A novel electron-acceptor moiety as a building block for efficient donor–acceptor based fluorescent organic lighting-emitting diodes†
Miao-Miao
Xue
,
Chen-Chao
Huang
,
Yi
Yuan
,
Ye-Xin
Zhang
,
Man-Keung
Fung
* and
Liang-Sheng
Liao
*
Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Institute of Functional Nano & Soft Materials (FUNSOM) & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou 215123, P. R. China. E-mail: lsliao@suda.edu.cn; mkfung@suda.edu.cn
First published on 1st December 2016
Abstract
A new electron-withdrawing moiety (BFPz) has been used for the first time as an acceptor in OLEDs and its corresponding core unit (2-Br-BFPz) was synthesized. Combined with an electron-donating moiety triphenylamine, a novel fluorescent material with a D–A structure named TPA-BFPz was synthesized. Encouragingly, the EQE values of non-doped and doped blue OLEDs reach 3.68% and 4.42%, respectively.
Fluorescent materials have been playing a critical role in organic light-emitting diodes (OLEDs) since Tang’s report on tris(8-hydroxyquinoline)-aluminum (Alq3)-based multilayer devices.1,2 In particular, with the introduction of the concepts of hybridized local and charge transfer (HLCT) and thermally activated delayed fluorescence (TADF) in recent years, the research based on fluorescent molecules with donor–acceptor (D–A) structures has become a hot topic.3–6 A compound with a D–A structure means the material is composed of both electron donor and acceptor moieties. However, electron-acceptor moieties with a strong electron-withdrawing capability are far from adequate.
Therefore, it is crucial to design desirable electron-accepting moieties. As we know, dibenzofuran, a classical tricyclic unit, has confined conjugation structures which allow delocalization of both the injected electrons/holes and hence possess outstanding carrier transporting ability.7–9 Nevertheless, its electron-withdrawing ability is hardly comparable with other prototypical moieties such as benzimidazole,10 triazine11 and borane derivatives,12 which have been widely used as electron acceptors in D–A based π-conjugated fluorescent molecules. On the other hand, pyrazine is also known to be an excellent electron-withdrawing molecule.13 Therefore, it would be an innovative strategy if a new electron-accepting moiety is synthesized based on a hybrid structure of dibenzofuran and pyrazine. In this way, two nitrogen atoms are embedded into the tricyclic system, as seen in Scheme 1. By combining this new electron-accepting moiety with a triphenylamine (TPA) group that possesses outstanding hole-transporting ability, a novel fluorescent molecule with a D–A structure is formed.
To obtain this novel D–A structure, a new core unit named 2-bromobenzofuro[2,3-b]pyrazine (2-Br-BFPz) was first synthesized as a precursor. 2-Br-BFPz was synthesized by the ring closing reaction of 5-bromo-3-(2-methoxyphenyl)pyrazin-2-amine prepared by the Suzuki coupling reaction of (2-methoxyphenyl)boronic acid and 3,5-dibromopyrazin-2-amine. The D–A fluorescent molecule, known as TPA-BFPz, was then prepared by the Suzuki coupling reaction of 2-Br-BFPz with N,N-diphenyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline. The synthetic yield of TPA-BFPz was ∼60%. Its detailed synthetic route is shown in Scheme S1 (ESI†) and the synthetic procedure is described in the ESI.† The thermal properties of TPA-BFPz were studied using thermal gravimetric analysis (TGA) and differential scanning calorimetry (DSC), as shown in Fig. S1 and Table S1 (ESI†). It can be seen that TPA-BFPz is thermally stable with a 5% weight-loss temperature (Td) of up to 350 °C and its glass-transition temperature (Tg) is 63 °C.
In order to probe the interaction between D (TPA) and A (BFPz) in the TPA-BFPz molecule, its photophysical properties were characterized. The UV-Vis spectrum of TPA-BFPz diluted in toluene solution (1 × 10−5 mol L−1) and the photoluminescence (PL) spectrum of TPA-BFPz prepared as a film are shown in Fig. 1a. There are two pronounced absorption peaks. The short-wavelength absorption peak at 308 nm can be ascribed to the n–π* transition of the triphenylamine center,14 and the longer wavelength at 394 nm can be attributed to the charge transfer (CT) transition from D (TPA) to A (BFPz). It can also be seen that TPA-BFPz (in film) shows a strong emission at 482 nm. Furthermore, the CT characteristic of TPA-BFPz would be quite obvious if different polar solvents were used in the fluorescence measurements. As shown in Fig. 1b and Table S2 (ESI†), the solvatochromic effect of the PL spectra of TPA-BFPz was observed in different polar solvents. When the solvent polarity was increased gradually from low polarity (e.g. hexane) to high polarity such as N,N-dimethylformamide (DMF), the PL spectra of TPA-BFPz become wider and exhibited a total red-shift as large as 135 nm, i.e., 428 nm in hexane (Hex.), 466 nm in toluene (Tol.) and 563 nm in DMF. But there was an exception that the PL spectra of TPA-BFPz exhibited a blue-shift phenomenon from dichloromethane (DCM) to tetrahydrofuran (THF) while the polarity of THF is larger than that of DCM. This may be because the large electronegativity of the oxygen (O) atom in the THF molecule may have a short-range effect with α-H in the pyrazine part of the TPA-BFPz molecule, which may reduce the energy of the ground state and then lead to the blue-shift phenomenon.15 For a better understanding of the solvent effects on PL behaviors, the UV-Vis absorption spectra of TPA-BFPz recorded in different solvents are also shown in Fig. S2 (ESI†). In order to unveil the solvatochromic effect chromatically, the fluorescence of TPA-BFPz dissolved by different polar solvents was observed under a UV lamp (365 nm), as shown in Fig. 1c. It is obvious that all the materials exhibit strong but diverse fluorescence emissions. The color change from deep blue in hexane to orange yellow in DMF indicates that TPA-BFPz is a favorable fluorescent material with a strong CT-state characteristic. Meanwhile, it also suggests that TPA-BFPz holds high sensitivity of its fluorescence emission to different types of solvents. Particularly, such a solvent effect almost makes it capable of emitting in the entire visible spectrum. With the aim to further investigate its fluorescence properties, the fluorescence quantum yields (ΦF) in solution and in film were tested. As a result, TPA-BFPz exhibited a strong fluorescence with ΦF up to 92% and 78% in DCM solution and in film, respectively. In addition, the transient PL of a neat film of TPA-BFPz was also tested, as shown in Fig. S3 (ESI†). It shows that TPA-BFPz has a short fluorescence lifetime of 6.9 ns without a “delayed fluorescence” phenomenon.
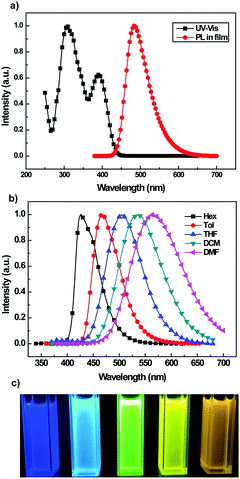 | ||
| Fig. 1 (a) UV-Vis and PL spectra of TPA-BFPz; (b) PL spectra of TPA-BFPz in different solvents; and (c) color of TPA-BFPz in different solvents under UV exposure with a wavelength of 365 nm. | ||
To gain a deeper insight into the relationship between D (TPA) and A (BFPz) at the molecular level, DFT calculations (B3LYP/6-31 g*) on the TPA-BFPz molecule were carried out. As illustrated in Fig. S4 (ESI†), due to the electron-donating ability of the TPA unit and the electron-accepting ability of the BFPz moiety, the highest occupied molecular orbital (HOMO) of TPA-BFPz is mainly distributed in the TPA group and the lowest unoccupied molecular orbital (LUMO) is mainly located in the BFPz moiety, but their molecular orbitals are partly overlapped in the pyrazine part of the BFPz group and the benzene ring of the TPA group. Furthermore, the TPA and BFPz groups are twist-linked with a slightly distorted angle (θD–A) of 21°. The distribution of molecular orbitals and small θD–A further enhance the radiative-transition rate and fluorescence efficiency.
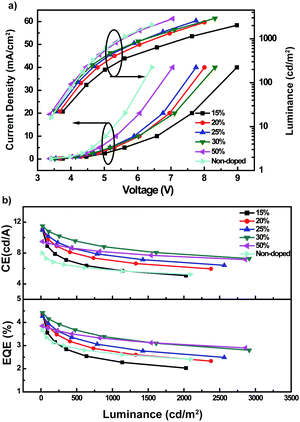
As TPA-BFPz possesses outstanding fluorescence properties as mentioned, we further explored it as an emitter for OLED applications. The HOMO energy level of TPA-BFPz was measured using ultraviolet photoelectron spectroscopy (UPS), which is estimated to be 5.62 eV, as shown in Fig. S5 (ESI†). Knowing the band gap of TPA-BFPz as determined from the UV–Vis spectrum in Fig. 1a, the LUMO energy level is calculated to be 2.79 eV. A non-doped OLED with a configuration of ITO/1,4,5,8,9,11-hexaazatriphenylene-hexacarbonitrile (HAT-CN, 10 nm)/di-[4-(N,N-ditolyl-amino)-phenyl]cyclohexane (TAPC, 40 nm)/TPA-BFPz (20 nm)/1,3,5-tri[(3-pyridyl)-phen-3-yl]benzene (TmPyPB, 45 nm)/8-hydroxyquinolinolatolithium (Liq, 2 nm)/Al was fabricated. In addition, the energy level diagram for each material used in the devices is summarized in Fig. S6 (ESI†). To our delight, the non-doped device has a low driving voltage of 3.4 V (at a current density of 0.2 mA cm−2) and a maximum current efficiency (CE), power efficiency (PE) and external quantum efficiency (EQE) of 8.0 cd A−1, 7.4 lm W−1 and 3.68%, respectively, with an electroluminescence (EL) peak at 484 nm (Fig. 2, Fig. S7 and S8, ESI†). In order to further improve the device performance, we optimized the device structure with a configuration of ITO/HAT-CN (10 nm)/TAPC (40 nm)/bis[2-(diphenylphosphino)phenyl]ether oxide (DPEPO):TPA-BFPz (X wt%, 20 nm)/TmPyPB (45 nm)/Liq (2 nm)/Al, in which DPEPO serves as a host material and X represents the doping concentration of TPA-BFPz in DPEPO. As shown in Fig. 2 and Fig. S7 (ESI†), all the devices have the same EL peak at 492 nm and driving voltages of less than 3.7 V when driven at 0.2 mA cm−2. All the doped devices show a better device performance than the non-doped OLED. But the EL spectra of the doped devices have a red-shift of about 8 nm compared to that of the non-doped one because DPEPO is a polar molecule. The corresponding device performance is summarized in Table 1. As shown in Table 1, the voltages tested at 0.2 mA cm−2 of devices decreased when the doping concentration of TPA-BFPz in DPEPO is increased, which indicated that TPA-BFPz might have good electron and hole transporting abilities. When the doping concentration of TPA-BFPz is 30%, the D–A based OLED exhibits maximum efficiencies of 11.5 cd A−1, 10.3 lm W−1 and 4.42%. The superior device performance implies that TPA-BFPz is a favorable D–A molecule and a promising blue fluorescent material for OLEDs.
| Devicesa | Voltageb (V) | CEmaxc cd/A | PEmaxc lm/W | EQEmaxc % | CIEd |
|---|---|---|---|---|---|
| a Doping concentration of TPA-BFPz in DPEPO. b Driving voltage (at 0.2 mA cm−2). c CEmax = max current efficiency, PEmax = power efficiency, and EQEmax = external quantum efficiency. d Measured at 5 mA cm−2. | |||||
| 15% | 3.7 | 11 | 9.2 | 4.4 | 0.19, 0.42 |
| 20% | 3.6 | 11 | 9.4 | 4.3 | 0.19, 0.43 |
| 25% | 3.5 | 11 | 9.7 | 4.27 | 0.19, 0.44 |
| 30% | 3.5 | 11.5 | 10.3 | 4.42 | 0.19, 0.44 |
| 50% | 3.4 | 9.5 | 8.9 | 3.85 | 0.18, 0.42 |
| Non-doped | 3.4 | 8 | 7.4 | 3.68 | 0.16, 0.35 |
In summary, an innovative electron-withdrawing moiety, 2-Br-BFPz, which possesses a highly confined conjugated structure, has been designed and synthesized. We have combined 2-Br-BFPz with the TPA group, which is a classical electron-donating unit, to form a novel fluorescent material with a D–A structure named TPA-BFPz. Interestingly, TPA-BFPz exhibits strong fluorescence and has been utilized as a blue fluorescent emitter for OLEDs. The EQE values for non-doped and doped devices reach 3.68% and 4.42%, respectively, which illustrates that the BFPz unit is a promising electron-withdrawing moiety. It is foreseen that the electron-withdrawing unit can be used for designing novel TADF or HLCT materials in the future.
We thank the financial support from the Natural Science Foundation of China (No. 61575136 and 61475106) and the Natural Science Foundation of Jiangsu Province (BK20151264). This project was also financially supported by the Collaborative Innovation Center (CIC) of Suzhou Nano Science and Technology and the Priority Academic Program Development of the Jiangsu Higher Education Institutions (PAPD).
Notes and references
- (a) C. W. Tang and S. A. VanSlyke, Appl. Phys. Lett., 1987, 51, 913 CrossRef CAS; (b) C. Adachi, T. Tsutsui and S. Saito, Appl. Phys. Lett., 1989, 55, 1489 CrossRef CAS; (c) J. Shi and C. W. Tang, Appl. Phys. Lett., 2002, 80, 3201 CrossRef CAS.
- M. A. Baldo, M. E. Thompson and S. R. Forrest, Nature, 2000, 403, 750 CrossRef CAS PubMed.
- (a) W. Li, Y. Pan, R. Xiao, Q. Peng, S. Zhang, D. Ma, F. Li, F. Shen, Y. Wang, B. Yang and Y. Ma, Adv. Funct. Mater., 2014, 24, 1609 CrossRef CAS; (b) S. Zhang, L. Yao, Q. Peng, W. Li, Y. Pan, R. Xiao and Y. Ma, Adv. Funct. Mater., 2015, 25, 1755 CrossRef CAS; (c) D. Yu, F. Zhao, Z. Zhang, C. Han, H. Xu, J. Li, D. Ma and P. Yan, Chem. Commun., 2012, 48, 6157 RSC.
- (a) Q. Zhang, J. Li, K. Shizu, S. Huang, S. Hirata, H. Miyazaki and C. Adachi, J. Am. Chem. Soc., 2012, 134, 14706 CrossRef CAS PubMed; (b) S. Wu, M. Aonuma, Q. Zhang, S. Huang, T. Nakagawa, K. Kuwabara and C. Adachi, J. Mater. Chem. C, 2014, 2, 421 RSC.
- J. Ye, Z. Chen, M. K. Fung, C. Zheng, X. Ou, X. Zhang, Y. Yuan and C. S. Lee, Chem. Mater, 2013, 25, 2630 CrossRef CAS.
- (a) L. Chen, Y. B. Jiang, H. Nie, R. R. Hu, H. S. Kwok, F. Huang, A. J. Qin, Z. J. Zhao and B. Z. Tang, ACS Appl. Mater. Interfaces, 2014, 6, 17215 CrossRef CAS PubMed; (b) G. W. Lin, H. R. Peng, L. Chen, H. Nie, W. W. Luo, Y. H. Li, S. M. Chen, R. R. Hu, A. J. Qin, Z. J. Zhao and B. Z. Tang, ACS Appl. Mater. Interfaces, 2016, 8, 16799 CrossRef CAS PubMed; (c) X. J. Zhan, Z. B Wu, Y. X. Lin, Y. J. Xie, Q. Peng, Q. Q Li, D. G. Ma and Z. Li, Chem. Sci., 2016, 7, 4355 RSC.
- S. Dong, C. Gao, Z. Zhang, Z. Jiang, S. Lee and L. Liao, Phys. Chem. Chem. Phys., 2012, 14, 14224 RSC.
- L. Deng, J. Li, G. Wang and L. Wu, J. Mater. Chem. C, 2013, 1, 8140 RSC.
- C. Han, G. Xie, H. Xu, Z. Zhang, L. Xie, Y. Zhao, S. Liu and W. Huang, Adv. Mater., 2011, 23, 2491 CrossRef CAS PubMed.
- C. H. Chen, W. S. Huang, M. Y. Lai, W. C. Tsao, J. T. Lin, Y. H. Wu, T. H. Ke, L. Y. Chen and C. C. Wu, Angew. Chem., Int. Ed., 2008, 47, 581 CrossRef PubMed.
- S. Y. Lee, T. Yasuda, H. Nomura and C. Adachi, Appl. Phys. Lett., 2012, 101, 093306 CrossRef.
- (a) K. Suzuki, S. Kubo, K. Shizu, T. Fukushima, A. Wakamiya, Y. Murata, C. Adachi and H. Kaji, Angew. Chem., Int. Ed., 2015, 127, 15446 CrossRef; (b) Y. J. Shiu, Y. C. Cheng, W. L. Tsai, C. C. Wu, C. T. Chao, C. W. Lu, Y. Chi, Y. T. Chen, S. Liu and P. T. Chou, Angew. Chem., Int. Ed., 2016, 55, 3017 CrossRef CAS PubMed.
- Y. Liu, L. S. Cui, M. F. Xu, X. B. Shi, D. Y. Zhou, Z. K. Wang, Z. Q. Jiang and L. S. Liao, J. Mater. Chem. C, 2014, 2, 2488 RSC.
- (a) Y. T. Tao, Q. Wang, Y. Shang, C. L. Yang, L. Ao, J. G. Qin, D. G. Ma and Z. G. Shuai, Chem. Commun., 2009, 77 RSC; (b) Z. Ge, T. Hayakawa, S. Ando, M. Ueda, T. Akiike, H. Miyamoto, T. Kajita and M. Kakimoto, Adv. Funct. Mater., 2008, 18, 584 CrossRef CAS.
- (a) W. Y. Hung, L. C. Chi, W. J. Chen, Y. M. Chen, S. H. Chou and K. T. Wong, J. Mater. Chem., 2010, 20, 10113 RSC; (b) D. Fan, Y. P. Yi, Z. D. Li, W. J. Liu, Q. Peng and Z. G. Shuai, J. Phys. Chem. A, 2015, 119, 5233 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental detail, physical measurements and other electronic format. See DOI: 10.1039/c6cc09486d |
| This journal is © The Royal Society of Chemistry 2017 |