 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and deposition of a Tröger’s base polymer on the electrode surface for potentiometric detection of a neuroblastoma tumor marker metabolite†
T. V.
Shishkanova
*a,
M.
Havlík
a,
M.
Dendisová
b,
P.
Matějka
b and
V.
Král
*a
aDepartment of Analytical Chemistry, University of Chemistry and Technology in Prague, 16628 Prague 6, Technická 5, Czech Republic. E-mail: tatiana.shishkanova@vscht.cz; shishkat@yahoo.com; kralv@vscht.cz
bDepartment of Physical Chemistry, University of Chemistry and Technology in Prague, 16628 Prague 6, Technická 5, Czech Republic
First published on 7th September 2016
Abstract
The development of novel diagnostic tools is a primary goal in bioanalytical chemistry. Here we report the synthesis of Tröger’s base functionalized with amino- and coumarin-units designed as a monomeric unit for the development of an electrochemical cancer sensor. The synthesized receptor was deposited onto a conducting support using electrochemical polymerization, characterized spectroscopically and tested potentiometrically towards metabolites used as tumor markers of neuroblastoma.
The development of detection methods for diseases with high mortality represents a crucial target of contemporary science. At present, the classical methodology for cancer detection is based on biopolymer analysis.1–5 A modern innovative approach is based on low-molecular weight non-classical tumor markers (TMs) and cancer cell metabolite identification.6–8 Despite the potential significance of detection of low-molecular weight non-classical TMs, there have been no reports on their determination using supramolecular analytical chemistry, especially potentiometry.9 Neuroblastoma, a neuroblastic tumor derived from the primordial neural crest, remains the leading cause of disease in infancy. A promising screening test for neuroblastoma could rely on the monitoring of urine catecholamine metabolites, namely homovanillic acid (HVA) and vanillylmandelic acid (VMA) (Fig. 1), which serve as TMs.10
Ultrasensitive diagnostic devices based on electrochemical principles could be quite a simple approach to screening the low-molecular-weight non-classical TMs of neuroblastoma. Recently, Nemiroski et al. have proposed universal mobile and highly effective electrochemical devices for the detection of (i) glucose in the blood for personal health, (ii) sodium in urine for clinical analysis, and (iii) a malarial antigen for clinical research.11 The compatibility of similar devices with any mobile phone or wearable electronics guarantees that sophisticated diagnostic testing can be performed by users with a broad spectrum of needs, resources, and levels of technical expertise. A few reports have been related to voltammetric determination of HVA and VMA without using supramolecular receptors.12
Since a number of biologically relevant molecules are electroactive, the selectivity of many electrochemical sensors based on electroactivity is limited. Therefore, a promising way to avoid overlapping electrochemical signals and increase selectivity is the development of a potentiometric sensor derived from a recognition receptor selective towards the low-molecular-weight target metabolite. We pioneered potentiometric determination of the metabolites for neuroblastoma screening.
Tröger's base is a molecule containing two arenes connected to the b and f sides of 1,5-methano-1,5-diazocine.13,14 Due to its unique structural features (C2-symmetry and rigid V-shape geometry) and the possibility of functionalization, Tröger's base is an attractive receptor for the development of optical and spectroscopic sensors.13–16 The sensor technology assumes immobilization of a supramolecular receptor onto the electrode interface between the transducer and the measured sample. The practically applicable approach is to immobilize a receptor into a suitable matrix. However, a non-covalently immobilized monomer can leach out from the matrix as a result of desorption and other physical effects occurring on the electrode surface in contact with the sample. In contrast, covalent immobilization of a receptor allows limiting the leaching out from the sensor surface and constructing microsensors for practical applications. In this communication, a Tröger's base derivative 6 (Scheme 1) was designed to allow us to deposit it electrochemically on the electrode surface and to detect the metabolite in question potentiometrically. The success of the supramolecular design was proven using spectroscopic and nanoscopic experiments, comprising both optical and mechanical measurements. Scheme 1 shows the synthesis of receptor 6.†
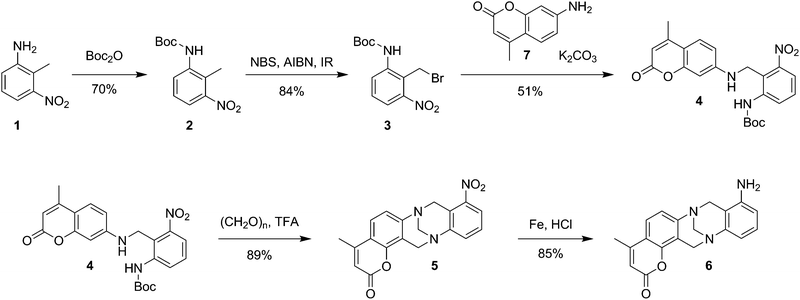 | ||
| Scheme 1 Stepwise preparation protocol of Tröger’s base functionalized with amino- and coumarin-units 6. | ||
We should note that this is not a case of preparation of a molecularly imprinted polymer. TMs are not present in the electropolymerization mixture.† A cyclic voltammogram of 6 on a Pt electrode (Fig. 2) exhibits two anodic peaks at ca. 0.55 and 1.02 V (from the 1st potential scan) and the reduction peak at ca. 0.47 V. The current intensity increased from 1 to 4 cycles, indicating the polymer growth (Fig. 2). In the subsequent scans, the current decreased, indicating the growth of the non-conducting polymeric film. The presence of the anodic peak at 1.02 V is related to the oxidation of the monomer and initiation of the electrosynthesis of the polymeric film.17 A couple (peaks Ia and Ib) is evident at a potential of ca. 0.55 V, corresponding to the reduction–oxidation of an adsorbed product.
 | ||
| Fig. 2 Cyclic voltammogram of a Tröger's base derivative 6 in CH3CN/TBAFB/HCl on a Pt electrode. The electrode potential was swept repeatedly between 0.0 and 1.2 V vs. Ag/AgCl at a scan rate of 50 mV s−1; the number of scans is 4.† | ||
Scanning near-field infrared microscopy (SNIM) combined with nanomechanical patterning shows that the polymeric film on the electrode surface is nanostructured and consists of “hills” and “valleys” with a maximal height profile of 0.5 μm (Fig. 3A). The amplitude of the infrared signal assigned to the aromatic C–C stretching mode suggests that the adhering film is porous, with grains located on the edges of both “hills” and “valleys” (Fig. 3B).
 | ||
| Fig. 3 SNIM images: 5 × 5 μm of the Au electrode surface. Mechanical amplitude signal (A) and optical signal (B). | ||
To confirm the formation of the polymer derived from 6, Fourier transform infrared (FTIR) spectra of the monomer and the polymer prepared by chemical polymerization in CH3CN were compared (Fig. 4). The polymer spectrum differs substantially from that of the monomer between 3400 and 3200 cm−1 and under 1600 cm−1. In contrast to the monomer spectrum, which exhibits bands at ∼3350 cm−1 and ∼3237 cm−1 assigned to a primary amino group, the polymer spectrum exhibits the band at ∼3217 cm−1 of the secondary amino group.18 The intensity ratio of bands at ca. 2960 cm−1 and 2930 cm−1 of asymmetric stretching vibrations of CH3 and CH2 groups, respectively, is reversed. These changes may be rationalized as follows: in the methylcoumarin unit, the possible delocalization of electrons in the lactone ring taking place during polymerization leads to the change in the dipole moment of CH3 vibrations and to the conformational changes. The bands at 1713 and 1716 cm−1 of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration of the coumarin unit are observed in both the monomer and polymer spectra, respectively. After polymerization, the intensity of the coupled N–H and C–C vibration band at 1683 cm−1 is remarkably increased to a level comparable to the C
O vibration of the coumarin unit are observed in both the monomer and polymer spectra, respectively. After polymerization, the intensity of the coupled N–H and C–C vibration band at 1683 cm−1 is remarkably increased to a level comparable to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration.19 The aromatic ring modes are shown as a set of bands under 1600 cm−1 (1596, 1469, 1288, 1152, 1067, 1050, 942, 829 and 707 cm−1) in both spectra with different intensities. We note that the C
O vibration.19 The aromatic ring modes are shown as a set of bands under 1600 cm−1 (1596, 1469, 1288, 1152, 1067, 1050, 942, 829 and 707 cm−1) in both spectra with different intensities. We note that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration at 1483 cm−1 assigned to the benzenoid units20 characteristic of polyaniline is observed in the polymer spectrum. The intense band at 1044 cm−1 results from the sulfate anion as a product of aniline oxidation by persulfate and somewhat overlaps with the band at ca. 1140 cm−1 assigned to protonated polyaniline.18 Hence the FTIR spectra reveal the polymerization of 6via its amino group according to the mechanism of aniline polymerization.
N stretching vibration at 1483 cm−1 assigned to the benzenoid units20 characteristic of polyaniline is observed in the polymer spectrum. The intense band at 1044 cm−1 results from the sulfate anion as a product of aniline oxidation by persulfate and somewhat overlaps with the band at ca. 1140 cm−1 assigned to protonated polyaniline.18 Hence the FTIR spectra reveal the polymerization of 6via its amino group according to the mechanism of aniline polymerization.
 | ||
| Fig. 4 FTIR spectra of the polymer (blue) and the monomer (red) from 4000 to 2000 cm−1 (A) and from 1900 to 600 cm−1 (B). Each spectrum was averaged from 256 scans with 4 cm−1 resolution. | ||
The electrochemically modified electrodes were tested for the potentiometric detection of TMs (Fig. 5).† A potentiometric response of −39 mV decade−1 towards VMA was found in the concentration range from 9.9 × 10−6 to 3.3 × 10−3 mol L−1 (the squared correlation coefficient was equaled 0.9914). The under-Nernstian behavior could be caused by the roughness of the polymeric film and the effect of the surface morphology of the supporting metallic material. In the case of HVA, the potentiometric response of the prepared electrode was insignificant (not shown). The tested TMs are structurally closely related (Fig. 1) and differ by the hydroxy group present in VMA. The potentiometric findings indicate that the electrode based on the polymer derived from 6 enables the selective detection of VMA in the presence of HVA (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KPot.VMA,
KPot.VMA,![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HVA = −0.9).† That a polymer film prepared from Tröger’s base prefers VMA to HVA may be a result of both its spatial arrangement and accessibility of binding sites for tested TMs into the polymeric film on the electrode surface. Actually, our spectroscopic findings confirmed that the conformational changes take place during electropolymerization. The hydroxyl groups of VMA (Fig. 1) may reinforce hydrogen bonding between VMA and the polymeric film.21 For infants (0–1 year old) and children (1–13 years old), the level of the analyzed TMs is (1.0–5.4) × 10−5 mol L−1 in urine.22 The content of TMs exceeding the above-mentioned range 10 times is a signal of concern.23 Experiments were conducted to measure the concentration of VMA added to an artificial urine.24 It was found to be (6 ± 2) × 10−5 mol L−1 (n = 3) VMA when 5 × 10−5 mol L−1 VMA was introduced. These results indicated that the constituents of the prepared urine sample do not interfere with the VMA detection and the proposed electrode based on the Troger's base polymer provides an alternative device for VMA determination.
HVA = −0.9).† That a polymer film prepared from Tröger’s base prefers VMA to HVA may be a result of both its spatial arrangement and accessibility of binding sites for tested TMs into the polymeric film on the electrode surface. Actually, our spectroscopic findings confirmed that the conformational changes take place during electropolymerization. The hydroxyl groups of VMA (Fig. 1) may reinforce hydrogen bonding between VMA and the polymeric film.21 For infants (0–1 year old) and children (1–13 years old), the level of the analyzed TMs is (1.0–5.4) × 10−5 mol L−1 in urine.22 The content of TMs exceeding the above-mentioned range 10 times is a signal of concern.23 Experiments were conducted to measure the concentration of VMA added to an artificial urine.24 It was found to be (6 ± 2) × 10−5 mol L−1 (n = 3) VMA when 5 × 10−5 mol L−1 VMA was introduced. These results indicated that the constituents of the prepared urine sample do not interfere with the VMA detection and the proposed electrode based on the Troger's base polymer provides an alternative device for VMA determination.
 | ||
| Fig. 5 (A) Potentiometric response and (B) calibration graph of the electrode modified with the polymer based on 6 towards VMA in 0.1 mol L−1 phosphate buffer at pH = 7. | ||
In conclusion, we have presented an innovative detection of a neuroblastoma TM which is based on specific and selective interaction with a polymer film (prepared from 6) and VMA. The detection was purely potentiometric. The geometry of electrochemically polymerized receptor 6 enabled it to interact specifically with VMA in contrast to HVA.
This work was supported by the Grant Agency of the Czech Republic (Project P206/15/02815S), Grant No. LH14008 (Kontakt II) and by the Technology Agency of the Czech Republic, Grant No. TE01020028.
Notes and references
- S. Ge, L. Ge, M. Yan, X. Song, J. Yu and J. Huang, Chem. Commun., 2012, 48, 9397 RSC.
- S. K. Basak and E. S. Srivatsan, in Cancer Biomarker: minimal and noninvasive early diagnosis and prognosis, Salivary biomarkers in early diagnosis of cancered. D. Barh, A. Carpi, M. Verma and M. Gunduz, CRC Press-Taylor & Francis group, 6000 Broken Sound Parkway NW, STE 300, Boca Raton, FL 33487-2742 USA, 2014, pp. 159–195 Search PubMed.
- G. K. Joshi, S. Deitz-McElyea, T. Liyanage, K. Lawrence, S. Mali, R. Sardar and M. Korc, ACS Nano, 2015, 9, 11075 CrossRef CAS PubMed.
- P. E Swanson, Appl. Immunohistochem. Mol. Morphol., 2015, 23, 81 CrossRef PubMed.
- S. Srivastava, B. J. Reid, S. Ghosh and B. S. Kramer, J. Cell. Physiol., 2016, 231, 1870 CrossRef CAS PubMed.
- Y. Lu, N. Li, L. Gao, Y.-J. Xu, C. Huang, K. Yu, Q. Ling, Q. Cheng, S. Chen, M. Zhu, J. Fang, M. Chen and C. N Ong, Cancer Res., 2016, 76, 2912 CrossRef CAS PubMed.
- K. Kim, S.-G. Yeo and B. C. Yoo, Cancer Res. Treat., 2015, 47, 78 CrossRef CAS PubMed.
- B. Muthuraj, S. Mukherjee, S. R. Chowdhury, C. R. Patra and P. K. Iyer, Biosens. Bioelectron., 2015 DOI:10.1016/j.bios.2015.12.036.
- (a) J. Wang, Biosens. Bioelectron., 2006, 21, 1887 CrossRef CAS PubMed; (b) B. Bohunicky and S. A. Mousa, Nanotechnol., Sci. Appl., 2011, 4, 1 CAS.
- T. Manickum, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2009, 877, 4140 CrossRef CAS PubMed.
- A. Nemiroski, D. C. Christodouleas, J. W. Hennek, A. A. Kumar, E. J. Maxwell and M. T. Fernandez-Abedul, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 11984 CrossRef CAS PubMed.
- (a) M. C. Blanco-Lopez, M. J. Lobo-Castanon, A. J. Miranda-Ordieres and P. Tunon-Blanco, Biosens. Bioelectron., 2003, 18, 353 CrossRef CAS PubMed; (b) Q. Li, C. Batchelor-McAuley and R. G. Compton, J. Phys. Chem., 2010, 114, 9713 CrossRef CAS PubMed.
- B. Dolensky, J. Elguero, V. Kral, C. Pardo and M. Valik, Adv. Heterocycl. Chem., 2007, 93, 1 CrossRef CAS.
- S. Sergeyev, Helv. Chim. Acta, 2009, 92, 415 CrossRef CAS.
- B. Baldeyrou, C. Tardy, C. Bailly, P. Colson, C. Houssier, F. Charmantray and M. Demeunynck, Eur. J. Med. Chem., 2002, 37, 315 CrossRef CAS PubMed.
- R. B. P. Elmes, M. Erby, S. A. Bright, D. C. Williams and T. Gunnlaugsson, Chem. Commun., 2012, 48, 2588 RSC.
- H. Lund and M. M. Baizer, Organic Electrochemistry, Marcel Dekker, New York, NY, 1991 Search PubMed.
- M. Trchová, Z. Morávková, I. Šeděnková and J. Stejskal, Chem. Pap., 2012, 66, 415 Search PubMed.
- E. Kavitha, N. Sundaraganesan and S. Sebastian, Indian J. Pure Appl. Phys., 2010, 48, 20 CAS.
- S. A. Hassoon, Int. J. Innovative Res. Sci., Eng. Technol., 2014, 3, 9763 Search PubMed.
- Y. Diñeiro, M. I. Menéndez, M. C. Blanco-López, M. J. Lobo-Castañón, A. J. Miranda-Ordieres and P. Tuñón-Blanco, Anal. Chem., 2005, 77, 6741 CrossRef PubMed.
- D. S. Wishart, R. Mandal, A. Stanislaus and M. Ramirez-Gaona, Metabolites, 2016, 6, 10 CrossRef PubMed.
- S. C. Gates, C. C. Sweeley, W. Krivit, D. DeWitt and B. E. Blaisdell, Clin. Chem., 1978, 24, 1680 CAS.
- N. Laube, B. Mohr and A. Hesse, J. Cryst. Growth, 2001, 233, 367 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of synthetic experimental. See DOI: 10.1039/c6cc06203b |
| This journal is © The Royal Society of Chemistry 2016 |

