 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A hexameric [MnIII18Na6] wheel based on [MnIII3O]7+ sub-units†
Maria
Manoli
a,
Ross
Inglis
b,
Stergios
Piligkos
c,
Lan
Yanhua
d,
Wolfgang
Wernsdorfer‡
d,
Euan K.
Brechin
*b and
Anastasios J.
Tasiopoulos
 *a
*a
aDepartment of Chemistry, University of Cyprus, 1678 Nicosia, Cyprus. E-mail: atasio@ucy.ac.cy; Fax: +357 22895451; Tel: +357 22892765
bEaStCHEM School of Chemistry, The University of Edinburgh, David Brewster Road, Edinburgh, EH9 3FJ, UK. E-mail: E.Brechin@ed.ac.uk
cDepartment of Chemistry, University of Copenhagen, Universitetsparken 5, Copenhagen, Denmark
dInstitut Néel, CNRS, BP-166, Grenoble Cedex 9, France
First published on 23rd September 2016
Abstract
A novel hexameric [MnIII18Na6] wheel-like aggregate consisting of [MnIII3O] triangles is reported. It is the second highest nuclearity oxime-based Mn cluster, the largest member of the recently-developed family of molecular oligomers based on [MnIII3O] triangles, and the only one with a wheel-like metal topology.
There is significant interest in the synthesis of large molecular aggregates due to their interesting physical properties and beautiful crystal structures.1,2 One of the main goals in the area of molecular magnetism involves the development of new methods for the utilization of magnetically interesting compounds as building-blocks for the construction of large clusters and multidimensional coordination polymers.2a,3 Despite the significant efforts to achieve this target, examples of such large clusters based on smaller magnetic clusters are still rather limited.2a,4 Amongst the most common building-blocks in manganese coordination chemistry are the homovalent [MnIII3(μ3-O)]7+ and heterovalent [MnIII2MnII(μ3-O)]6+ oxo-centred metal triangles which are often found as the main structural components in large compounds with complex structures.2,5 Efforts aimed at developing high nuclearity clusters based on [MnIII3O] sub-units were intensified after the discovery of discrete [MnIII3(μ3-O)]7+ clusters displaying ferromagnetic exchange interactions and single-molecule magnetism (SMM) behaviour,6 the isolation of a family of [MnIII6] SMMs that includes a member with a record energy barrier to magnetization reversal for transition metal SMMs,7 and several other clusters displaying interesting magnetic properties originating from tightly linked [Mn3(μ3-O)]n+ triangles sharing one or more of their edges.8 More recently there has been significant interest in the construction of oligomeric clusters consisting of covalently linked oxime-based [MnIII3O]7+ building-blocks. These investigations have afforded some dimeric [Mn3]2 and tetrameric [Mn3]4 aggregates displaying tetrahedral or rectangular core topologies.9
We have been interested in the development of new synthetic methods for the construction of high nuclearity Mn clusters. One of these methods, that involves the combination of phenolic oximes with diols, has afforded two structurally impressive complexes that describe a [Mn32] double-decker wheel,10a and an 1-D coordination polymer containing a [Mn40] octagonal super-structure.10b We now report the synthesis, structure and magnetic behaviour of the hexameric [MnIII3Na]6 molecular wheel [MnIII18Na6(μ3-O)6(sao)18Br12(H2O)18(DMF)6] (1) (sao2− is the dianion of salicylaldoxime) which is the second largest oxime-based Mn cluster known to date, the largest member of the family of molecular oligomers based on [Mn3] triangles, and the only one with a wheel-like metal topology.
The reaction of MnBr2·4H2O, 2-(hydroxymethyl)phenol (hpH2) and saoH2 in the presence of sodium cyanate (NaOCN) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in a 4
1 molar ratio in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeCN/DMF solvent mixture leads to the formation of 1 in ∼45% yield.§ The molecular structure§ of 1 (Fig. 1 and 2) contains a [MnIII18Na6] wheel-like cluster consisting of six crystallographically equivalent oxime-based [MnIII3O]7+ triangles linked through six Na+ ions. The [MnIII3Na] repeat unit of 1 contains one oxo-centred triangular arrangement of three MnIII ions and a Na+ ion attached to it via the O-atoms of the sao2− ligands, which occupy the edges of the triangle. The axial coordination sites of the MnIII ions are occupied by one bridging H2O molecule, and terminal Br− ions (2), H2O (2) and DMF (1) molecules. The MnIII ions are hexa-coordinated with distorted octahedral coordination geometries and all display the expected Jahn–Teller (JT) axial elongation, with the JT axes being perpendicular to the plane of the [Mn3] triangle. Two μ3-, one μ4-sao2−, and one bridging H2O ligand connect each [MnIII3O] triangle with two neighbouring Na+ ions, which in turn are linked to the next [MnIII3O] sub-units forming the [Mn18Na6] wheel. Two sao2− ligands bridge in a η1:η1:η2:μ3-mode to two MnIII ions and one Na+ ion, and one bridges in a η2:η1:η2:μ4-fashion to two MnIII and two Na+ ions. The phenyl rings of twelve of the sao2− ligands are located outside the [Mn18Na6] wheel, whereas those of the remaining six occupy the central cavity creating a hydrophobic shell (Fig. 2, top). The Mn–O–N–Mn torsion angles are rather small, ranging from 6.4 to 12.9°, in agreement with the values observed for other sao2−-based [MnIII3O] triangles.6,7 The neighbouring [MnIII3O] sub-units are significantly tilted as revealed from the angle between the planes defined by the Mn3+/O2− ions of adjacent [MnIII3O] units which is ∼52.2° (Fig. 2, bottom). As a result the aggregate of 1 deviates significantly from planarity. The [MnIII3O] triangles are well separated from each other, the shortest inter-triangle Mn⋯Mn distance being ∼6.23 Å. Complex 1 is of nano-sized dimensions with an outer diameter of ∼2.8 nm (Fig. 2, top) and a thickness of ∼0.8 nm. Clearly, complex 1 is a large cluster and indeed is one of the largest oxime-based Mn complexes known, being smaller only than the [Mn32] double-decker wheel.10a Close examination of the packing of 1 reveals a parallel arrangement of [Mn18Na6] molecules in the crystal and the formation of columns running along the c-axis of the cell (Fig. S1, ESI†).
1 MeCN/DMF solvent mixture leads to the formation of 1 in ∼45% yield.§ The molecular structure§ of 1 (Fig. 1 and 2) contains a [MnIII18Na6] wheel-like cluster consisting of six crystallographically equivalent oxime-based [MnIII3O]7+ triangles linked through six Na+ ions. The [MnIII3Na] repeat unit of 1 contains one oxo-centred triangular arrangement of three MnIII ions and a Na+ ion attached to it via the O-atoms of the sao2− ligands, which occupy the edges of the triangle. The axial coordination sites of the MnIII ions are occupied by one bridging H2O molecule, and terminal Br− ions (2), H2O (2) and DMF (1) molecules. The MnIII ions are hexa-coordinated with distorted octahedral coordination geometries and all display the expected Jahn–Teller (JT) axial elongation, with the JT axes being perpendicular to the plane of the [Mn3] triangle. Two μ3-, one μ4-sao2−, and one bridging H2O ligand connect each [MnIII3O] triangle with two neighbouring Na+ ions, which in turn are linked to the next [MnIII3O] sub-units forming the [Mn18Na6] wheel. Two sao2− ligands bridge in a η1:η1:η2:μ3-mode to two MnIII ions and one Na+ ion, and one bridges in a η2:η1:η2:μ4-fashion to two MnIII and two Na+ ions. The phenyl rings of twelve of the sao2− ligands are located outside the [Mn18Na6] wheel, whereas those of the remaining six occupy the central cavity creating a hydrophobic shell (Fig. 2, top). The Mn–O–N–Mn torsion angles are rather small, ranging from 6.4 to 12.9°, in agreement with the values observed for other sao2−-based [MnIII3O] triangles.6,7 The neighbouring [MnIII3O] sub-units are significantly tilted as revealed from the angle between the planes defined by the Mn3+/O2− ions of adjacent [MnIII3O] units which is ∼52.2° (Fig. 2, bottom). As a result the aggregate of 1 deviates significantly from planarity. The [MnIII3O] triangles are well separated from each other, the shortest inter-triangle Mn⋯Mn distance being ∼6.23 Å. Complex 1 is of nano-sized dimensions with an outer diameter of ∼2.8 nm (Fig. 2, top) and a thickness of ∼0.8 nm. Clearly, complex 1 is a large cluster and indeed is one of the largest oxime-based Mn complexes known, being smaller only than the [Mn32] double-decker wheel.10a Close examination of the packing of 1 reveals a parallel arrangement of [Mn18Na6] molecules in the crystal and the formation of columns running along the c-axis of the cell (Fig. S1, ESI†).
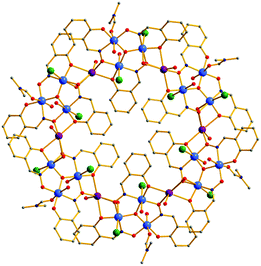 | ||
| Fig. 1 Representation of the molecular structure of 1. Colour code: Mn blue; Na, purple; Br, green; O, red; N, dark blue; C, grey. H atoms are omitted for clarity. | ||
The direct current (dc) molar magnetic susceptibility, χ (where χ = M/B; and M is the magnetization), of polycrystalline 1·3DMF·30H2O was measured in an applied magnetic field, B, of 0.1 T, over the 5–300 K temperature range. The data is plotted in Fig. 3 per [MnIII3] triangle. At room temperature, the χT product of 1 is 7.8 cm3 mol−1 K. This value is lower than that expected from the spin-only contribution to the magnetism of a MnIII trinuclear unit (9.0 cm3 mol−1 K, with gMn = 2.00), assuming that the magnetic properties of 1 arise as the superposition of the magnetic properties of six non-interacting [MnIII3] units. Upon cooling, the χT product decreases continuously to reach 2.5 cm3 mol−1 K per [MnIII3] at T = 5 K. This behaviour is indicative of antiferromagnetic interactions within the [MnIII3] units. To better define the magnetic properties of 1, variable-temperature-and-variable-field (VTVB) magnetization data were collected in the field range 0.5–7.0 T and in the temperature range 2–7 K. These data are shown as M/μBversus μBB/kT in the inset of Fig. 3.
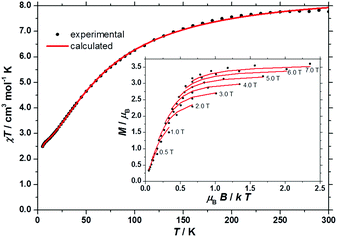 | ||
| Fig. 3 χT product of 1versus T plotted per [MnIII3] triangle. Inset: Variable temperature-and-variable-field (VTVB) magnetization data of 1 in the field range 0.5 to 7.0 T and in the temperature range 2 to 7 K. The experimental data are shown as black circles. The calculated curves, obtained from full matrix diagonalization of spin-Hamiltonian (1) for isolated MnIII3 triangles, are shown as solid red lines. | ||
For the interpretation of the magnetic properties of 1, we consider that they arise as a superposition of the magnetic properties of six non interacting [MnIII3] units. Thus, we used spin-Hamiltonian (1):
 | (1) |
Concluding, an aesthetically pleasing nano-sized hexameric wheel-like cluster containing oxime-based [MnIII3(μ3-O)]7+ sub-units is reported. It is the largest member of the recently-developed family of molecular oligomers consisting of [MnIII3] triangles and the only one with a wheel-like metal topology.9 Complex 1 is also the only oxime-based [MnIII3]n (n > 2) molecular oligomer where the sub-units are connected via a diamagnetic metal ion; in all other oligomeric complexes they are linked through bulky organic ligands. Because the Na+ ions are intimately associated with the [MnIII3] triangles, 1 can also be described as a heterometallic [M24] cluster, being the second-largest oxime-containing Mn cluster reported to date. This compound was prepared by a method involving the use of a combination of a phenolic oxime with a diol in reactions with Mn salts, although only the oxime appears in the final product, as was also the case for the largest known oxime – based Mn cluster, the Mn32 double-decker wheel.10a Reactions repeated without the diol present do not form 1. Clearly this synthetic method is proving invaluable for the isolation of high nuclearity clusters. Further investigations are in progress targeting [Mn18Na6] analogues with different oximes and alternative [diamagnetic and paramagnetic] connecting metals ions. The formation of complex 1 therefore opens up new avenues in the roadmap of molecular oligomers based on [MnIII3] triangles that should provide access to structurally and magnetically novel complexes.
This work was supported by the Cyprus Research Promotion Foundation Research Grant “ANABAΘMIΣH/ΠAΓIO/0308/12” which is co-funded by the Republic of Cyprus and the European Regional Development Fund. We also thank the University of Cyprus for an internal research grant to AJT. EKB thanks the Villum Foundations (Denmark) for a Velux Visiting Professorship.
Notes and references
- (a) C. J. Milios and R. E. P. Winpenny, Struct. Bonding, 2015, 164, 1 CrossRef CAS; (b) R. Bagai and G. Christou, Chem. Soc. Rev., 2009, 38, 1011 RSC; (c) D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268 CrossRef CAS PubMed; (d) Y.-Z. Zheng, G.-J. Zhou, Z. Zheng and R. E. P. Winpenny, Chem. Soc. Rev., 2014, 43, 1462 RSC; (e) M. Nakano and H. Oshio, Chem. Soc. Rev., 2011, 40, 3239 RSC; (f) X.-Y. Wang, C. Avendano and K. R. Dunbar, Chem. Soc. Rev., 2011, 40, 3213 RSC; (g) G. Aromí, D. Aguila, P. Gamez, F. Luis and O. Roubeau, Chem. Soc. Rev., 2012, 41, 537 RSC.
- (a) C. Papatriantafyllopoulou, E. E. Moushi, G. Christou and A. J. Tasiopoulos, Chem. Soc. Rev., 2016, 45, 1597 RSC; (b) G. E. Kostakis, A. M. Ako and A. K. Powell, Chem. Soc. Rev., 2010, 39, 2238 RSC; (c) A. Escuer, J. Esteban, S. P. Perlepes and T. C. Stamatatos, Coord. Chem. Rev., 2014, 275, 87 CrossRef CAS; (d) S. P. Watton, P. Fuhrmann, L. E. Pence, A. Ganeschi, A. Cornia, G. L. Abbati and S. J. Lippard, Angew. Chem., Int. Ed. Engl., 1997, 36, 2774 CrossRef CAS; (e) G. L. Abbati, A. Cornia, A. C. Fabretti, A. Ganeschi and D. Gatteschi, Inorg. Chem., 1998, 37, 1430 CrossRef CAS; (f) G. L. Abbati, A. Cornia, A. C. Fabretti, W. Malavasi, L. Schenetti, A. Ganeschi and D. Gatteschi, Inorg. Chem., 1997, 36, 6443 CrossRef CAS.
- I.-R. Jeon and R. Clérac, Dalton Trans., 2012, 41, 9569 RSC.
- (a) M. Manoli, S. Alexandrou, L. Pham, G. Lorusso, W. Wernsdorfer, M. Evangelisti, G. Christou and A. J. Tasiopoulos, Angew. Chem., Int. Ed., 2016, 55, 679 CrossRef CAS PubMed; (b) M. Charalambous, E. E. Moushi, C. Papatriantafyllopoulou, W. Wernsdorfer, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, Chem. Commun., 2012, 48, 5410 RSC.
- (a) E. K. Brechin, Chem. Commun., 2005, 5141 RSC; (b) A. J. Tasiopoulos and S. P. Perlepes, Dalton Trans., 2008, 5537 RSC.
- (a) R. Inglis, S. M. Taylor, L. F. Jones, G. S. Papaefstathiou, S. P. Perlepes, S. Datta, S. Hill, W. Wernsdorfer and E. K. Brechin, Dalton Trans., 2009, 9157–9168 RSC and references therein; (b) C. J. Milios, R. Inglis, L. F. Jones, A. Prescimone, S. Parsons, W. Wernsdorfer and E. K. Brechin, Dalton Trans., 2009, 2812–2822 RSC; (c) T. C. Stamatatos, D. Foguet-Albiol, C. C. Stoumpos, C. P. Raptopoulou, A. Terzis, W. Wernsdorfer, S. P. Perlepes and G. Christou, J. Am. Chem. Soc., 2005, 127, 15380 CrossRef CAS PubMed; (d) J. Cano, T. Cauchy, E. Ruiz, C. J. Milios, C. C. Stoumpos, T. C. Stamatatos, S. P. Perlepes, G. Christou and E. K. Brechin, Dalton Trans., 2008, 234 RSC.
- (a) R. Inglis, C. J. Milios, L. F. Jones, S. Piligkos and E. K. Brechin, Chem. Commun., 2012, 48, 181 RSC; (b) C.-I. Yang, Z.-Z. Zhang and S.-B. Lin, Coord. Chem. Rev., 2015, 289–290, 289 CrossRef CAS; (c) C. J. Milios, A. Vinslava, W. Wernsdorfer, S. Moggach, S. Parsons, S. P. Perlepes, G. Christou and E. K. Brechin, J. Am. Chem. Soc., 2007, 129, 2754 CrossRef CAS PubMed.
- (a) A. Escuer, B. Cordero, M. Font-Bardia, S. J. Teat and O. Roubeau, Eur. J. Inorg. Chem., 2016, 1232 CrossRef CAS; (b) R. Vicente, M. S. El Fallah, B. Casanovas, M. Font-Bardia and A. Escuer, Inorg. Chem., 2016, 55, 5735 CrossRef CAS PubMed.
- (a) T. N. Nguyen, W. Wernsdorfer, K. A. Abboud and G. Christou, J. Am. Chem. Soc., 2011, 133, 20688 CrossRef CAS PubMed; (b) A. M. Mowson, T. N. Nguyen, K. A. Abboud and G. Christou, Inorg. Chem., 2013, 52, 12320 CrossRef CAS PubMed; (c) J. M. Frost, S. Sanz, T. Rajeshkumar, M. B. Pitak, S. J. Coles, G. Rajaraman, W. Wernsdorfer, J. Schnack, P. J. Lusby and E. K. Brechin, Dalton Trans., 2014, 43, 10690 RSC; (d) T. N. Nguyen, M. Shiddiq, T. Ghosh, K. A. Abboud, S. Hill and G. Christou, J. Am. Chem. Soc., 2015, 137, 7160 CrossRef CAS PubMed; (e) T. N. Nguyen, W. Wernsdorfer, M. Shiddiq, K. A. Abboud, S. Hill and G. Christou, Chem. Sci., 2016, 7, 1156 RSC.
- (a) M. Manoli, R. Inglis, M. J. Manos, V. Nastopoulos, W. Wernsdorfer, E. K. Brechin and A. J. Tasiopoulos, Angew. Chem., Int. Ed., 2011, 50, 4441 CrossRef CAS PubMed; (b) M. Manoli, R. Inglis, M. J. Manos, G. S. Papaefstathiou, E. K. Brechin and A. J. Tasiopoulos, Chem. Commun., 2013, 49, 1061 RSC.
- H. Press, S. A. Teukolsky, W. T. Vetterling and B. P. Flannery, Numerical Recipes in C: The Art of Scientific Computing, Cambridge University Press, Cambridge, 2nd edn, 1992 Search PubMed.
- E. Manolopoulou, C. C. Stoumpos, M. Siczek, T. Lis, E. K. Brechin and C. J. Milios, Eur. J. Inorg. Chem., 2010, 483 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, structural figures and TGA data. CCDC 1498633. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc06644e |
| ‡ Current address: KIT, Physikalisches Institut, Wolfgang-Gaede-Str. 1D-76131 Karlsruhe, Germany. |
§ The diol does not appear in the final product, but its presence in the reaction mixture is essential for the formation of 1. Vacuum-dried solid analysed (C, H, N) as 1·3DMF·30H2O (see also Fig. S2 and the corresponding discussion in ESI†). Calcd (found): C 29.95 (29.85), H 4.09 (3.75), N 6.16 (6.41). Crystal data for 1: C144H168Br12Mn18N24Na6O66, M = 5334.47, trigonal, a = b = 44.310(2) Å, c = 21.224(1) Å, V = 36088(2) Å3, T = 100(2) K, space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, Z = 6, ρcalcd = 1.473 g cm−3, 24 c, Z = 6, ρcalcd = 1.473 g cm−3, 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 939 reflections collected, 7153 reflections used (Rint = 0.0357), R1 [I > 2σ(I)] = 0.0688, wR2 (all data) = 0.2175. The asymmetric unit also contains severely disordered solvent molecules that could not be modeled properly. Thus, the SQUEEZE program was used to eliminate the contribution of the electron density in the disordered solvent region from the overall intensity data. 939 reflections collected, 7153 reflections used (Rint = 0.0357), R1 [I > 2σ(I)] = 0.0688, wR2 (all data) = 0.2175. The asymmetric unit also contains severely disordered solvent molecules that could not be modeled properly. Thus, the SQUEEZE program was used to eliminate the contribution of the electron density in the disordered solvent region from the overall intensity data. |
| This journal is © The Royal Society of Chemistry 2016 |

