Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Umpolung synthesis of branched α-functionalized amines from imines via photocatalytic three-component reductive coupling reactions†
Angel L.
Fuentes de Arriba
,
Felix
Urbitsch
and
Darren J.
Dixon
*
Department of Chemistry, Chemistry Research Laboratory, University of Oxford, 12 Mansfield Road, Oxford, UK. E-mail: Darren.dixon@chem.ox.ac.uk
First published on 24th November 2016
Abstract
A three component reductive coupling reaction of a (hetero)aromatic amine, a (hetero)aromatic aldehyde and an electron deficient olefin catalysed by eosin Y under green LED light irradiation, for the direct generation of γ-amino acid derivatives, is described. This new umpolung synthesis of amines, which exploits the high nucleophilicity of a putative α-amino radical intermediate, generated via single electron reduction of the in situ generated imine from the Hantzsch ester terminal reductant, is efficient, operationally simple, broad in scope and offers a complementary strategy to existing synthetic approaches.
Chiral α-branched amines and their derivatives are commonplace in pharmaceuticals, agrochemicals and biologically relevant natural products (Fig. 1).1 Accordingly, the development of new and general methods for their synthesis, through new C–C bond forming processes is important and timely from both academic and industrial perspectives. Furthermore, the possibility to carry out such transformations as multicomponent coupling reactions is attractive for the ready generation of structural analogues in discovery processes.2 Such chiral α-branched amine motifs can be accessed from electrophilic imine substrates, through direct addition of carbon-centered nucleophiles such as organometallic reagents and electron rich π-nucleophiles. However, free radical chemistry offers the possibility to reverse the polarity of imine derivatives; the formal addition of a hydrogen atom to the C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N π-bond can generate a nucleophilic α-amino radical able to react with alkenes and alkynes.3 However, to date these approaches have been limited by the way the radical is generated, typically requiring the specific preparation of radical precursors from imines and subsequent use of, for example, stoichiometric quantities of tin reagents.4 Recently, under photoredox catalysis, α-amino radicals have been generated through the decarboxylation of α-amino acids,5 α-amino trifluoroborates6 or hydrogen atom transfer from protected primary and secondary amines.7–9 Also, α-amino radicals have been generated from electron rich tertiary amines by sequential loss of an electron from the nitrogen lone pair, followed by an α-proton. However, the resulting α-amino radical is readily oxidised by the photocatalyst and undergoes a second single-electron oxidation to generate an iminium intermediate which can be subsequently trapped with various nucleophiles.10 Intercepting the α-amino radical in reactions with, for example, electron poor alkenes is far less common, and to date has been limited to N-aryltetrahydroisoquinolines,8 and phenyl methyl amines.9 Photoredox reactions in which imine moieties have been used as a trap for nucleophilic radicals are limited to a few reports.11
N π-bond can generate a nucleophilic α-amino radical able to react with alkenes and alkynes.3 However, to date these approaches have been limited by the way the radical is generated, typically requiring the specific preparation of radical precursors from imines and subsequent use of, for example, stoichiometric quantities of tin reagents.4 Recently, under photoredox catalysis, α-amino radicals have been generated through the decarboxylation of α-amino acids,5 α-amino trifluoroborates6 or hydrogen atom transfer from protected primary and secondary amines.7–9 Also, α-amino radicals have been generated from electron rich tertiary amines by sequential loss of an electron from the nitrogen lone pair, followed by an α-proton. However, the resulting α-amino radical is readily oxidised by the photocatalyst and undergoes a second single-electron oxidation to generate an iminium intermediate which can be subsequently trapped with various nucleophiles.10 Intercepting the α-amino radical in reactions with, for example, electron poor alkenes is far less common, and to date has been limited to N-aryltetrahydroisoquinolines,8 and phenyl methyl amines.9 Photoredox reactions in which imine moieties have been used as a trap for nucleophilic radicals are limited to a few reports.11
Despite these recent advances we recognised that a direct method for the generation of ‘free’ α-amino radicals would offer a wealth of untapped synthetic potential. Our plan was to identify then develop a new reductive photochemical system that, directly from an in situ formed imine, would efficiently generate ‘free’ nucleophilic α-amino radical species capable of undergoing a range of carbon–carbon bond forming processes (Fig. 1). Through its modular design such a process would be useful for library and target synthesis alike and herein we wish to report our findings.
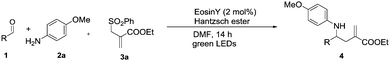
As a model reaction we chose the reductive coupling of benzaldehyde 1a, anisidine 2a and sulfone 3a.12 Following a series of preliminary studies performed to identify new reactivity, Ru(bpy)3Cl2, [Ir(dF(CF3)ppy)2(dtbbpy)][PF6] and [Ir(dtbbpy)(ppy)2][PF6], were found to efficiently promote the desired reaction in DMSO under blue LED light irradiation using Hantzsch ester as the terminal reductant. Pleasingly, the use of organic dyes such as eosin Y (EO) and rose bengal (RB) under green LED light irradiation, also promoted the desired reaction with similarly high yields. After further optimisation studies (see ESI†) the allylated product was isolated in 92% yield.
The three-component reductive coupling reaction was found to tolerate a wide range of aromatic aldehydes to afford 4-amino 4-aryl-2-methylene carboxylic esters in very good yields typically within 12 to 15 hours (Table 1). The best results were achieved with electron rich aromatic rings such as p-methoxy phenyl (entry 3) or p-dimethylamino phenyl (entry 4). A wide range of ortho substituents such methyl (entry 9), bromine (entry 5) and fluorine (entry 6) were well-tolerated as well. Oxygen substitution in the ortho position (entries 7 and 8) increased the amount of competing reductive amination. For 2-methoxy substitution (entry 8) this problem could be circumvented by using more powerful LEDs (10 W) in place of normal green LEDs strip. This preferentially accelerated the productive three-component reaction resulting in enhanced product yield.
| Entry | R | 4 | Yieldb (%) |
|---|---|---|---|
| a Reactions were carried out with 0.2 mmol of 1 and 2a, 0.3 mmol of Hantzsch ester, 0.5 mmol of 3a and 2 mol% of catalyst under green strip LEDs irradiation (1 W) in DMF. b Isolated yield. c Reaction irradiated with green 10 W LEDs. d Reaction time: 7 days. e Reaction time: 3 days. f Reaction time: 2 days. g Imine was preformed. | |||
| 1 | Ph | 4a | 87 |
| 2 | 2-Naphtyl | 4b | 85 |
| 3 | 4-MeOC6H4 | 4c | 80 |
| 4 | 4-Me2NC6H4 | 4d | 97 |
| 5 | 2-BrC6H4 | 4e | 74 |
| 6 | 2-FC6H4 | 4f | 85 |
| 7 | 2-OHC6H4 | 4g | 64c |
| 8 | 2-MeOC6H4 | 4h | 57 (86)c |
| 9 | 2-MeC6H4 | 4i | 94 |
| 10 | 4-CNC6H4 | 4j | 54d |
| 11 | 4-CF3C6H4 | 4k | 0 (82)c |
| 12 | 3-CNC6H4 | 4l | 85f |
| 13 | 4-CO2MeC6H4 | 4m | 63e (78)c |
| 14 | 4-Pyridyl | 4n | 65e |
| 15 | 3-Pyridyl | 4o | 83 |
| 16 | 2-Pyridyl | 4p | 53f |
| 17 | 2-Furyl | 4q | 84f |
| 18 | 2-Thiofuryl | 4r | 87f |
| 19 | 1-Methyl-1H-imidazol-2-yl | 4s | 79f |
| 20 | 1H-Indol-2-yl | 4t | 52e,g |
Pleasingly, electron deficient aromatic aldehydes yielded the desired product (entries 10–13) with acceptable yield although some competing overreduction was observed in all cases. While the reaction with 4-cyanobenzaldehyde generated the product with 54% yield (entry 10), switching the cyano substituent to the meta position, thus increasing the electron density of the aldehyde starting material, reduced the reaction time from 7 days to 2 days and the yield recovered to 85% (entry 12). Whereas employing more powerful LEDs had no influence on the reaction of the p-cyano substituent, a notable increase in yield was observed for 4-(methoxycarbonyl)phenyl (entry 13, single crystal X-ray diffraction studies of compound 4m are shown in the ESI†)13 and trifluoromethylphenyl (entry 11). Importantly, heteroaromatic aldehydes, such as pyridine, furan, thiophene, imidazole and indole, also successfully partook in the reaction. As already witnessed with CN substituent, (entries 10 and 12), the position of the heteroatom in the aromatic ring influenced the amount of overreduction observed. While more electron deficient 4- and 2-pyridinecarboxaldehyde produced yields of 50–60% (entries 14 and 16), 3-pyridinecarboxaldehyde improved the product yield to 83% (entry 15). Additionally, the rate of the productive reaction diminished for these substrates and accordingly the reaction time was increased to 3 days (entry 14) or 2 days (entry 16) respectively. Electron-rich 5-membered heterocycles such as furan (entry 17), thiophene (entry 18) and 1-methyl-1H-imidazole (entry 19) also required a reaction time of 2 days, yet afforded good yields. Especially noteworthy is the tolerance of indole (entry 20) as the heteroaryl substituent. Although the imine had to be preformed due to the very slow reaction between indole-2-carbaldehyde and p-anisidine under standard conditions, a 52% yield was obtained after 3 days before the reaction stalled; a remarkable outcome given that unprotected pyrroles or easily abstracted hydrogens often prove detrimental to photoredox reactions.14
Regarding the amine component, reactivity was witnessed in the presence of both electron-rich and electron-poor aromatic amines as well as heteroaromatic amines (Table 2, entries 1–4). Although ortho substituents were well-tolerated, with O-methoxyphenyl (entry 1) and 2-pyridyl substituents (entry 4) the preformation of the imine was necessary but the standard reaction conditions still afforded the desired products in excellent yields in both cases. Preformation of the imine was also necessary when electron poor p-nitroaniline was used as amine component; although in this case the reaction yield was diminished to 46%.
Next, we explored the range of allyl sulfones that were tolerated in the reaction (Table 2, entries 5–7). A tert-butyl ester derivative (entry 5) performed in the reaction without significant loss of yield (82%). However, other carbonyl groups such as ketones, carboxylic acids or carboxamides all failed to afford the product (ESI†). Interestingly, other electron withdrawing groups such as cyano (entry 6) and diethylphosphonate (entry 7) performed in the reaction well, yielding the expected product with moderate yield. The developed reaction proved to be robust to scale up; a gram scale synthesis of 4a proceeded in a yield of 95% which represents an improvement when compared to the reaction performed on 0.2 mmol scale.
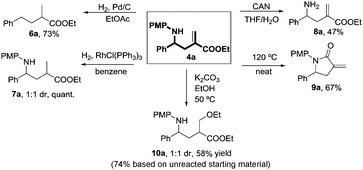
Three-component coupling product 4a is a versatile and modifiable building block rich in synthetic potential (Fig. 2).15 α-Methyl-γ-phenyl carboxylic compounds (6a) are easily accessible through simple hydrogenolysis and some derivatives have been studied as agonists for antimicrobial peptide systems.16 The PMP protecting group could be easily removed under standard conditions to provide the parent α-phenyl-γ-aminoesters that could find application in medicinal chemistry programmes as γ-aminobutyric acid (GABA) derivatives. Also, α-alkylidene γ-lactam 9a can be formed simply by heating compound 4a in the absence of solvent. Such lactams have already shown potential as inhibitors of homoserine transacetylase (HTA)17 and have been studied as less toxic alternatives to α-methylene γ-lactones in cancer treatment.18 Our new methodology allows a fast and easy approach to the synthesis of libraries of α-methylene γ-lactams in only two steps. The double bond can be further manipulated through hydrogenation to give 7a or through addition of nucleophiles such as ethanol yielding 10a.
To interrogate the reaction pathway to the three-component coupling product, control and cross-over experiments where performed. LED irradiation and eosin Y were necessary for the reaction to proceed. Also, the reaction was inhibited when TEMPO was added, supporting the participation of radical intermediates in the transformation (ESI†). As phenyl benzyl amines have been used in photoredox catalysis as reductive quenchers,19 the appearance of the overreduction product in the photoredox reactions with the less reactive imines20 raised the possibility that this compound could be, in fact, a potential species suffering single electron oxidation by eosin Y to generate an alpha amino radical21,22 ready to add into allyl sulfone 3a. However, subjection of a mixture of reductive amination product 11, allyl sulfone 3a and eosin Y in DMSO to green LED light irradiation gave no reaction, ruling out a tandem reductive then oxidative pathway to 4a (Fig. 3). Interestingly, a similar reaction set-up but in the presence [Ir(dF(CF3)ppy)2(dtbbpy)][PF6] as photocatalyst and blue LED light irradiation, afforded the reaction product as expected in 63% NMR yield.
An attempted cross-over experiment in which both an imine and p-tolualdehyde-derived amine 11 were present in the reaction mixture confirmed that under these conditions only the imine was capable of giving rise to the reaction product (Fig. 3).
Stern–Volmer quenching experiments (ESI†) showed a significant quenching of eosin Y fluorescence by Hantzsch ester, whereas sulfone or imine produced a much less pronounced effect. On the basis of these observations, we propose the reaction mechanism as depicted in Fig. 4. Under the reaction conditions, incident light excites EO, which undergoes a rapid intersystem crossing to the lowest energy triplet state,22 thus providing an oxidising species (E01/2 = +0.83 V vs. SCE in CH3CN)22 which reduces the Hantzsch ester to the radical cation, HE˙+.23 At this point EO radical-anion, EO˙− (E01/2 = −1.06 vs. SCE in CH3CN)22 is oxidized by the imine (E01/2 = −1.90 V vs. SCE in CH3CN),24 but in order for this process to take place spontaneously, the intervention of transient proton donor is necessary to significantly lower the redox potential of the imine by proton coupled electron transfer (PCET).25 A possible candidate might be Hantzsch ester radical cation (HE˙+). In agreement with this hypothesis it was observed that reducing agents other than the Hantzsch ester afforded no product or very low conversion. The generated α-amino-radical would then be poised to add to the sulfone C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C to form the product and benzene sulfinyl radical (E01/2 = +0.40 vs. SCE)26 which can be further reduced by HE, HE˙+ or EO˙−. On–off light experiments demonstrated that no product formation was occurring in the dark, thus supporting our hypothesis (ESI†). However, a short length radical chain mechanism cannot be completely excluded (see ESI†).27,28
C to form the product and benzene sulfinyl radical (E01/2 = +0.40 vs. SCE)26 which can be further reduced by HE, HE˙+ or EO˙−. On–off light experiments demonstrated that no product formation was occurring in the dark, thus supporting our hypothesis (ESI†). However, a short length radical chain mechanism cannot be completely excluded (see ESI†).27,28
In summary, we have developed a new three-component reductive coupling reaction of (hetero)arylamines, (hetero)aromatic aldehydes and electron deficient olefins catalysed by eosin Y under green LED light irradiation, employing Hantzsch ester as the terminal reductant.27 The reaction is easy to perform, broad in scope and provides a convenient umpolung alternative to amine synthesis from imines. The Hantzsch ester was found to play a critical role in generating the reactive nucleophilic radical intermediates and cross-over experiments confirmed the imine – and not the amine resulting from imine reduction – was the source of the α-amino radical. Work to expand the breadth of photochemical umpolung imine coupling reactions is ongoing in our laboratories and the results will be disclosed in due course.
We thank Marie Curie Actions-MSCA-IF-EF-ST to A. L. F. A. (660125), F. U. is supported by the EPSRC Centre for Doctoral Training in Synthesis for Biology and Medicine (EP/L015838/1). We also thank Heyao Shi for X-ray structure determination and Dr Amber L. Thompson and Dr Kirsten E. Christensen (Oxford Chemical Crystallography Service) for X-ray mentoring and help.
Notes and references
- T. L. Lemke, Review of Organic Functional Groups: Introduction to Medicinal Organic Chemistry, Lippincott Williams & Williams, Baltimore, 4th edn, 2003 Search PubMed.
- B. E. Blass, Basic Principles of Drug Discovery and Development, Academic Press, Boston, 2015 Search PubMed.
- (a) P. Renaud and L. Giraud, Synthesis, 1996, 913 CrossRef CAS; (b) J. M. Aurroecoechea and R. Suero, ARKIVOC, 2004, 10 Search PubMed; (c) M. Szostak and D. J. Procter, Angew. Chem., Int. Ed., 2012, 51, 9238 CrossRef CAS PubMed.
- X. Xia, S. Ji, F. Wang, D. Huang, K.-H. Wang, Y. Su and Y. Hu, Chin. J. Org. Chem., 2015, 35, 1040 CrossRef CAS.
- (a) Z. Zuo and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 5257 CrossRef CAS PubMed; (b) A. Noble and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 11602 CrossRef CAS PubMed; (c) Z. Zuo, H. Cong, W. Li, J. Choi, G. C. Fu and D. W. C. MacMillan, J. Am. Chem. Soc., 2016, 138, 1832 CrossRef CAS PubMed.
- D. R. Heitz, K. Rizwan and G. A. Molander, J. Org. Chem., 2016, 81, 7308 CrossRef CAS PubMed.
- M. H. Shaw, V. W. Shurtleff, J. A. Terrett, J. D. Cuthbertson and D. W. C. MacMillan, Science, 2016, 352, 1304 CrossRef CAS PubMed.
- (a) P. Kohls, D. Jadhav, G. Pandey and O. Reiser, Org. Lett., 2012, 14, 672 CrossRef CAS PubMed; (b) L. R. Espelt, E. M. Wiensch and T. P. Yoon, J. Org. Chem., 2013, 78, 4107 CrossRef PubMed; (c) J. Xuan, T.-T. Zeng, Z.-J. Feng, Q.-H. Deng, J.-R. Chen, L.-Q. Lu, W.-J. Xiao and H. Alper, Angew. Chem., Int. Ed., 2015, 54, 1625 CrossRef CAS PubMed.
- (a) Y. Miyake, K. Nakajima and Y. Nishibayashi, J. Am. Chem. Soc., 2012, 134, 3338 CrossRef CAS PubMed; (b) S. Zhu, A. Das, L. Bui, H. Zhou, D. Curran and M. Rueping, J. Am. Chem. Soc., 2013, 135, 1823 CrossRef CAS PubMed; (c) E. Fava, A. Millet, M. Nakajima, S. Loescher and M. Rueping, Angew. Chem., Int. Ed., 2016, 55, 6776 CrossRef CAS PubMed; (d) C. K. Prier and D. W. C. MacMillan, Chem. Sci., 2014, 5, 4173 RSC.
- (a) A. G. Condie, J. C. González-Gómez and C. R. J. Stephenson, J. Am. Chem. Soc., 2010, 132, 1464 CrossRef CAS PubMed; (b) S. Cai, X. Zhao, X. Wang, Q. Liu, Z. Li and D. Z. Wang, Angew. Chem., Int. Ed., 2012, 51, 8050 CrossRef CAS PubMed; (c) M. Rueping, S. Zhu and R. M. Koenigs, Chem. Commun., 2011, 47, 12709 RSC; (d) G. Zhao, C. Yang, L. Guo, H. Sun, C. Chen and W. Xia, Chem. Commun., 2012, 48, 2337 RSC; (e) M. Rueping, C. Vila, R. M. Koenigs, K. Poscharny and D. C. Fabry, Chem. Commun., 2011, 47, 2360 RSC; (f) M. Rueping, S. Zhu and R. M. Koenigs, Chem. Commun., 2011, 47, 8679 RSC; (g) D. B. Freeman, L. Furst, A. G. Condie and C. R. J. Stephenson, Org. Lett., 2012, 14, 94 CrossRef CAS PubMed; (h) Z.-Q. Wang, M. Hu, X.-C. Huang, L.-B. Gong, Y.-X. Xie and J.-H. Li, J. Org. Chem., 2012, 77, 8705 CrossRef CAS PubMed; (i) S. Zhu and M. Rueping, Chem. Commun., 2012, 48, 11960 RSC; (j) M. Rueping, R. M. Koenigs, K. Poscharny, D. C. Fabry, D. Leonori and C. Vila, Chem. – Eur. J., 2012, 18, 5170 CrossRef CAS PubMed; (k) Y. Pan, S. Wang, C. W. Kee, E. Dubuisson, Y. Yang, K. P. Loh and C.-H. Tan, Green Chem., 2011, 13, 3341 RSC; (l) W. Fu, W. Guo, G. Zou and C. Xu, J. Fluorine Chem., 2012, 140, 88 CrossRef CAS; (m) J. F. Franz, W. B. Kraus and K. Zeitler, Chem. Commun., 2015, 51, 8220 RSC; (n) H. Bartling, A. Eisenhofer, B. König and R. M. Gschwind, J. Am. Chem. Soc., 2016, 138, 11860 CrossRef CAS PubMed.
- (a) D. Hager and D. W. C. MacMillan, J. Am. Chem. Soc., 2014, 136, 16986 CrossRef CAS PubMed; (b) J. L. Jeffrey, F. P. Petronijevic and D. W. C. MacMillan, J. Am. Chem. Soc., 2015, 137, 8404 CrossRef CAS PubMed; (c) M. Nakajima, E. Fava, S. Loecher, Z. Jiang and M. Rueping, Angew. Chem., Int. Ed., 2015, 54, 8828 CrossRef CAS PubMed; (d) E. Fava, A. Millet, M. Nakajima, S. Loescher and M. Rueping, Angew. Chem., Int. Ed., 2016, 55, 6776 CrossRef CAS PubMed; (e) D. Uraguchi, N. Kinoshita, T. Kizu and T. Ooi, J. Am. Chem. Soc., 2015, 137, 13768 CrossRef CAS PubMed.
- (a) J. Zhang, Y. Li, F. Zhang, C. Hu and Y. Chen, Angew. Chem., Int. Ed., 2016, 128, 1904 CrossRef; (b) M.-H. Larraufie, R. Pellet, L. Fensterbank, J.-P. Goddard, E. Lacote, M. Malacria and C. Ollivier, Angew. Chem., Int. Ed., 2011, 50, 4463 CrossRef CAS PubMed.
- Low temperature single X-ray diffraction data were collected for 4m using a (Rigaku) Oxford Diffraction Supernova diffractometer. Data were reduced using CrysAlisPro and solved using Superflip [ L. Palatinus and G. Chapuis, J. Appl. Crystallogr., 2007, 40, 786 CrossRef CAS ] before within CRYSTALS [ P. W. Betteridge, J. R. Carruthers, R. I. Cooper, K. Prout and D. J. Watkin, J. Appl. Crystallogr., 2003, 36, 1487 CrossRef; R. I. Cooper, A. L. Thompson and D. J. Watkin, J. Appl. Crystallogr., 2010, 43, 1100 CrossRef ]. CCDC 1507899.
- C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed.
- (a) F. Pan, J.-M. Chen, Z. Zhuang, Y.-Z. Fang, S. X.-A. Zhang and W.-W. Liao, Org. Biomol. Chem., 2012, 10, 2214 RSC; (b) F. Pan, J.-M. Chen, T.-Y. Qin, S. X.-A. Zhang and W.-W. Liao, Eur. J. Org. Chem., 2012, 5324 CrossRef CAS.
- G. H. Gudmundsson, B. Agerberth, E. Steingrimsson, R. Stromberg and R. Raqib, PCT Int. Appl., WO 2009087474 A2 20090716, 2009 Search PubMed.
- T. G. Elford, A. Ulaczyk-Lesanko, G. De Pascale, G. D. Wright and D. G. Hall, J. Comb. Chem., 2009, 11, 155 CrossRef CAS PubMed.
- C. Belaud, C. Roussakis, Y. Letourneux, M. El Alami and J. Villieras, Synth. Commun., 1985, 15, 1233 CrossRef CAS.
- (a) J. Hu, J. Wang, T. H. Nguyen and N. Zheng, Beilstein J. Org. Chem., 2013, 9, 1977 CrossRef PubMed; (b) J. W. Beatty and C. R. J. Stephenson, Acc. Chem. Res., 2015, 48, 474 CrossRef PubMed.
- Hantzsch ester has been commonly used to perform imine reduction, selected references: (a) G. Li, Y. Liang and J. C. Antilla, J. Am. Chem. Soc., 2007, 129, 5830 CrossRef CAS PubMed; (b) Z. Zhang and P. R. Schreiner, Synlett, 2007, 1455 CAS; (c) Q. P. B. Nguyen and T. H. Kim, Synthesis, 2012, 1977 CAS; (d) D. Menche, J. Hassfeld, J. Li, G. Menche, A. Ritter and S. Rudolph, Org. Lett., 2006, 8, 741 CrossRef CAS PubMed; (e) D. Menche and F. Arikan, Synlett, 2006, 841 CrossRef CAS.
- D. D. M. Wayner, J. J. Dannenberg and D. Griller, Chem. Phys. Lett., 1986, 131, 189 CrossRef CAS.
- D. P. Hari and B. König, Chem. Commun., 2014, 50, 6688 RSC.
- X. Wei, L. Wang, W. Jia, S. Du, L. Wu and Q. Liu, Chin. J. Chem., 2014, 32, 1245 CrossRef CAS.
- H. G. Roth, N. A. Romero and D. A. Nicewicz, Synlett, 2016, 714 CAS.
- (a) D. C. Miller, K. T. Tarantino and R. R. Knowles, Top. Curr. Chem., 2016, 374 Search PubMed; (b) L. J. Rono, H. G. Yayla, D. Y. Wang, M. F. Armstrong and R. R. Knowles, J. Am. Chem. Soc., 2013, 135, 17735 CrossRef CAS PubMed; (c) K. T. Tarantino, P. Liu and R. R. Knowles, J. Am. Chem. Soc., 2013, 135, 10022 CrossRef CAS PubMed.
- A. U. Mayer, K. Strakova, T. Slanina and B. König, Chem. – Eur. J., 2016, 22, 8694 CrossRef PubMed.
- During the preparation of this manuscript an [Ir(dtbbpy)(ppy)2][PF6] catalysed photoredox reaction between allylsulfones and aldehydes, ketones and aldimines was reported by: L. Qi and Y. Chen, Angew. Chem., Int. Ed., 2016, 55, 13312 CrossRef CAS PubMed.
- M. A. Cismeia and T. P. Yoon, Chem. Sci., 2015, 6, 5426 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data, fluorescence experiments and copies of 1H and 13C NMR spectra. CCDC 1507899. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc09172e |
| This journal is © The Royal Society of Chemistry 2016 |