45% missing OH reactivity, relative to the total measured OH reactivity. Professor Brune suggested that this important discrepancy is not due to the measurement of the HO2 radical, potential interferences in sampling or the sampling of transported plumes; but rather it may be due to either emissions of unknown organic species that accompany the NO emissions (which may account for the missing OH reactivity), or the OH + NO + O2 → HO2 + NO2 reaction kinetics (resolving the HO2 discrepancy).The second talk was given by Professor William Bloss on the interpretation of in situ HONO measurements via the photochemical steady state (DOI: C5FD00224A). HONO, NOx and HOx measurements at an urban background site were reported, supporting the presence of additional HONO sources in the atmosphere. Spatially resolved measurements of HONO and NOx abundance were obtained using a mobile instrument laboratory. The measurements of the spatial variability of HONO in different environments (urban, suburban and rural) show pronounced changes in abundance, particularly in proximity to major roads within urban areas. Professor Bloss indicated that photo-stationary steady state (PSS) analyses near major roads in urban areas are potentially problematic. However, in rural and some suburban regions, the measurements show areas of homogeneity in HONO and NOx abundance, suggesting that PSS analyses are most probably valid. This approach in measurements prompted the discussion for the need of fast time resolution VOC measurements in order to obtain the spatial variability of OH.
Dr Shengrui Tong presented the third talk of the session and discussed the HONO formation mechanism in Beijing during the winter period (DOI: C5FD00163C). Continuous HONO measurements were made at an urban and a suburban site and illustrated similar variations in concentration, albeit being higher at the urban site. The correlation of HONO with CO and NOx indicated direct emissions of HONO at the urban site. Calculations suggest that a mean direct emissions contribution of up to 49% of the total mean HONO concentration was observed at the urban site, while a significantly smaller contribution of 10% was observed at the suburban area. Dr Tong showed that the formation of HONO in urban and suburban areas is significantly different, and can be affected by homogeneous/heterogeneous reactions, NO2 conversion efficiency – influenced by PM2.5 concentrations and relative humidity, and other meteorological conditions. High concentrations of NO in urban areas led to a significant contribution of the homogeneous reaction to the production of HONO; while the production of HONO from heterogeneous reactions was 10 times larger at the suburban site during haze periods.
The final talk of the session was by Dr Saewung Kim who presented OH reactivity at an urban site and a suburban forest region in Seoul, South Korea – a rapidly emerging megacity in Eastern Asia (DOI: C5FD00230C). Approximately 50% and 35% of the total OH reactivity observed at the urban site was accounted for by NOx and VOCs, respectively. The comprehensive VOC data suggest that isoprene accounts for 25–47% of the total OH reactivity attributed to VOCs. At the urban site almost all the OH reactivity could be accounted for, using the trace gas data available. However, for the suburban forest region only 30% of the OH reactivity could be accounted for. Dr Kim argued that biogenic VOC (BVOC) emissions and their oxidation products may be responsible for this missing OH reactivity, but some delegates debated that the amount of unmeasured and unknown VOCs and their oxidation products could account for a substantial amount of the missing OH reactivity. It was argued that if all of the unknown VOCs were identified then the missing OH reactivity would be reduced dramatically. However, there is no known instrument that can identify the entire matrix of compounds in the atmosphere and thus, the discussion of powerful instrumental techniques from the first session of talks re-emerged, as delegates continued discussions into the coffee room, after the session was concluded.
Session 3: Urban case studies
Session 3 of the meeting was chaired by Professor Urs Baltensperger (Paul Scherer Institute) and included talks by Professor Spyros Pandis (Carnegie Mellon University), Dr Dominik van Pinxteren (TROPOS Leipzig), Dr Zongbo Shi (University of Birmingham; presenting on behalf of Professor Kebin He (Tsinghua University)), Dr Maria Cruz Minguillón (Institute of Environmental Assessment and Water Research), Professor Constantinos Sioutas (University of Southern California), Dr Martin Shafer (University of Wisconsin-Madison), Dr Christian Ehlers (Research Centre Julich), Dr David Carslaw (Ricardo-AES and University of York) and Dr James Lee (University of York).During this session Professor Baltensperger proposed a new Faraday Discussion question answering mechanism that would enable early-career researchers to be given priority if they wanted to ask questions. It was found that the proposal of raising two hands for doctoral researchers and one hand for experienced researchers led to more active participation from the younger delegates.
The session was opened by Professor Spyros Pandis who presented ‘a tale of five cities’ (DOI: C5FD00212E). By showing observations from five case studies (Athens, Paris, Pittsburgh, Los Angeles and Mexico City) over a relatively long time scale, he demonstrated how changes in emissions by industrial and transportation sources affect the urban atmosphere. He also showed that the contribution of “usual suspects” to fine PM, such as local transportation and industry, has been decreasing due to air pollution control whereas that of secondary aerosols, a large fraction of which comes from long range transport, has been increasing in all five cities. Sources such as wood burning and cooking also show a significant increase in contribution to urban PM2.5. Professor Pandis concluded that atmospheric reactions, leading to the formation of secondary aerosols, play a vital role in urban air pollution in developed countries, making the source apportionment of PM more challenging. It also makes it more difficult to further reduce the PM pollution as emission control from a much larger area (e.g., 500 km) needs to be considered. A lot of the following discussions focused on non-traditional aerosol sources such as cooking and the uncertainty in Positive Matrix Factorisation (PMF) attribution.
The second talk by Dr Dominik van Pinxteren presented the source apportionment of size-resolved aerosol particles in Leipzig, Germany (DOI: C5FD00228A). A number of size-fractionated particulate matter samples from four sites were analysed for inorganic ions, organic and elemental carbon, water soluble organic carbon, trace metals, and organic compounds. PMF was used for source apportionment. They found a major difference in the sources of PM of different sizes, where traffic appears to be the dominant source for quasi-ultrafine particles (0.05–0.14 μm), regional aerosols (mostly secondary in origin) for PM from 0.14 to 1.2 μm and urban sources for large particles (3.5–10 μm). Dr van Pinxteren concluded that the amounts of traffic related pollutants such as soot were lower than those in 2000, suggesting that the low emission zone is helping to improve the air quality in Leipzig.
After lunch, Dr Zongbo Shi presented on behalf of Professor Kebin He, who was unable to attend. In this paper, Li et al. took advantage of a unique opportunity for strict air pollution control in Beijing and wider northern China for the China Victory Day Parade to examine how emission control affected the aerosol chemistry (DOI: C6FD00004E). Their receptor modelling analysis showed that compared with the pre-emission control period, POAs from traffic and cooking emissions decreased by 42% whereas SOAs reduced by 59%. They demonstrated that under similar weather conditions, PM1 mass concentrations reduced by 52–57% on average during the emission control period (Fig. 5). Interestingly, the bulk submicron aerosol composition remained similar prior to and during the emission control periods. Li et al. further showed that the molar ratios of sulfate or nitrate to the sums of the sulfate and SO2 or nitrate and NO2 were lower during the emission control periods and argued that the emission control suppressed the secondary formation of sulfate and nitrate, a claim subjected to lively discussions by some of the delegates.
 | ||
| Fig. 5 Estimated influence of emission controls on mass concentrations of PM1 and its major chemical species in Beijing (DOI: C6FD00004E). | ||
Dr Maria Cruz Minguillón then presented the impact of traffic emission on aerosol composition by comparing aerosol characteristics between two periods with high and low traffic (DOI: C5FD00182J). She presented clear evidence on the impact of vehicle emissions on VOCs (particularly the aromatic hydrocarbons), black carbon (BC), and OC, as well as the fraction of non-fossil OC. OA source apportionment based on 14C and Aerosol Chemical Speciation Monitor (ACSM) analyses revealed that the fossil OC was mainly secondary (>70%) except for the last sample, when the fossil secondary OC only represented 51% of the total fossil OC. Dr Minguillón also showed very intriguing data indicating that the fraction of non-fossil secondary OC increased from 37% of total secondary OC for the first sample (low traffic) to 60% for the last sample (high traffic). Dr Minguillón argued that this enhanced formation of non-fossil SOAs could be attributed to the reaction of BVOC precursors with NOx emitted from road traffic (or from its nocturnal derivative nitrate that enhances night-time semi-volatile oxygenated OAs (SV-OOAs)), since NO2 concentrations increased from 19 to 42 mg m−3 from the first to the last day of sampling. The delegates contributed to some stimulating discussion as to why the contribution of non-fossil fuel OC was higher during the period with higher traffic.
The next two papers focused on the oxidative potential of particulate matter. Professor Constantinos Sioutas presented the first study by Shirmohammadi et al. who attempted to apportion the sources of oxidative species in airborne particles and examined the relative importance of tailpipe and non-tailpipe emissions on the oxidative potential of ambient particles in Los Angeles (DOI: C5FD00166H). Professor Sioutas showed that DTT (dithiothreitol) activity was strongly correlated with organic tracers of primary vehicle tailpipe emissions such as PAHs and hopanes as well as EC. Through multiple linear regression (MLR) analysis on DTT activity and PM sources identified by a Molecular Marker-Chemical Mass Balance (MM-CMB) model, he suggested that primary biogenic emissions, secondary organic carbon (SOC) and vehicular abrasion in the PM2.5 size range, along with vehicular abrasion and vehicle tailpipe emissions in the PM0.18 size range were the major sources driving the DTT activity levels in central LA. By comparing with DTT data from previous studies in central Los Angeles, Professor Sioutas argued that non-tailpipe emissions have become an important concern for public health.
Thereafter, Professor Martin Shafer presented a study on the oxidative potential of size-fractionated atmospheric aerosol (DOI: C5FD00196J). He determined the oxidative potentials of particulate matter in different size ranges using several assays: an in vitro rat alveolar macrophage model, a chemical ROS probe (DTT), and cytokine induction (TNFa) to examine relationships between PM-induced reactive oxygen species (ROS). ROS and DTT activity was remarkably uniform in the samples across all sites, and he argued that the chemical drivers of oxidative activity are relatively similar for the 18 urban sites and 3 background sites across the European continent. PM3 dominated the size distribution of both ROS (86% of the total) and DTT activity (76% of the total). ROS and DTT activities, which were highly correlated, were primarily related to soluble fractions but not to any of the measured inorganic elements in the PM3. In contrast, another oxidative potential matrix, cytokine induction, was almost exclusively driven by the insoluble components of the PM extracts, which is in contrast to the macrophage ROS production data. Following the two presentations on oxidative potentials, there was an intense discussion in regards to the sources of oxidative potential (OP) and the relevance of different OP metrics in human health impact assessment.
The last sub-session of the ‘urban case studies’ included three discussion papers. The first one was given by Dr Christian Ehlers who reported the long term observational datasets of nitrogen oxides and hydrocarbons in Germany between 1994 and 2014 (DOI: C5FD00180C). He showed that traffic emissions of VOCs have decreased by an average of 14.5% per year. NOx emissions, however, have stayed almost the same over the same period. The VOC composition was used to determine the major emission source of hydrocarbons in urban areas, where the results indicated that petrol cars with temporarily reduced catalyst efficiency were responsible for the emissions. Their observations in the vicinity of main roads in German cities show a decrease in the ratio of OH reactivities of VOCs and NO2 (RVOC/RNO2) by a factor of 7.5 over the time period of 1994–2014. This reduction led to a drastic decrease in local ozone production driven by the faster reduction in hydrocarbons. Dr Ehlers suggested that the overall reduction of ozone production benefits from the low decrease of NOx emissions from road traffic. He also showed modelling results indicating the potential benefit of reducing NOx emissions and avoiding enhanced ozone production and thus, meeting the NO2 limit.
Dr David Carslaw then presented modelling and measurement data on primary NO2 emissions from vehicle exhausts in the UK (DOI: C5FD00162E). He analysed the ambient NOx data, NO2 measurements and vehicle emission remote sensing data obtained in London, to better understand the recent trends in the NO2/NOx ratio from road vehicles. Evidence showed that NO2 concentrations had decreased from 2010 despite less evidence of a reduction in the total NOx. The decrease was shown to be driven by relatively large reductions in the amount of NO2 directly emitted by vehicles. Their analysis of NOx and NO2 vehicle emission remote sensing data showed that these reductions have mostly been driven by reduced NO2/NOx emission ratios from heavy duty vehicles and buses, rather than light duty vehicles. Interestingly, evidence from the analysis of Euro 4 and 5 diesel passenger cars show that the NO2/NOx ratio decreases with increasing vehicle age. For example the NO2/NOx ratio decreased from 29.5 ± 2.0% in Euro 5 diesel cars up to one year old to 22.7 ± 2.5% for four year old vehicles (Fig. 6). At some roadside locations the reductions in primary NO2 have had a large effect on reducing both the annual mean concentration and number of hourly exceedances of the European Limit Values of NO2.
 | ||
Fig. 6 Trend in NO2/NOx ratio for Euro 4 and 5 diesel cars by vehicle age based on a total sample of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 721 vehicles from the 2012 and 2013 remote sensing data (DOI: C5FD00162E). The uncertainties show the 95% confidence interval in the mean. 721 vehicles from the 2012 and 2013 remote sensing data (DOI: C5FD00162E). The uncertainties show the 95% confidence interval in the mean. | ||
The final talk of the day was given by Dr James Lee, who presented spatially resolved flux measurements of NOx (DOI: C5FD00170F). The eddy-covariance technique was used to estimate the emission flux and footprint of NOx from London from an aircraft. The estimated emissions were compared to the emission data from the National Atmospheric Emissions Inventory (NAEI) with scaling factors used to account for the actual time of day, day of the week and month of the year of the measurement. Dr Lee argued that the NOx emissions in London were significantly underestimated in the NAEI, mainly due to its under-representation of real world road traffic emissions, echoing the introductory lecture (DOI: C6FD00065G). By comparing with the enhanced London Atmospheric Emissions Inventory (LAEI) which attempted to take into account real world driving emission factors and road measurement data, Dr Lee showed that the measurement to inventory agreement was substantially improved. However, there was still an underestimation by the LAEI of 30–40% compared with flux measurements, suggesting significant improvements are still required in the NOx emissions inventory. This study highlighted the critical importance of obtaining independent measurements of pollutant emission rates from vehicles during on road driving conditions and using these data in emission inventories, rather than relying on emissions data obtained during artificial test driving conditions or provided by vehicle manufacturers.
The conference banquet
The conference banquet was held at the Royal Society, a short 10 min walk from Burlington House. The banquet offered both an excellent three course meal and the opportunity to network and further discuss atmospheric science. Following an inspiring speech regarding the history of the Faraday Discussions by Professor Andy Mount (the chair of the Faraday Discussion committee), a Spiers Memorial Award medal was presented to Professor Urs Baltensperger for leading major advances in understanding the formation, composition and processes affecting atmospheric organic aerosols. Professor Baltensperger demonstrated his Latin language expertise and thanked all his colleagues (previous and present alumni) for their invaluable contribution to research in aerosol composition, formation and processes (Fig. 7) (http://www.rsc.org/ScienceAndTechnology/Awards/SpiersMemorialAward/). | ||
| Fig. 7 Professor Urs Baltensperger awarded with the Spiers Memorial Award medal and his former and present group members (photo by Zhe Tian). | ||
Thereafter, Professor Roy Harrison thanked all the delegates for enthusiastically contributing to all of the discussions during and after the scheduled meetings. He then announced that the joint poster award winners were Chuanping Meng from Macquarie University, Australia, and Edna Cabrera-Martinez from the University of Reading for their excellent posters titled ‘Characterization of organic molecular markers in PM2.5 aerosol at an urban site in Sydney’ and ‘Ozonolysis of ultrasonically levitated droplets containing unsaturated carboxylic acids’, respectively. Professor Harrison then reminded all delegates to continue to contribute to the last day of discussions scheduled for the following morning. The banqueting dinner was concluded with the traditional and interesting ‘loving cup’ ceremony, which includes bowing.
Session 4: Numerical modelling strategies for the urban atmosphere
The last day of the conference and the final session focused on numerical modelling strategies for the urban atmosphere. Speakers in the first half of the session included Dr Sasha Madronich (NCAR, USA), Dr Irina Nikolova (University of Birmingham, UK) and Dr Shupeng Zhu (ENPC, France). Dr Sasha Madronich presented a study of non-linear partitioning and organic volatility distributions of urban aerosols derived from measurements in Mexico City and Paris, and simulations with the GECKO-A explicit chemical model (DOI: C5FD00209E). This linearity indicates that the OA pollution can be addressed by rational and proportionate reductions in emissions. The OA chemistry may be more dynamic than previously thought in terms of formation and removal with the likely outcome that volatility distributions also evolve over the lifetime span of the aerosol. The change in the volatility distribution is mainly driven by photochemistry, causing redistribution of organic material from higher to lower volatilities. However, there are limitations, e.g. the diversification of intermediate organic compounds is very broad and filling those volatility bins may bring high sensitivity, but it was argued that specific compounds may be of interest but not essential to know for empirical models. The sensitivity exponents estimated from the volatility distributions are local, i.e. they predict the response of gas-particle partitioning to small changes in the total amount without any reference to the history of the air parcel. A global sensitivity analysis would take into account the response of OA to changes in the initial conditions, emissions or changes in the nature of the seed.The next talk was given by Dr Irina Nikolova on the topic of modelling component evaporation and composition change of traffic-induced ultrafine particles (diameter less than 0.1 μm) during their transport from street canyon to urban background areas (DOI: C5FD00164A). The study showed the associated composition changes of multicomponent SVOC particles in realistic ambient conditions and compared the results with observations from London. A significant reduction in particle diameter was simulated in line with the observations due to the fast SVOC evaporation from the particle surface. Nucleation mode particles have little or no SVOC constituents after evaporation during advection, while SVOCs are still present in the Aitken and the accumulation mode particles. The existence of a non-volatile core to the particle was discussed among the delegates. Currently, the composition of the non-volatile core is not fully understood. The relatively high sulphur content (less than 50 ppm) in the fuel may contribute to the constituents of that core. In addition, trace metals from the lubricating oil (such as Ca, Fe) may contribute to the composition of that core as well. Most of the hydrocarbon species evaporate from the unburned lubricating oil droplets heated at high temperatures. Such a metallic core could be coated with semi-volatile species upon leaving the tailpipe. The study by Dr Nikolova does not specify the content of the non-volatile core; however, the health-related response to that core may be different depending on its composition.
The last speaker of that session was Dr Shupeng Zhu. He presented his findings on particle diversity and mixing state over greater Paris, in a model-measurement inter-comparison study (DOI: C5FD00175G). He argued that the assumption of considering internally mixed particles for urban-scale simulations may not be valid due to the shorter time scales involved. A size-composition resolved aerosol model (SCRAM) was developed and the internal and external mixing state assumption was tested. He concluded that the traditional aerosol model that assumes internally mixed particles may be suitable for regional scale modelling, while in urbanised areas the internal mixing assumption does not hold and needs to be re-evaluated. However, it was argued during the discussions that inorganic–organic interactions (mixing of inorganic components with insoluble and water soluble organics) were not considered in the study and this may have an effect on the multicomponent thermodynamics and partitioning. Dr Zhu acknowledged the suggestion and mentioned that they will consider the dynamics of condensation/evaporation of organic matter and inorganic–organic interactions using AIOMFAC (Aerosol Inorganic–Organic Mixtures Functional groups Activity Coefficients; http://www.aiomfac.caltech.edu/about.html, last access April 2016).
After the refreshing morning tea, speakers of the second half of the session included Professor Alison Tomlin (University of Leeds, UK), Dr Lisa Whalley (National Centre for Atmospheric Science, UK) and Dr Andreas Skouloudis (European Commission, Italy). Professor Tomlin presented their new findings in the treatment of uncertainties in reactive pollution dispersion models at urban scales (DOI: C5FD00159E). The predictions of NO2 concentrations within urban streets are an important asset to evaluate strategies to reduce exposure to NO2. However, those predictions are subject to uncertainties and need to be cautiously considered when using models for decision making. NO2 predictions are highly sensitive to the activation energy for the NO + O3 reaction as well as the wall roughness length. However, the most important physical parameter under peak traffic conditions is the background wind direction. It was discussed that accurate reference measurements for wind direction are a crucial part of the air quality assessment for in-street locations. The wind direction above roof tops is not a routine measurement in the UK and it is typically measured outside the actual city, e.g. in airports.
The next talk in the second half of the session was given by Dr Lisa Whalley, focussing on the assessment of chemical schemes and constraints in air quality models used to predict ozone in London against detailed box modelling, incorporating the Master Chemical Mechanism (MCMv3.2) (DOI: C5FD00218D). The objective of their study was to analyse the ability of less explicit chemical schemes such as the Generic Reaction Set (GRS) and the Common Representative Intermediates (CRIv2-R5) mechanisms to predict ozone compared to the more explicit MCMv3.2. The ozone simulated with the less explicit schemes, GRS and CRIv2-R5, is highly sensitive to HONO chemistry in the winter and to a lesser extent in the summer. The advantage provided by the two simplified chemistry mechanisms is in the computer processing time. The run time when using CRIv2-R5 and GRS decreases by a factor of 1000 and 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, respectively, in comparison with MCMv3.2. The discussion acknowledged the idea of including a robust chemical complexity. However, regulatory models such as ADMS-Urban would not be able to cope with such a computational burden because it is primarily designed to run on a single core machine. Inclusion of all known chemistry of the urban atmosphere with full complexity in numerical models is still impossible due to the limited computational resources. It was pointed that methods for chemical model reduction exist and have been applied in other fields, e.g. combustion modelling. Such methods reduce the size of the reaction mechanisms and retain the relevant and important chemical complexity.
000, respectively, in comparison with MCMv3.2. The discussion acknowledged the idea of including a robust chemical complexity. However, regulatory models such as ADMS-Urban would not be able to cope with such a computational burden because it is primarily designed to run on a single core machine. Inclusion of all known chemistry of the urban atmosphere with full complexity in numerical models is still impossible due to the limited computational resources. It was pointed that methods for chemical model reduction exist and have been applied in other fields, e.g. combustion modelling. Such methods reduce the size of the reaction mechanisms and retain the relevant and important chemical complexity.
Discussions about chemical and meteorological complexities raised the topic of emission complexity. High resolution models require an input of high resolution emission inventories. Therefore, there is a need to develop bottom-up emission inventories. The bottom-up approach could inspire new modelling development marking a new era in the field of air quality simulations and predictions. Dr Andreas Skouloudis, the last speaker of the session, discussed his findings on the topic of verifiable emission reductions in European urban areas with air quality models (DOI: C5FD00189G). The study dealt with a modelling methodology for describing the air quality in base and target years. The verification with real world measurements of a number of pollutants in the target year shows maximum results were achieved with appropriate emission reductions. An example of the reduction in NO2 and benzene in 11 European cities is plotted in Fig. 8. Also shown is a remaining problem with ozone in view of the local emission inventories provided by the local authorities. However, it is likely that further emission reductions may not provide further pollutant concentration reductions, but they may steer the problem into another source category. Dynamic emission factors and finer emission-resolved inventories are desperately needed as well as filling the gap between regional and fine urban scale models (in-street models). It was argued during the discussion that regulatory measures and decisions are based on regional large scale domains. However, these may not be appropriate in population-based epidemiological studies because they may lead to exposure misclassification.
 | ||
| Fig. 8 The change (in %) between 1995 and 2010 for the average concentrations of NO2, benzene and ozone in 11 European cities on the basis of numerically calculated values (DOI: C5FD00189G). | ||
Concluding remarks lecture
Finally, Professor Jose-Luis Jimenez (University of Colorado) summarised the three days of talks and discussions and presented the concluding remarks lecture (DOI: C6FD90019D). He gave his talk on the latest news, gaps, revolutionary instrumentation methods and dangers in the field of urban chemistry. There was good news, in that the ‘parade blue’ emission control study in China showed some outstanding effects in improving air quality (DOI: C6FD00004E). However it was stressed that there are major challenges in reducing the air pollution without stopping economic growth in developing countries. Implementation of new technologies does not guarantee a cleaner air and a reduction in air pollution levels. An example was given following the work of Carslaw and co-authors (DOI: C5FD00162E), where the introduction of diesel catalysts and filters showed an increase of NO2 concentration. A new look into the cooking habits and potentially incomplete emission inventories were also topics of discussion following the talk of Pandis et al. (DOI: C5FD00212E). There are still missing sources and/or processes in the urban environment (DOI: C5FD00190K, C5FD00204D, C5FD00230C, C5FD00182J, C5FD00170F). The urban impact on larger scales and vice versa is still not fully understood, and thus it is important to link urban studies with those at regional and even global scales. Efforts and more work are needed in developing countries to better understand air pollution. Alternative techniques such as low cost sensors (DOI: C5FD00201J) were mentioned several times throughout the Faraday Discussion meeting spreading the fear that they will potentially be wasted resources and lead to a deprivation of good quality data. Furthermore, the balance between the number of field campaigns and the time needed to sufficiently analyse the wealth of the increasingly complex generated data needs to be found. Nonetheless, instrumental revolution has allowed us to access a new dimension of knowledge in the chemistry of the urban atmosphere. Comprehensive aerosol characterisation is taking a new turn with what is being called ‘a quantum leap’ in organic speciation (DOI: C5FD00185D) and airborne fluxes (DOI: C5FD00170F). New atmospheric measurement instruments have also helped us to close many of the previous gaps, e.g., re-attaching the O3 problem in far greater detail, nucleation, organic aerosol formation, and deposition of gas phase species.The concluding remarks were followed by well-deserved acknowledgements to the RSC staff, Jack Busby and Susannah May, who organized the meeting and did an amazing job in accurately recording the questions, and were extremely efficient in sending the questions to the questioner (Jack or Susannah sent an email to the questioner within minutes!). Professor Roy Harrison also thanked all the delegates for actively contributing to the discussions and Zhe Tian and Ruixin Xu (University of Birmingham) for facilitating the discussions and reminded all of the delegates to post their questions and answers on the Faraday Discussion forum. After a final lunch for the Faraday Discussion meeting, the delegates went their separate ways with plenty to think about following an intense but stimulating three days of discussion.
Acknowledgements
Z. S. acknowledges support from the Natural Environment Research Council (NE/I021616/1) and the University of Birmingham Fellowship scheme. M. S. A. and I. N. acknowledges support from the European Research Council as part of the FASTER project (ERC-2012-AdG; 320821). We are grateful to Jack Busby, Susannah May and Emily Finney from the RSC for providing useful information on the manuscript.References
- J. Almeida, et al., Molecular understanding of sulphuric acid-amine particle nucleation in the atmosphere, Nature, 2013, 502, 359–363, DOI:10.1038/nature12663.
- J. Kirkby, et al., Ion-induced nucleation of pure biogenic particles, Nature, 2016, 533, 521–526, DOI:10.1038/nature17953.
- J. Trostl, et al., The role of low-volatility organic compounds in initial particle growth in the atmosphere, Nature, 2016, 533, 527–531, DOI:10.1038/nature18271.
- F. Bianchi, et al., New particle formation in the free trosphere: A question of chemistry and timing, Science, 2016, 352, 1109–1112, DOI:10.1126/science.aad5456.
- F. Canonaco, M. Crippa, J. G. Slowik, U. Baltensperger and A. S. H. Prévôt, SoFi, an Igor based interface for the efficient use of the generalized multilinear engine (ME-2) for source apportionment: application to aerosol mass spectrometer data, Atmos. Meas. Tech., 2013, 6, 3649–3661 Search PubMed.
| This journal is © The Royal Society of Chemistry 2016 |

