 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
SmCpR2-mediated cross-coupling of allyl and propargyl ethers with ketoesters and a telescoped approach to complex cycloheptanols†
Mateusz P.
Plesniak
,
Xavier
Just-Baringo
,
Fabrizio
Ortu
 ,
David P.
Mills
* and
David J.
Procter
*
,
David P.
Mills
* and
David J.
Procter
*
School of Chemistry, The University of Manchester, Oxford Rd, Manchester, M13 9PL, UK. E-mail: david.mills@manchester.ac.uk; david.j.procter@manchester.ac.uk
First published on 25th October 2016
Abstract
A highly regio- and diastereoselective cross-coupling of allyl/propargyl ethers and δ-ketoesters, mediated by SmCpR2 reagents, delivers decorated δ-lactones. Screening of the Cp ligands on Sm(II) was employed to achieve high regio and diastereocontrol in some cases. Crucially, SmI2 gave unsatisfactory results in the transformation. The process has been exploited in a telescoped approach to complex cycloheptanols in which two Sm(II) reagents act in turn on the simple starting materials.
The latter half of the twentieth century saw the emergence of a new generation of metallic reductive ET reagents1 for substrate activation.2 At the vanguard of this new order was samarium(II) iodide (SmI2, Kagan's reagent)3 and since its first use in synthesis by Kagan,4 it has become indispensable.5 Unfortunately, some SmI2-mediated processes proceed with unsatisfactory levels of stereo- and regiocontrol and the identification of alternative Sm(II) reagents that deliver improved results is desirable.3–5
δ-Lactones are important building blocks for synthesis and are motifs found in many bioactive targets.6 Inspired by the seminal studies of Evans on the properties of SmCp*27 and of Kagan8 and Takaki9 on the allylation of simple, unfunctionalised ketones using SmCp2 and SmCp*2(THF)2, respectively, we envisaged a route to lactones 1 from allyl ethers 2 and δ-ketoesters 3 (Scheme 1). In stark contrast to SmI2, SmCpR2 reagents have found limited use in preparative organic synthesis as ET reagents despite applications in organometallic synthesis and polymer science.7–10 Crucially, SmI2 proved to be unreactive towards allyl ethers and gave unsatisfactory results with allylic halides in the proposed coupling-lactonisation: δ-lactones were obtained with poor regio- and diastereocontrol.11 Herein, we describe the optimisation of a SmCpR2-mediated approach to decorated δ-lactones 1 from allyl and propargyl ethers 2 and δ-ketoesters 3.12 To our knowledge, this is the first study examining the influence of substituents on the Cp ring on SmCpR2-mediated C–C bond-formation. Furthermore, we report a telescoped approach to complex cycloheptanols 4 (Scheme 1).
 | ||
| Scheme 1 A SmCpR2-mediated approach to decorated δ-lactones: reductive coupling of allyl/propargyl ethers and δ-ketoesters. A telescoped approach allows access to complex cycloheptanols. | ||
We began by preparing a family of SmCpR2 reagents 5a–f, including novel complexes 5c, 5e and 5f, with varying steric and electronic properties (Scheme 2). In line with Takaki's findings,9 the high reducing ability of 5a,b,e,f allowed readily prepared and stable allyl/propargyl ethers 2 to be used as substrates in the reductive cross-coupling (SmI2–THF, ca. −1.8 V vs. SCE; SmI2–H2O, ca. −2.2 V vs. SCE; SmCp*2(THF)2, −2.2 V vs. SCE).13 We therefore used the coupling-lactonisation of 2a and bifunctional ketone 3a to evaluate the Sm(II)CpR2 complexes with the aim of identifying a Sm(II) reagent capable of mediating a regio- and diastereoselective process. Notably, the use of SmCpR2 reagents allowed flexibility with regard to solvent choice – SmI2 is almost exclusively used in THF – and toluene was found to provide optimal reactivity and selectivity in the coupling.
 | ||
| Scheme 2 Tailoring the SmCpR2 reagent for the regio- and diastereoselective coupling-lactonisation to give anti-1a. The minor regioisomer is 1a′ (not shown). | ||
Pleasingly, Sm(II) reagents 5a,b and 5e,f gave the desired lactone isomer 1a with high regiocontrol and with a preference for the formation of anti-1a, as confirmed by X-ray crystallographic analysis (Scheme 2).14 A proposed mechanism for the coupling is shown in Scheme 3.9
 | ||
| Scheme 3 Proposed mechanism and origin of regio- and diastereocontrol in the SmCpR2-mediated coupling of 2a and 3a. R = (CH2)3CO2Et. | ||
To control the reactivity of allylic samarium(III) intermediate 6a, we proposed that larger Cp ligands would favour reaction through η1 organosamarium(III) 6cvia transition structure 7 to give the desired branched product 8. However, if the Cp ligands were too large, the cyclic transition structure 7 may be disrupted and diastereocontrol lost. As the use of SmCp*2(THF)25a gave anti-1a with moderate dr 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17, we fine-tuned the steric characteristics of the SmCpR2 reagents by varying the nature and number of silylsubstituents on the Cp ligands. While bis-TMSCp ligands in 5b (TMS – trimethylsilyl) gave excellent regiocontrol, lower diastereoselectivity was obtained in the coupling. Interestingly, tris-TMSCp complex 5c and SmCp25d proved unreactive. This is likely due to the steric hindrance associated with 5c and the lower reducing ability of 5d. Switching to the bulkier DPMSCp ligands in 5e (DPMS = diphenylmethylsilyl) gave anti-1a with very good regiocontrol (91
17, we fine-tuned the steric characteristics of the SmCpR2 reagents by varying the nature and number of silylsubstituents on the Cp ligands. While bis-TMSCp ligands in 5b (TMS – trimethylsilyl) gave excellent regiocontrol, lower diastereoselectivity was obtained in the coupling. Interestingly, tris-TMSCp complex 5c and SmCp25d proved unreactive. This is likely due to the steric hindrance associated with 5c and the lower reducing ability of 5d. Switching to the bulkier DPMSCp ligands in 5e (DPMS = diphenylmethylsilyl) gave anti-1a with very good regiocontrol (91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) and diastereocontrol (92
9) and diastereocontrol (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 dr). Bulkier TPSCp ligands in 5f gave anti-1a with lower diastereocontrol (TPS = triphenylsilyl). The ability to isolate and characterise SmCpR2 reagents by X-ray crystallography, and thus understand the environment around the Sm(II) centre, is an advantage when tailoring their properties (Fig. 1). Complexes 5e and 5f are the first SmCpR2 systems bearing monosubstituted Cp ligands to be structurally characterised by X-ray crystallography.14,15
8 dr). Bulkier TPSCp ligands in 5f gave anti-1a with lower diastereocontrol (TPS = triphenylsilyl). The ability to isolate and characterise SmCpR2 reagents by X-ray crystallography, and thus understand the environment around the Sm(II) centre, is an advantage when tailoring their properties (Fig. 1). Complexes 5e and 5f are the first SmCpR2 systems bearing monosubstituted Cp ligands to be structurally characterised by X-ray crystallography.14,15
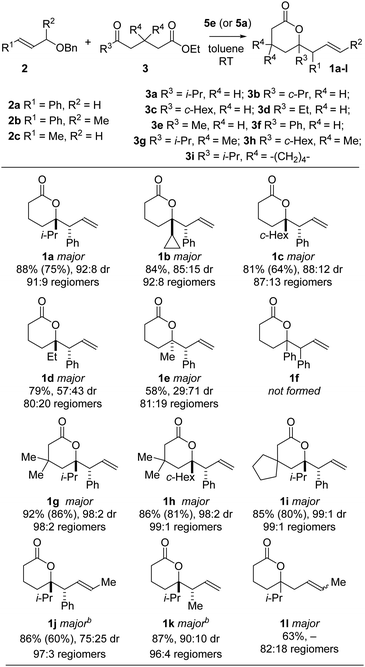
The scope of the SmCpR2-mediated coupling was explored using various allyl ethers 2 and ketoesters 3 (Scheme 4). Using Sm(DPMSCp)2(THF) 5e, lactone adducts 1a–c and 1g–i were obtained with high diastereocontrol (up to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and high regiocontrol (up to 99
1) and high regiocontrol (up to 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in good yield (81–92%). Little diastereocontrol was observed in the coupling of ethyl ketone 3d to give 1d. The reaction of methyl ketone 3e resulted in a reversal of the observed diastereoselectivity and the formation of syn-1e, lending support to the six-membered transition state proposed for the reaction, in which the larger ketone substituent preferentially adopts an equatorial orientation (cf.Scheme 3). No lactone product was observed when analogous phenyl ketone 3f was subjected to the cross-coupling procedure. Interestingly, the cross-coupling of (E)-((but-2-en-1-yloxy)methyl)benzene 2c with 3a, using 5e, gave lactone 1l as the major regioisomeric product. However, switching to the use of SmCp*2THF25a allowed the opposite lactone regioisomer 1k to be obtained from the same combination of coupling partners. Thus, fine-tuning of SmCpR2 reagents allowed partners to be united in complementary fashion. SmCp*2(THF)25a was also found to be the optimal reagent for the cross-coupling of propargylic ether 2a′ with keto esters 3 to give allenyl lactones 1m–r in good to excellent yields (52–93%) and excellent regiocontrol in all cases (99
1) in good yield (81–92%). Little diastereocontrol was observed in the coupling of ethyl ketone 3d to give 1d. The reaction of methyl ketone 3e resulted in a reversal of the observed diastereoselectivity and the formation of syn-1e, lending support to the six-membered transition state proposed for the reaction, in which the larger ketone substituent preferentially adopts an equatorial orientation (cf.Scheme 3). No lactone product was observed when analogous phenyl ketone 3f was subjected to the cross-coupling procedure. Interestingly, the cross-coupling of (E)-((but-2-en-1-yloxy)methyl)benzene 2c with 3a, using 5e, gave lactone 1l as the major regioisomeric product. However, switching to the use of SmCp*2THF25a allowed the opposite lactone regioisomer 1k to be obtained from the same combination of coupling partners. Thus, fine-tuning of SmCpR2 reagents allowed partners to be united in complementary fashion. SmCp*2(THF)25a was also found to be the optimal reagent for the cross-coupling of propargylic ether 2a′ with keto esters 3 to give allenyl lactones 1m–r in good to excellent yields (52–93%) and excellent regiocontrol in all cases (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Scheme 5).
1) (Scheme 5).
Interestingly, the coupling of allyl/propargyl ethers 2 and ketoesters 3 can be telescoped with our previously reported lactone radical cyclisations using SmI2–H2O to deliver decorated cycloheptanols 4. The versatility, selectivity, and mutual compatibility of Sm(II) reagents16 is key to the success of this telescoped ET approach that converts simple substrates to complex products. For example coupling of allyl ether 2a and ketoesters 3a/c using SmCpR2 reagent 5e, followed by addition of SmI2–H2O (5 fold excess to ensure complete conversion) and in situ ester–alkene radical cyclisation (via transition structure I),17 gave the desired 7-membered carbocycles 4a and 4b which were isolated as single diastereoisomers in good overall yield (Scheme 6).18 The intermediate δ-lactones 1a/c are the most complicated substrates used to date in such radical cyclisations.17,18 These radical cyclisations require ET to the ester carbonyl of complex δ-lactones 1 – a process made possible by the mixing of SmI2 in THF with H2O.17 No cyclization was observed using SmCpR2.11 Pleasingly, the analogous one pot process employing propargyl ether 2a′ and 3a/c/j allowed both the chemo- and diastereoselectivity of the process to be switched: addition of Cp*2Sm(THF)25a followed by SmI2–H2O (1.7 fold excess) triggered cross-coupling and an ester–allene radical cyclisation (via transition structure II)17,18 and resulted in the formation of cycloheptan-1,4-diol products 4c, 4d and 4e, rather than hemiketal products, and with the opposite relative stereochemistry at the highlighted stereocentre.19 Cycloheptanols 4c, 4d and 4e were isolated as single diastereoisomers in good to high overall yield. The two complementary processes result in the formation of four contiguous stereocenters and two new carbon–carbon bonds in one pot.
 | ||
| Scheme 6 A telescoped approach to complex cycloheptanols from simple starting materials using two Sm(II) reagents. | ||
In summary, we have optimised an approach to substituted δ-lactones that involves the regio- and diastereoselective coupling of allyl/propargyl ethers and ketoesters, mediated by SmCpR2 reagents. Screening of the Cp ligands on Sm(II) was employed to achieve high regio and diastereocontrol in some cases. Crucially, SmI2 gave unsatisfactory results in the transformation. The process has been exploited in a telescoped approach to complex cycloheptanols in which two Sm(II) reagents act in turn on the simple starting materials.
We acknowledge the EPSRC (Studentship to M. P.; Established Career Fellowship to D. J. P.; Postdoctoral Fellowship to X. J.-B.) and The Leverhulme Trust (Fellowship to D. J. P.).
Notes and references
- C. Chatgilialoglu and A. Studer, Encyclopedia of Radicals in Chemistry, Biology and Materials, Wiley-Blackwell, Chichester, UK, 2012 Search PubMed.
- (a) A. Gansäuer and H. Bluhm, Chem. Rev., 2000, 100, 2771 CrossRef; (b) J. Streuff and A. Gansäuer, Angew. Chem., Int. Ed., 2015, 54, 14232 CrossRef CAS PubMed; (c) W. J. Evans, Inorg. Chem., 2007, 46, 3435 CrossRef CAS PubMed; (d) M. Szostak and D. J. Procter, Angew. Chem., Int. Ed., 2012, 51, 9238 CrossRef CAS PubMed; (e) K. Takai, Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Ltd, 2001 Search PubMed; (f) A. Fürstner, Chem. Rev., 1999, 99, 991 CrossRef; (g) T. Hirao, Chem. Rev., 1997, 97, 2707 CrossRef CAS PubMed; (h) Z. L. Shen, S. Y. Wang, Y. K. Chok, Y. H. Xu and T. P. Loh, Chem. Rev., 2013, 113, 271 CrossRef CAS PubMed; (i) J. Justicia, L. A. de Cienfuegos, A. G. Campaña, D. Miguel, V. Jakoby, A. Gansäuer and J. M. Cuerva, Chem. Soc. Rev., 2011, 40, 3525 RSC.
- D. J. Procter, R. A. Flowers and T. Skrydstrup, Organic Synthesis Using Samarium Diiodide: A Practical Guide, RSC Publishing, Cambridge, 2010 Search PubMed.
- (a) J. L. Namy, P. Girard and H. B. Kagan, Nouv. J. Chim., 1977, 1, 5 CAS; (b) P. Girard, J. L. Namy and H. B. Kagan, J. Am. Chem. Soc., 1980, 102, 2693 CrossRef CAS.
- (a) M. Szostak, N. J. Fazakerley, D. Parmar and D. J. Procter, Chem. Rev., 2014, 114, 5959 CrossRef CAS PubMed; (b) K. C. Nicolaou, S. P. Ellery and J. S. Chen, Angew. Chem., Int. Ed., 2009, 48, 7140 CrossRef CAS PubMed; (c) M. Szostak, M. Spain, D. Parmar and D. J. Procter, Chem. Commun., 2012, 48, 330 RSC; (d) R. A. Flowers II, Synlett, 2008, 1427 CrossRef; (e) A. Dahlén and G. Hilmersson, Eur. J. Inorg. Chem., 2004, 3393 CrossRef; (f) H. B. Kagan and J. L. Namy, Influence of solvents or additives on the organic chemistry mediated by diiodosamarium, in Lanthanides: Chemistry and Use in Organic Synthesis, ed. S. Kobayashi, Springer Berlin Heidelberg, Heidelberg, 1999, p. 155 Search PubMed.
- For selected, recent examples, see: (a) T. E. Smith, M. Djang, A. J. Velander, C. W. Downey, K. A. Carroll and S. van Alphen, Org. Lett., 2004, 6, 2317 CrossRef CAS PubMed; (b) R. Doran, L. Duggan, S. Singh, C. D. Duffy and P. J. Guiry, Eur. J. Org. Chem., 2011, 7097 CrossRef CAS; V. Boucard, G. Broustal and J. M. Campagne, Eur. J. Org. Chem., 2007, 225 Search PubMed; (c) G. J. Florence, N. M. Gardner and I. Paterson, Nat. Prod. Rep., 2008, 25, 342 RSC; (d) A. Albrecht, Ł. Albrecht and T. Janecki, Eur. J. Org. Chem., 2011, 2747 CrossRef CAS; (e) D. Belmessieri, L. C. Morrill, C. Simal, A. M. Z. Slawin and A. D. Smith, J. Am. Chem. Soc., 2011, 133, 2714 CrossRef CAS PubMed; (f) S. Bera, C. G. Daniliuc and A. Studer, Org. Lett., 2015, 17, 4940 CrossRef CAS PubMed; (g) K. V. Chuang, C. Xu and S. E. Reisman, Science, 2016, 353, 912 CrossRef CAS PubMed.
- (a) W. J. Evans, I. Bloom, W. E. Hunter and J. L. Atwood, J. Am. Chem. Soc., 1981, 103, 6507 CrossRef CAS; (b) W. J. Evans, J. W. Grate, H. W. Choi, I. Bloom, W. E. Hunter and J. L. Atwood, J. Am. Chem. Soc., 1985, 107, 941 CrossRef CAS; (c) W. J. Evans, J. W. Grate, L. A. Hughes, H. Zhang and J. L. Atwood, J. Am. Chem. Soc., 1985, 107, 3728 CrossRef CAS; (d) W. J. Evans, L. A. Hughes, D. K. Drummond, H. Zhang and J. L. Atwood, J. Am. Chem. Soc., 1986, 108, 1722 CrossRef CAS; (e) W. J. Evans and D. K. Drummond, J. Am. Chem. Soc., 1986, 108, 7440 CrossRef CAS; (f) W. J. Evans and D. K. Drummond, J. Am. Chem. Soc., 1989, 111, 3329 CrossRef CAS; (g) W. J. Evans, T. A. Ulibarri and J. W. Ziller, J. Am. Chem. Soc., 1990, 112, 219 CrossRef CAS; (h) W. J. Evans, T. A. Ulibarri and J. W. Ziller, J. Am. Chem. Soc., 1990, 112, 2314 CrossRef CAS; (i) W. J. Evans, T. A. Ulibarri and J. W. Ziller, Organometallics, 1991, 10, 134 CrossRef CAS; (j) W. J. Evans, R. A. Keyer and J. W. Ziller, Organometallics, 1993, 12, 2618 CrossRef CAS; (k) W. J. Evans, C. A. Seibel and J. W. Ziller, Inorg. Chem., 1998, 37, 770 CrossRef CAS; (l) W. J. Evans, C. A. Seibel, J. W. Ziller and R. J. Doedens, Organometallics, 1998, 17, 2103 CrossRef CAS.
- (a) J. Collin, J. L. Namy, C. Bied and H. B. Kagan, Inorg. Chim. Acta, 1987, 140, 29 CrossRef CAS; (b) J. L. Namy, J. Collin, J. Zhang and H. B. Kagan, J. Organomet. Chem., 1987, 328, 81 CrossRef CAS; (c) C. Bied, J. Collin and H. B. Kagan, Tetrahedron, 1992, 48, 3877 CrossRef CAS; (d) J. Collin, J. L. Namy, F. Dallemer and H. B. Kagan, J. Org. Chem., 1991, 56, 3118 CrossRef CAS; (e) H. B. Kagan, J. Collin, J. L. Namy, C. Bied, F. Dallemer and A. Lebrun, J. Alloys Compd., 1993, 192, 191 CrossRef CAS.
- (a) Y. Makioka, K. Koyama, T. Nishiyama, K. Takaki, Y. Taniguchi and Y. Fujiwara, Tetrahedron Lett., 1995, 36, 6283 CrossRef CAS; (b) K. Takaki, M. Maruo and T. Kamata, J. Org. Chem., 1996, 61, 8332 CrossRef CAS PubMed; (c) K. Takaki, T. Kusudo, S. Uebori, T. Nishiyama, T. Kamata, M. Yokoyama, K. Takehira, Y. Makioka and Y. Fujiwara, J. Org. Chem., 1998, 63, 4299 CrossRef CAS.
- (a) W. J. Evans, J. Alloys Compd., 2009, 488, 493 CrossRef CAS; (b) W. J. Evans, Coord. Chem. Rev., 2000, 206, 263 CrossRef; (c) G. A. Molander and J. A. C. Romero, Chem. Rev., 2002, 102, 2161 CrossRef CAS PubMed.
- See ESI†.
- Selected recent studies on the allylation of ketones: (a) T. Nakano, K. Endo and Y. Ukaji, Chem. – Asian J., 2016, 11, 713 CrossRef CAS PubMed; (b) Y. C. Teo, J. D. Goh and T. P. Loh, Org. Lett., 2005, 7, 2743 CrossRef CAS PubMed; (c) T. Takeda, M. Yamamoto, S. Yoshida and A. Tsubouchi, Angew. Chem., Int. Ed., 2012, 51, 7263 CrossRef CAS PubMed; (d) J. Yadav, G. R. Stanton, X. Fan, J. R. Robinson, E. J. Schelter, P. J. Walsh and M. A. Pericas, Chem. – Eur. J., 2014, 20, 7122 CrossRef CAS PubMed; (e) M. Raducan, R. Alam and K. J. Szabó, Angew. Chem., Int. Ed., 2012, 51, 13050 CrossRef CAS PubMed; (f) R. Alam, T. Vollgraff, L. Eriksson and K. J. Szabó, J. Am. Chem. Soc., 2015, 137, 11262 CrossRef CAS PubMed; (g) Y. Yamamoto and N. Asao, Chem. Rev., 1993, 93, 2207 CrossRef CAS ; Processes using SmI2: ; (h) A. Krief and A. M. Laval, Chem. Rev., 1999, 99, 745 CrossRef CAS PubMed.
- (a) W. J. Evans, S. L. Gonzales and J. W. Ziller, J. Am. Chem. Soc., 1994, 116, 2600 CrossRef CAS; (b) M. Szostak, M. Spain and D. J. Procter, J. Org. Chem., 2014, 79, 2522 CrossRef CAS PubMed.
- See ESI† for CCDC numbers.
- For a SmCpR3 complex bearing monsubstituted Cp ligands, see: M. E. Fieser, M. R. MacDonald, B. T. Krull, J. E. Bates, J. W. Ziller, F. Furche and W. J. Evans, J. Am. Chem. Soc., 2015, 137, 369 CrossRef CAS PubMed.
- Alternative allylation procedures using transition metals are not compatible with a telescoped process. Transition metal salts lead to the decomposition of the SmI2–H2O reagent essential for the second stage of the process. See ESI†.
- X. Just-Baringo and D. J. Procter, Acc. Chem. Res., 2015, 48, 1263 CrossRef CAS PubMed.
- (a) I. Yalavac, S. E. Lyons, M. R. Webb and D. J. Procter, Chem. Commun., 2014, 50, 12863 RSC; (b) D. Parmar, H. Matsubara, K. Price, M. Spain and D. J. Procter, J. Am. Chem. Soc., 2012, 134, 12751 CrossRef CAS PubMed; (c) K. D. Collins, J. M. Oliveira, G. Guazzelli, B. Sautier, S. De Grazia, H. Matsubara, M. Helliwell and D. J. Procter, Chem. – Eur. J., 2010, 16, 10240 CrossRef CAS PubMed.
- 4a and 4b are obtained as hemiketals as the α-methyl group on the top face hinders further reduction. In the formation of 4a,b the stereochemistry at the highlighted position is established during the cyclisation event. In the formation of 4c,d,e the stereochemistry at the highlighted position is established during conjugate reduction of a cycloheptenone intermediate, post cyclisation. Thus different relative stereochemical outcomes are observed. This is consistent with our previous findings. See ref. 18b.
Footnote |
| † Electronic supplementary information (ESI) available: Control experiments, full experimental details, NMR spectra, and CCDC numbers for X-ray structures. CCDC 1472300–1472305. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc07318b |
| This journal is © The Royal Society of Chemistry 2016 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif)
