Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synchronous exfoliation and assembly of graphene on 3D Ni(OH)2 for supercapacitors†
Liguo
Ma
a,
Maojun
Zheng
*ab,
Shaohua
Liu
 *c,
Qiang
Li
a,
Yuxiu
You
a,
Faze
Wang
a,
Li
Ma
d and
Wenzhong
Shen
a
*c,
Qiang
Li
a,
Yuxiu
You
a,
Faze
Wang
a,
Li
Ma
d and
Wenzhong
Shen
a
aKey Laboratory of Artificial Structure and Quantum Control, Ministry of Education, Department of Physics and Astronomy, Shanghai Jiao Tong University, Shanghai, 200240, P. R. China. E-mail: mjzheng@sjtu.edu.cn; Fax: +86-021-54741040
bCollaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing, 210093, P. R. China
cCenter for Advancing Electronics Dresden (cfaed) & Department of Chemistry and Food Chemistry, Dresden, 01062, Germany. E-mail: shaohua.liu@tu-dresden.de
dSchool of Chemistry and Chemical Technology, Shanghai Jiao Tong University, Shanghai, 200240, P. R. China
First published on 21st October 2016
Abstract
Nowadays, new approaches to fabricate high-performance electrode materials are of vital importance in the renewable energy field. Here, we present a facile synthesis procedure of 3D Ni(OH)2/graphene hybrids for supercapacitors via synchronous electrochemical-assisted exfoliation and assembly of graphene on 3D Ni(OH)2 networks. With the assistance of an electric field, the electrochemically exfoliated high-quality graphene can be readily, uniformly assembled on the surfaces of 3D Ni(OH)2. When serving as electrode materials for supercapacitors, the resulting 3D Ni(OH)2/graphene composites exhibited excellent specific capacitance (263 mF cm−2 at 2 mA cm−2), remarkable rate capability and super-long cycle life (retention of 94.1% even after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 continuous charge–discharge cycles), which may be attributed to their highly porous, stable 3D architecture as well as uniform, firm anchoring of ultrathin graphene on their surfaces. Therefore, our approach provides a facile strategy for the large-scale synthesis of high-quality graphene based composites towards various applications.
000 continuous charge–discharge cycles), which may be attributed to their highly porous, stable 3D architecture as well as uniform, firm anchoring of ultrathin graphene on their surfaces. Therefore, our approach provides a facile strategy for the large-scale synthesis of high-quality graphene based composites towards various applications.
Supercapacitors with high capacitance and long lifespan have attracted considerable interest as energy-storage devices in recent years. At present, two kinds of supercapacitors have been developed based on the charge storage mechanism: electric double-layer capacitors and pseudocapacitors. By comparison, pseudocapacitors governed by Faradaic reactions at the electrode materials, such as RuO2,1 MnO2,2 NiO,3 Co3O4,4 and CuO,5 have higher theoretical capacitance. Despite that, those electrode materials usually suffer from poor stability, low conductivity and large volume change during charge/discharge processes.6 Among the various redox-active materials, Ni(OH)2 is an attractive candidate for high-performance supercapacitors because of its low-cost, various morphologies, high theoretical capacitance, ready availability and good stability in alkaline electrolytes.7 In attempts to further improve the electrochemical performance of Ni(OH)2-based electrodes, Ni(OH)2/carbon composites involving high surface-area conductive materials (carbon nanotubes,8 activated carbon9 and graphene10) have been explored. However, the fabrication of Ni(OH)2/carbon electrodes usually needs multi-step procedures. Taking Ni(OH)2/graphene as an example, graphene needs to be firstly prepared by Hummer's method or modified Hummer's method, and subsequently grows on Ni(OH)2 surfaces by hydrothermal treatment.11 Obviously, such fabrication processes are complicated and costly for supercapacitor applications.
Recently, high-quality graphene can be produced by a simple, fast and green electrochemical exfoliation method. Müllen et al.12 manufactured high-quality few-layer graphene through anodic exfoliation. Yan et al.13 used a Na2SO4-containing electrolyte and other metal sulfate salts to exfoliate graphite and further fabricate Fe2O3–, Co3O4– and V2O5–graphene hybrids. Ji et al.14 produced NiO quantum dots/graphene flakes for supercapacitor applications by a green, one-step alternating voltage method. Nevertheless, one-step fabrication of Ni(OH)2/graphene hybrid materials by synchronous electrochemical-assisted exfoliation and assembly for supercapacitor electrodes still remains unrealized so far.
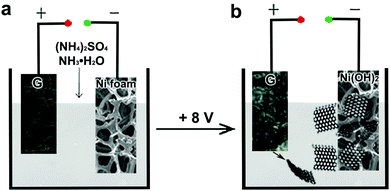
Here, for the first time, we demonstrate a facile approach for the preparation of Ni(OH)2/graphene composites via in situ assembly of exfoliated graphene on 3D Ni(OH)2 surfaces. As illustrated in Fig. 1, ultrathin high-quality graphene was obtained by the solution-based electrochemical exfoliation with a two-electrode system, using graphite flakes, Ni foam and a mixed solution containing NH3·H2O and (NH4)2SO4 (Fig. 1a) as the anode, cathode and electrolyte, respectively. When a direct current voltage of 8 V was applied to a two-electrode setup, vigorous bubbles were produced at electrodes, anodic graphite began to dissociate to form graphene sheets, suspended in the electrolyte (Fig. 1b). Meanwhile, the cathodic Ni foam firstly transformed into Ni(OH)2 through NH3·H2O treatment following reactions (1) and (2):
| Ni + 6NH3 + 2H2O → [Ni(NH3)6]2+ + 2OH− + H2↑ | (1) |
| Ni2+ + 2OH− → Ni(OH)2 | (2) |
The morphology and structure of the exfoliated graphene sheets were investigated by SEM, TEM and Raman spectroscopy shown in Fig. S1–S3 (ESI†), which clearly demonstrate the formation of high-quality few-layer graphene.12 Further, graphene obtained by electrochemical-assisted exfoliation would spontaneously absorb and assemble on the cathodic 3D Ni(OH)2 due to electrostatic interaction. Despite high quality, the resulting graphene derived from electrochemical-assisted exfoliation still possesses a few negative charges around its edges,12 which would prompt their assembly on the Ni(OH)2 surfaces with the assistance of an electric field. Notably, the Ni foam is not only the nickel source for the growth of Ni(OH)2 thin layers, but also a skeleton to support the active materials of Ni(OH)2/graphene composites, which affords a good electrical contact and strong mechanical adherence to their interfaces.15
The structure of the Ni(OH)2/graphene composites was further characterized by Raman spectroscopy (Fig. 2a). The Raman spectrum of the Ni(OH)2/graphene composites presents three obvious characteristic vibration bands at 1350 cm−1, 1583 cm−1 and 2700 cm−1 corresponding to the D, G and 2D band of graphene, respectively. In addition, the Ni(OH)2/graphene composites also present four peaks from Ni(OH)2, at around 315 and 450 cm−1 (longitudinal optical, A1g(T) and Eg(T)), 983.5 cm−1 (phonon mode, Eg(R)) and 3580 cm−1 (internal hydroxyl symmetric stretching mode, A1g(I)).16
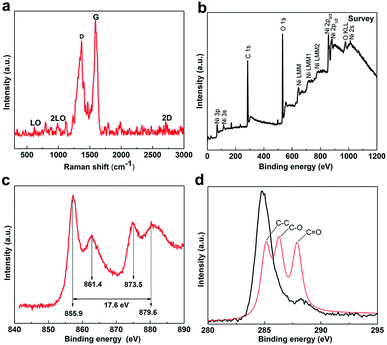 | ||
| Fig. 2 (a) Raman pattern (excitation laser wavelength: 532 nm) and (b–d) XPS spectra of the Ni(OH)2/graphene samples. | ||
X-ray photoelectron spectroscopy (XPS) was used to estimate various chemical states of bonded elements. Typical spectra of the as-prepared 3D Ni(OH)2/graphene samples are presented in Fig. 2b–d. A single peak at around 840.8 eV presents the Ni LMM Auger spectrum.17 The Ni 2p XPS spectrum shows two major peaks centered at around 874.9 eV and 857.3 eV, corresponding to Ni 2p1/2 and Ni 2p3/2, respectively. The characteristic of the Ni(OH)2 phase with a spin-energy separation of 17.6 eV coincides with a previous report.18 Moreover, a strong peak of the O 1s spectrum at 532.3 eV is also associated with hydroxide groups (OH−), suggestive of the presence of Ni(OH)2.18 Additionally, the XPS spectrum of C 1s shows three signals of C–C (284.6 eV), C–O (286.7 eV) and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.6 eV), which are possibly from the covalent oxygen groups on graphene (Fig. 2d).
O (288.6 eV), which are possibly from the covalent oxygen groups on graphene (Fig. 2d).
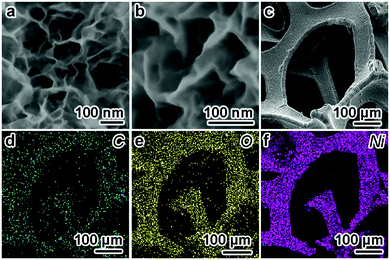
The morphologies of the Ni(OH)2/graphene composites were investigated by scanning electron microscopy (SEM). Fig. 3a and b show representative SEM images of Ni(OH)2/graphene, revealing a highly porous architecture, which is composed of nanoflakes with a thickness of 20–30 nm. No collapse of the Ni foam struts or obstruction of the pores is observed, indicating the strong mechanical strength of the Ni(OH)2/graphene and the uniform dispersion of the Ni(OH)2 nanosheets. Furthermore, the elemental mappings of C, O and Ni were investigated from a small region on the foam (Fig. 3c–f), indicating a continuous, uniform distribution of Ni(OH)2 and graphene on the 3D Ni foam surfaces. The corresponding EDS mapping also revealed that the C element content of the Ni(OH)2/graphene composites is about 8% (atomic ratio, Table S1 in the ESI†), whereas there was no C element in the pristine Ni(OH)2 without graphene on their surfaces (Fig. S4, ESI†). Fig. S5 (ESI†) shows that the sheet morphology of graphene and Ni(OH)2 (black arrow markings), and a corresponding selected-area electron-diffraction pattern only indicate the typical sixfold symmetry expected for graphene. This result indicates an amorphous morphology of Ni(OH)2. In addition, except for the peaks associated with the Ni foam substrate, no peaks are present in the X-ray diffraction pattern of Ni(OH)2/graphene in Fig. S6 (ESI†), which further confirms the amorphous nature of the sample.
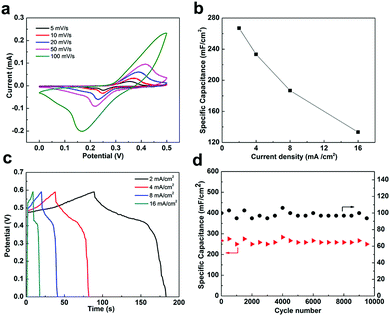
Further, we evaluated the electrochemical performance of the as-prepared Ni(OH)2/graphene composites in a three-electrode test cell. The capacitive behavior of the electrode material is generally characterized using cyclic voltammetry (CV) curves. Fig. 4a presents typical CV curves of the Ni(OH)2/graphene samples in a 1 M KOH electrolyte at various scan rates between 0.0 and 0.5 V. Two strong redox peaks can be observed in each curve. For electric double-layer capacitors, CV curves appear nearly rectangular. Our results thus indicate that the capacitance characteristics are mainly due to Faradaic redox reactions. For the Ni(OH)2 electrode materials, the surface Faradaic reactions will proceed as:
| Ni(OH)2 + OH− ↔ NiOOH + H2O + e− |
To further evaluate the electrochemical properties and estimate the stable potential windows of the as-prepared Ni(OH)2/graphene composites, galvanostatic charging and discharging of the films in 1 M KOH solution were performed using a Pt counter electrode and a saturated calomel reference electrode. The charge–discharge curves (Fig. 4b) at different current densities (2–16 mA cm−2) between 0.0 and 0.6 V display slight nonlinearities that are very distinct from those of typical pure double-layer capacitors. This result indicates the occurrence of Faradaic reactions in the Ni(OH)2/graphene composites.
The specific capacitance of the Ni(OH)2/graphene electrode can be calculated from the discharge curve according to C = I × Δt/(A × ΔV),19 where I is the discharge current (A), Δt is the discharge time (s), A is the area of the active material in the electrode (cm2) and ΔV is the potential change during discharge (V). The specific capacitances obtained were 267, 233, 187, and 133 mF cm−2 at current densities of 2, 4, 8 and 16 mA cm−2, respectively. Clearly, the specific capacitance gradually decreases with increasing current density.
Good cycling stability is another important characteristic for high-performance supercapacitors. Fig. 4d reveals the cycle performance of the Ni(OH)2/graphene samples measured at a current density of 2 mA cm−2 for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Afterward, the capacitance retention was 94.1% of the initial capacitance, indicating excellent long-term stability of the Ni(OH)2/graphene composite electrode, which exceed those of the previously reported Ni(OH)2-based materials (usually with a capacitance retention of 51.0–63.2% within 10
000 cycles. Afterward, the capacitance retention was 94.1% of the initial capacitance, indicating excellent long-term stability of the Ni(OH)2/graphene composite electrode, which exceed those of the previously reported Ni(OH)2-based materials (usually with a capacitance retention of 51.0–63.2% within 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles).15,20 By comparison, the 3D Ni(OH)2 film without graphene presented a capacitance retention of only 41.4% even after 1000 cycles. These results highly highlight the vital role of the incorporation of graphene into electrochemical active electrode materials (Fig. S7, ESI†). Obviously, Ni(OH)2/graphene has the smaller equivalent series resistance (ESR) value than Ni(OH)2 as shown in Fig. S8 (ESI†), reflecting the enhancement in the electronic and ionic conductivities of Ni(OH)2 with the presence of graphene. This result indicates that Ni(OH)2/graphene composites exhibit good current rate properties, which are consistent with the cycle performance results.
000 cycles).15,20 By comparison, the 3D Ni(OH)2 film without graphene presented a capacitance retention of only 41.4% even after 1000 cycles. These results highly highlight the vital role of the incorporation of graphene into electrochemical active electrode materials (Fig. S7, ESI†). Obviously, Ni(OH)2/graphene has the smaller equivalent series resistance (ESR) value than Ni(OH)2 as shown in Fig. S8 (ESI†), reflecting the enhancement in the electronic and ionic conductivities of Ni(OH)2 with the presence of graphene. This result indicates that Ni(OH)2/graphene composites exhibit good current rate properties, which are consistent with the cycle performance results.
In summary, we present a facile strategy to synchronously exfoliate graphite and assemble the high-quality graphene on 3D Ni(OH)2 surfaces. High-quality graphene and the unique architecture of 3D skeleton provided Ni(OH)2/graphene hybrids with high conductivity, strong mechanical stability, ion diffusivity as well as the capability to accommodate volume changes during Faradaic reactions. As a result, the resulting 3D Ni(OH)2/graphene composites exhibited excellent specific capacitance, remarkable rate capability and super-long cycle life when used as electrode materials for supercapacitors. Such a simple and cost-effective synthetic approach would open new doors for the development of a serial of high-quality graphene based materials, and offer promising applications for high-performance energy-storage devices.
We acknowledge the support of the National 863 Program 2011AA050518 and the Natural Science Foundation of China (Grant no. 11174197, 11574203, and 61234005).
Notes and references
- J. Zhang, J. Jiang, H. Li and X. S. Zhao, Energy Environ. Sci., 2011, 4, 4009–4015 CAS.
- Z. Lei, J. Zhang and X. S. Zhao, J. Mater. Chem., 2012, 22, 153–160 RSC.
- J.-W. Lang, L.-B. Kong, W.-J. Wu, Y.-C. Luo and L. Kang, Chem. Commun., 2008, 4213–4215 RSC.
- X.-H. Xia, J.-P. Tu, Y.-J. Mai, X.-L. Wang, C.-D. Gu and X.-B. Zhao, J. Mater. Chem., 2011, 21, 9319–9325 RSC.
- S. K. Shinde, D. P. Dubal, G. S. Ghodake and V. J. Fulari, RSC Adv., 2015, 5, 4443–4447 RSC.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- (a) X. Xiong, D. Ding, D. Chen, G. Waller, Y. Bu, Z. Wang and M. Liu, Nano Energy, 2015, 11, 154–161 CrossRef CAS; (b) A. Harvey, X. He, I. J. Godwin, C. Backes, D. McAteer, N. C. Berner, N. McEvoy, A. Ferguson, A. Shmeliov, M. E. G. Lyons, V. Nicolosi, G. S. Duesberg, J. F. Donegan and J. N. Coleman, J. Mater. Chem. A, 2016, 4, 11046–11059 RSC; (c) G.-W. Yang, C.-L. Xu and H.-L. Li, Chem. Commun., 2008, 6537–6539 RSC; (d) Y. F. Yuan, X. H. Xia, J. B. Wu, J. L. Yang, Y. B. Chen and S. Y. Guo, Electrochim. Acta, 2011, 56, 2627–2632 CrossRef CAS.
- Z. Tang, C. H. Tang and H. Gong, Adv. Funct. Mater., 2012, 22, 1272–1278 CrossRef CAS.
- Q. Huang, X. Wang, J. Li, C. Dai, S. Gamboa and P. J. Sebastian, J. Power Sources, 2007, 164, 425–429 CrossRef CAS.
- (a) S. Yang, X. Wu, C. Chen, H. Dong, W. Hu and X. Wang, Chem. Commun., 2012, 48, 2773–2775 RSC; (b) J. Yan, Z. Fan, S. Wei, G. Ning, W. Tong, Z. Qiang, R. Zhang, L. Zhi and W. Fei, Adv. Funct. Mater., 2012, 22, 2632–2641 CrossRef CAS; (c) S. Min, C. Zhao, G. Chen and X. Qian, Electrochim. Acta, 2014, 115, 155–164 CrossRef CAS; (d) C. H. Wu, S. X. Deng, H. Wang, Y. X. Sun, J. B. Liu and H. Yan, ACS Appl. Mater. Interfaces, 2014, 6, 1106–1112 CrossRef CAS PubMed.
- (a) J. Yan, W. Sun, T. Wei, Q. Zhang, Z. Fan and F. Wei, J. Mater. Chem., 2012, 22, 11494–11502 RSC; (b) J. Xie, X. Sun, N. Zhang, K. Xu, M. Zhou and Y. Xie, Nano Energy, 2013, 2, 65–74 CrossRef CAS; (c) H. Wang, H. S. Casalongue, Y. Liang and H. Dai, J. Am. Chem. Soc., 2010, 132, 7472–7477 CrossRef CAS PubMed; (d) C. Jiang, B. Zhan, C. Li, W. Huang and X. Dong, RSC Adv., 2014, 4, 18080–18085 RSC.
- K. Parvez, Z.-S. Wu, R. Li, X. Liu, R. Graf, X. Feng and K. Müllen, J. Am. Chem. Soc., 2014, 136, 6083–6091 CrossRef CAS PubMed.
- W. Zhang, Y. Zeng, N. Xiao, H. H. Hng and Q. Yan, J. Mater. Chem., 2012, 22, 8455–8461 RSC.
- M. Jing, C. Wang, H. Hou, Z. Wu, Y. Zhu, Y. Yang, X. Jia, Y. Zhang and X. Ji, J. Power Sources, 2015, 298, 241–248 CrossRef CAS.
- L. Wang, X. Li, T. Guo, X. Yan and B. K. Tay, Int. J. Hydrogen Energy, 2014, 39, 7876–7884 CrossRef CAS.
- C. Murli, S. M. Sharma, S. K. Kulshreshtha and S. K. Sikka, Phys. Chem. Chem. Phys., 2001, 307, 111–116 CAS.
- M. C. Biesinger, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, Phys. Chem. Chem. Phys., 2012, 14, 2434–2442 RSC.
- H. B. Li, M. H. Yu, F. X. Wang, P. Liu, Y. Liang, J. Xiao, C. X. Wang, Y. X. Tong and G. W. Yang, Nat. Commun., 2013, 4(5), 54–56 Search PubMed.
- M. Jin, G. Zhang, F. Yu, W. Li, W. Lu and H. Huang, Phys. Chem. Chem. Phys., 2013, 15, 1601–1605 RSC.
- J. Ji, L. L. Zhang, H. Ji, Y. Li, X. Zhao, X. Bai, X. Fan, F. Zhang and R. S. Ruoff, ACS Nano, 2013, 7, 6237–6243 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc07645a |
| This journal is © The Royal Society of Chemistry 2016 |