Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ion beam induced 18F-radiofluorination: straightforward synthesis of gaseous radiotracers for the assessment of regional lung ventilation using positron emission tomography†
V.
Gómez-Vallejo
,
A.
Lekuona
,
Z.
Baz
,
B.
Szczupak
,
U.
Cossío
and
J.
Llop
*
Molecular Imaging Unit, CIC biomaGUNE, Paseo Miramón 182, 20009 San Sebastián, Guipúzcoa, Spain. E-mail: jllop@cicbiomagune.es
First published on 7th September 2016
Abstract
A simple, straightforward and efficient method for the synthesis of [18F]CF4 and [18F]SF6 based on an ion beam-induced isotopic exchange reaction is presented. Positron emission tomography ventilation studies in rodents using [18F]CF4 showed a uniform distribution of the radiofluorinated gas within the lungs and rapid elimination after discontinuation of the administration.
Imaging methods visualizing areas of impaired ventilation may become a powerful tool in the early/differential diagnoses of lung diseases. Currently, clinical ventilation studies are mainly performed using planar gamma camera imaging or single photon emission computerized tomography (SPECT). Gases such as 81mKr1 and more often radiolabelled aerosols generated either from water-soluble agents ([99mTc]-DTPA)2 or solid particles (Technegas)3 are used as contrast agents. After inhalation, 81mKr is distributed according to the regional ventilation. However, limited access and short half-life (T1/2 = 13 s) restrict its use. Liquid radio-aerosols and Technegas show central airway deposition and peripheral hotspot formation in patients with obstructive lung diseases. Moreover, both planar gamma camera imaging and SPECT have limitations in terms of sensitivity, spatiotemporal resolution and quantification.
The high sensitivity and quantitative nature of Positron Emission Tomography (PET) may result in a valuable alternative to assess lung ventilation. Positron emitter-labelled gases such as neon-19 (19Ne) and [13N]N2 have been successfully applied to ventilation studies.4 Unfortunately, the short half-lives of both isotopes (T1/2 = 17.4 s and 9.97 min for 19Ne and 13N, respectively) have restricted routine application in the clinical setting. Indeed, the use of PET in the context of lung ventilation studies has been historically thwarted by the lack of appropriate radiotracers.
Previous promising investigations using MRI with fluorinated gases as contrast agents in both animal models5 and human subjects6 inspired us in the potential use of 18F-labelled gases for the non-invasive assessment of regional lung ventilation using PET. Contrary to aerosols and suspensions, which often separate and clump upon settling, gases distribute uniformly at equilibrium. Hence, the preparation of inert, non-toxic, and water-insoluble fluorinated gases radiolabelled with 18F (T1/2 = 109.7 min) may result in an extremely useful tool for the ultra-sensitive, in vivo and non-invasive assessment of regional lung ventilation.
Our first target compound was [18F]CF4, whose non-radioactive analogue (CF4) is non-toxic after inhalation (lethal concentration is low in rat = 890![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm/15 minutes),‡ has very low solubility in water and is chemically inert. The production of this radioactive gas was first envisaged by following a well-established procedure under non-radioactive conditions, based on the reaction of silicon carbide (SiC) with F2. However, the fact that F2 is used in large excess in this synthetic approach, which is difficult to implement under radioactive conditions, together with the unease around producing and manipulating [18F]F2, encouraged us in the pursuit of simple and straightforward alternative synthetic strategies, which may ultimately facilitate the future application of the novel radiotracers in the clinical setting. Taking into account that the specific radioactivity (amount of radioactivity per unit mass) of the labelled compounds should not be an issue in the assessment of lung ventilation (ventilation studies with fluorinated gases performed with MRI require an extremely high concentration of the fluorinated agent, see for example ref. 6), isotopic exchange reactions appeared appropriate.
000 ppm/15 minutes),‡ has very low solubility in water and is chemically inert. The production of this radioactive gas was first envisaged by following a well-established procedure under non-radioactive conditions, based on the reaction of silicon carbide (SiC) with F2. However, the fact that F2 is used in large excess in this synthetic approach, which is difficult to implement under radioactive conditions, together with the unease around producing and manipulating [18F]F2, encouraged us in the pursuit of simple and straightforward alternative synthetic strategies, which may ultimately facilitate the future application of the novel radiotracers in the clinical setting. Taking into account that the specific radioactivity (amount of radioactivity per unit mass) of the labelled compounds should not be an issue in the assessment of lung ventilation (ventilation studies with fluorinated gases performed with MRI require an extremely high concentration of the fluorinated agent, see for example ref. 6), isotopic exchange reactions appeared appropriate.
Isotopic exchange reactions of fluorocarbons with 18F were already attempted in the early 50s using conventional chemical methods with very poor results.7 Despite the exchange of 18F with CF4 could be observed in the presence of metallic salts acting as catalysts, the fraction exchanged was extremely low even at high temperatures.8 No exchange could be observed for SF6 even in the presence of metal catalysts.9
During the 70s and the 80s, recoil 18F atoms were used in a variety of gas-phase kinetic investigations.10 Recoil 18F atoms, generated via different nuclear reactions, proved to undergo isotopic exchange reactions and the formation of [18F]CF4 and [18F]SF6 was reported.11
In this context, we first anticipated that the irradiation of CF4 with protons with an appropriate energy should produce in situ recoil 18F atoms via the 19F(p,pn)18F nuclear reaction, capable of undergoing an isotopic exchange reaction with surrounding CF4 molecules to form [18F]CF4. With this aim, cyclotron (IBA Cyclone 18/9)-accelerated protons with a nominal energy of 18 MeV were used to irradiate a mixture of CF4/neon (method A; see Fig. S1 (ESI†) for the general configuration of the experimental set-up). In brief, the target was filled with CF4 to a final pressure of P1 = 2–4 bar, topped with neon to 20 bar and subjected to proton irradiation (integrated current C1 = 4–8 μA h). The target gas was finally collected in a liquid nitrogen cooled cryogenic trap. Higher concentrations of CF4 during irradiation and increased integrated current values (C1) lead to higher amounts of [18F]CF4 (Table 1, entries 1–4); however, the production yields were relatively low and only 0.80 ± 0.06 GBq of the radiotracer could be produced with an integrated current of 8 μA h. Cross-sectional values for the nuclear reaction 19F(p,pn)18F, which has an energy threshold of approximately 10 MeV and reaches a maximum at cross-sectional values of around 150 mb between 20 and 30 MeV,12 suggest that the limiting step in the production of [18F]CF4 using method A is the generation of the radionuclide, which is limited due to the maximum energy available in our cyclotron (18 MeV).
| Entry | P 1 (bar) | C 1 (μA h) | C 2 (μA h) | C 3 (μA h) | Act. (GBq) |
|---|---|---|---|---|---|
| a Production method A. b Production method B; P1: CF4 filling pressure under method A; C1: integrated current, method A; C2: integrated current, first irradiation, method B; C3: integrated current, second irradiation, method B. | |||||
| 1a | 2 | 4 | NA | NA | 0.27 ± 0.03 |
| 2a | 2 | 8 | NA | NA | 0.49 ± 0.04 |
| 3a | 4 | 4 | NA | NA | 0.58 ± 0.04 |
| 4a | 4 | 8 | NA | NA | 0.80 ± 0.06 |
| 5b | NA | NA | 1 | 1 | 2.30 ± 0.08 |
| 6b | NA | NA | 1 | 2 | 2.47 ± 0.08 |
| 7b | NA | NA | 1 | 4 | 2.75 ± 0.13 |
| 8b | NA | NA | 4 | 4 | 8.43 ± 0.59 |
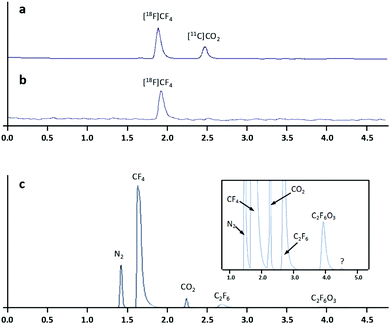
Gas chromatography analysis of the radioactive gases showed the presence of two radioactive species at the end of the cryogenic trapping step (Fig. 1a) identified as [18F]CF4 and [11C]CO2. Although the source of 11C has not been demonstrated, this radionuclide may be generated due to the presence of N2 in the target during irradiation via the 14N(p,α)11C nuclear reaction, as N2 is present as an impurity in the CF4 gas bottle. In situ reaction with O2 absorbed in the target walls may result in the formation of [11C]CO2. Importantly, [11C]CO2 could be easily removed by passing the irradiated gases through a soda lime trap before cryogenic trapping (Fig. 1b). Mass spectrometry analysis confirmed the presence of 5 major species, corresponding to N2, CF4, CO2, C2F6, and C2F6O3, respectively (Fig. 1c). One very minor unidentified peak was also observed (Fig. 1c, inset). The determination of the amount of activity present in the collection reservoir at 30 min intervals for 4 hours confirmed the radionuclidic purity of the radiofluorinated gas (calculated T1/2 = 109.5 min).
In order to improve the production yields, we envisioned the synthesis of the labelled species using a dual beam approach (method B). In brief, the process consisted of four steps: (i) the target was filled with [18O]O2 at P = 20 bar and was irradiated with protons (integrated current C2 = 1–4 μA h); (ii) the target gas was removed by cryogenic retrieval; (iii) the target was filled with CF4 to 4 bar, topped with neon to 20 bar and irradiated with protons (integrated current C3 = 1–4 μA h); and (iv) the target gas was cryogenically trapped as above.
In this process, during the first irradiation of pure [18O]O2, 18F is generated in a high yield, because the cross-sectional values of the nuclear reaction 18O(p,n)18F have an energy threshold of around 2 MeV and reach a maximum at energies of around 6 MeV (cross-sectional values at this energy is around 500 mb).13 During the cryogenic recovery of the 18O-enriched oxygen, fluorine-18 remains absorbed on the walls of the target chamber, and is ready to undergo isotopic exchange reactions in the following step. Indeed, the subsequent irradiation of CF4/Ne resulted in the formation of [18F]CF4. Similar chromatographic profiles to those obtained under method A were found (see Fig. S2, ESI†). A clear correlation between the amount of [18F]CF4 and the integrated current used for the first irradiation was observed, as expected (entries 7 and 8, Table 1). Interestingly, the integrated current for the second irradiation had a minor effect on the amount of [18F]CF4, suggesting that the isotopic exchange reaction is relatively fast (Table 1, entries 5–7).
In previous works, the mechanism of formation of [18F]CF4 was described as a result of a substitution reaction of a “hot” 18F atom with CF4, leading to the vibrationally excited molecule CF318F*, which ultimately deactivates via collision with the surrounding molecules.14 In our case, the reaction mechanism should differ from that previously described. Because the incident protons impact majorly in the gas, hot 18F atoms are only produced by the 19F(p,pn)18F nuclear reaction, which has a minor contribution to the final amount of [18F]CF4. Hence, we hypothesize that the ionic species CF3+, which is unreactive towards CF4, is formed during the irradiation of the CF4/Ne mixture, and reacts with an 18F atom absorbed on the walls of the target chamber, leading to the rapid formation of [18F]CF4. With this in our hands, 8.43 ± 0.59 GBq of [18F]CF4 could be produced in short irradiation times. These results are extraordinary, especially considering that full optimization of the experimental conditions was not carried out. An increase in the CF4 filling pressure or application of higher integrated current values in the first irradiation may lead to higher production yields.
With the aim of proving the suitability of our method for the preparation of other fluorinated gases, the synthesis of [18F]SF6 was approached using the same experimental conditions (methods A and B) but replacing the CF4 gas bottle by an SF6 gas bottle. Under method A, an integrated current of 4 μA h resulted in the formation of 0.36 ± 0.05 GBq of [18F]SF6, while under method B, an integrated current of 4 μA h in both irradiations resulted in 6.77 ± 0.21 GBq of pure [18F]SF6. The analysis of the radioactive gas by radio GC-MS after purification using a soda lime trap confirmed the presence of only one radioactive species identified as [18F]SF6. MS analysis confirmed the presence of 4 species that were identified as N2, CF4, F3N, and SF6. These results are extremely positive, as they suggest that the strategy reported here might be extrapolated to the preparation of other radiolabelled fluorinated gases.
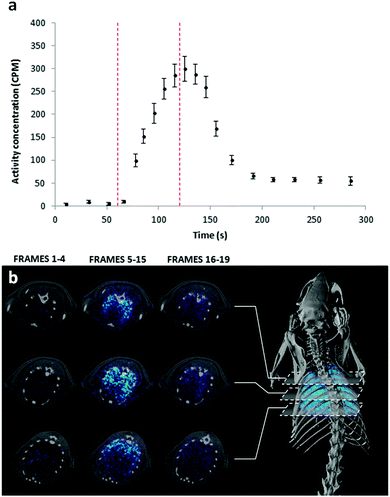
To prove the suitability of the radiofluorinated gases for the assessment of lung ventilation, in vivo PET studies combined with Computerised Tomography (CT) imaging were conducted using [18F]CF4 and a simple administration protocol based on dilution of the radioactive gas in the carrier oxygen (Fig. S3, ESI†). The time–activity curve in the lungs showed a sharp increase just after the onset of the administration of [18F]CF4 (Fig. 2a). When the delivery was discontinued, almost complete elimination from the lungs was achieved in a few seconds. The time-averaged PET images clearly show uniform distribution of the gas within the lungs (Fig. 2b). The use of [18F]CF4 as a ventilation marker has two major advantages: (i) the non-radioactive analogue is chemically inert, non-toxic, and has poor solubility in water; hence, a low translocation to the blood and remote organs is expected. Additionally, the gas is rapidly exhaled. Altogether, these factors contribute to a minimisation of the radiation dose posed on the subject; and (ii) the relatively long half-life of 18F and the efficient production method reported here should enable centralized production and distribution to nearby imaging centres, facilitating a potential translation into the clinical setting. Of note, such a translation may require the development of tailored gas administration systems capable of collecting the radioactive gases exhausted by the patients.
In conclusion, we report here the unprecedented, highly efficient, simple and easy-to-automate preparation of [18F]CF4 and [18F]SF6 following an ion beam induced chemical reaction based on a double proton irradiation approach. The methodology might be extended to other fluorinated gases. [18F]CF4 has proven suitable for the determination of regional lung ventilation using PET-CT. The radiofluorinated gases reported here may become powerful tools in the diagnostic, prognostic or evaluation of response to treatment for a wide variety of lung diseases. Future works will focus on the refinement of the experimental set-up and evaluation of [18F]CF4 as a ventilation marker using animal models of impaired lung ventilation.
This work was supported by Departamento de Desarrollo Económico y Competitividad of the Basque Government, under the Elkartek 2015 program, project biomagune 2015, ref. KK-2015/0000088.
Notes and references
- F. Fazio and T. Jones, Br. Med. J., 1975, 3, 673 CrossRef CAS PubMed.
- J. Palmer, U. Bitzén, B. Jonson and M. Bajc, J. Nucl. Med., 2001, 42, 1288 CAS.
- W. M. Burch, P. J. Sullivan and C. J. McLaren, Nucl. Med. Commun., 1986, 7, 865 CrossRef CAS PubMed.
- C. G. Rhodes and J. M. B. Hughes, Eur. Respir. J., 1995, 8, 1001 CAS.
- (a) A. W. Scholz, U. Wolf, M. Fabel, N. Weiler, C. P. Heussel, B. Eberle, M. David and W. G. Schreiber, Magn. Reson. Imaging, 2009, 27, 549 CrossRef PubMed; (b) J. M. Pérez-Sánchez, R. Pérez de Alejo, I. Rodríguez, M. Cortijo, G. Peces-Barba and J. Ruiz-Cabello, Magn. Reson. Med., 2005, 54, 460–463 CrossRef PubMed.
- U. Wolf, A. Scholz, M. Terekhov, K. Muennemann, K. Kreitner, C. Werner, C. Dueber and W. G. Schreiber, Proc. Intl. Soc. Mag. Reson. Med., 2008, 16 Search PubMed.
- J. E. Boggs, E. R. Van Artsdalen and A. R. Brosi, J. Am. Chem. Soc., 1955, 77, 6505 CrossRef CAS.
- T. A. Gens, J. A. Wethington Jr, A. R. Brosi and E. R. Van Artsdalen, J. Am. Chem. Soc., 1957, 79, 1001–1002 CrossRef CAS.
- M. T. Rogers and J. J. Katz, J. Am. Chem. Soc., 1952, 74, 1375 CrossRef CAS.
- N. Colebourne and R. Wolfgang, J. Chem. Phys., 1963, 38, 2782 CrossRef.
- (a) M. B. Knickelbein, K. D. Knierim and K. W. Root, Chem. Phys., 1984, 83, 235 CrossRef CAS; (b) F. S. Rowland, J. A. Cramer, R. S. Iyer, R. Milstein and R. L. Williams, Radiopharmaceuticals and Labeled Compounds, IAEA, Vienna, 1973, vol. 1, p. 383 Search PubMed.
- E. Běták, R. Mikołajczak, J. Staniszewska, S. Mikołajewski, E. Rurarz and J. Wojtkowska, Nukleonika, 2011, 56, 269 Search PubMed.
- IAEA Technical Repots Series no. 468, Cyclotron Produced Radionuclides: Physical Characteristics and Production Methods, p. 109.
- A. Bishop, N. Satyamurthy, G. Bida, G. Hendry, M. Phelps and J. R. Barrio, Nucl. Med. Biol., 1996, 23, 189 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details for ion beam-induced reactions and in vivo experiments. See DOI: 10.1039/c6cc06249k |
| ‡ Data obtained from Material Safety Data Sheet from Air Liquide, prepared to U.S. OSHA, CMA, ANSI and Canadian WHMIS Standards. As stated in this document, exposures to high concentrations of this gas may cause sensitization of the heart to adrenaline and nor-adrenaline. The compound is not considered nor suspected to be a cancer-causing agent. It is not irritating, and it is not known to cause sensitization. The most significant hazard associated with this gas is inhalation of oxygen-deficient atmospheres. |
| This journal is © The Royal Society of Chemistry 2016 |