Open Access Article
Open Access ArticleMultifaceted magnetization dynamics in the mononuclear complex [ReIVCl4(CN)2]2−†
Xiaowen
Feng
a,
Jun-Liang
Liu
 bcd,
Kasper S.
Pedersen
cde,
Joscha
Nehrkorn‡
f,
Alexander
Schnegg
f,
Karsten
Holldack
g,
Jesper
Bendix
h,
Marc
Sigrist
ij,
Hannu
Mutka
i,
Dumitru
Samohvalov
cd,
David
Aguilà
cd,
Ming-Liang
Tong
bcd,
Kasper S.
Pedersen
cde,
Joscha
Nehrkorn‡
f,
Alexander
Schnegg
f,
Karsten
Holldack
g,
Jesper
Bendix
h,
Marc
Sigrist
ij,
Hannu
Mutka
i,
Dumitru
Samohvalov
cd,
David
Aguilà
cd,
Ming-Liang
Tong
 b,
Jeffrey R.
Long
*akl and
Rodolphe
Clérac
*cd
b,
Jeffrey R.
Long
*akl and
Rodolphe
Clérac
*cd
aDepartment of Chemistry, University of California Berkeley, California 94720, USA. E-mail: jrlong@berkeley.edu
bMOE Key Lab of Bioinorganic and Synthetic Chemistry, School of Chemistry, Sun Yat-Sen University, Guangzhou 510275, P. R. China
cCNRS, CRPP, UPR 8641, F-33600 Pessac, France. E-mail: clerac@crpp-bordeaux.cnrs.fr
dUniv. Bordeaux, CRPP, UPR 8641, F-33600 Pessac, France
eCNRS, ICMCB, UPR 9048, F-33600 Pessac, France
fBerlin Joint EPR Laboratory, Institut für Nanospektroskopie, Helmholtz-Zentrum für Materialien und Energie, 12489 Berlin, Germany
gHelmholtz-Zentrum für Materialien und Energie, Inst. f. Methoden und Instrumente der Forschung mit Synchrotronstrahlung, 12489 Berlin, Germany
hDepartment of Chemistry, University of Copenhagen, DK-2100 Copenhagen, Denmark
iInstitut Laue-Langevin, 38042 Grenoble Cedex 9, France
jInstitute of Chemistry, Academia Sinica, Nankang, 11529 Taipei, Taiwan, ROC
kMaterials Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, California 94720, USA
lDepartment of Chemical and Biomolecular Engineering, University of California Berkeley, Berkeley, California 94720, USA
First published on 5th October 2016
Abstract
The mononuclear complex (Bu4N)2[ReIVCl4(CN)2]·2DMA (DMA = N,N-dimethylacetamide) displays intricate magnetization dynamics, implying Orbach, direct, and Raman-type relaxation processes. The Orbach relaxation process is characterized by an energy barrier of 39 K (27 cm−1) that is discussed based on high-field electron paramagnetic resonance (EPR), inelastic neutron scattering and frequency-domain THz EPR investigations.
For paramagnetic molecules with a spin ground state of ST, the presence of anisotropy may result in a magnetic bistability through the stabilization of the MS = +ST and −ST microstates, which are separated by an energy barrier, Δ, thereby leading to slow dynamics of the magnetization. As a family, such molecules are referred to as single-molecule magnets (SMMs).1,2 Although the first generations of SMMs were polynuclear complexes characterized by ever larger spin ground states,3 theoretical and experimental results have established that an increase in the spin ground state is compensated by a corresponding decrease in total magnetic anisotropy.4 To overcome this problem, new approaches have emerged, such as using molecules containing a single paramagnetic lanthanide or actinide ion, both known for their often pronounced magnetic anisotropy.5,6 The effective energy barriers for magnetization reversal, Δeff, for these molecules have been reported to exceed 1000 K (695 cm−1),6 which is an order of magnitude larger than the original Mn12 SMM.2
More recently, it was shown that mononuclear first-row transition metal complexes can display significant magnetic anisotropy, originating from a near-orbitally degenerate ground state where the orbital angular momentum is left unquenched.7 Moreover, taking advantage of the Kramers theorem,8 fast quantum tunneling could be effectively avoided by using half-integer spin metal ions. Following this strategy, several mononuclear SMMs based on first-row transition metal ions were reported.9 For example, a recent linear two-coordinate FeI complex displayed a Δeff of 354 K (246 cm−1), and a magnetic hysteresis up to 6.5 K, thereby challenging high-performing lanthanide systems.10 In addition to the synthetic challenges of generating such low-coordinate complexes, mononuclear SMMs of first-row transition metal ions have the intrinsic disadvantage of possessing weak spin–orbit coupling and thus may have a relatively small magnetic anisotropy. To meet this challenge, recent attention turned to 5d metal ions, for which strong spin–orbit coupling can be combined with the tunability of the electronic structure through the chemically controllable geometry and ligand field.11
Although SMM behavior seemed to be reserved for systems with D < 0 (easy-axis type anisotropy; with Ĥ = DŜT,z2), an increasing number of complexes featuring D > 0 (easy-plane type anisotropy) in conjunction with SMM properties have been reported.12 However, for molecules with half-integer spin, a doubly degenerate ground state is always assured by the Kramers theorem, regardless of the sign of D. Thus, in principle, observation of slow magnetization dynamics via an Orbach process involving at least three MS microstates is always possible for a half-integer spin system, irrespective of the MS compositions of the ground-state doublet.13 Indeed, several SMMs with D > 0 have been reported to exhibit this type of relaxation, despite a complete mismatch between the calculated and experimentally extracted energy barriers,12c,e,f thereby pointing towards the presence of alternative relaxation processes.
Recently, rhenium(IV) complexes (Bu4N)2[Re(ox)X4] (ox = oxalate, X = Cl, Br), (PPh4)2[ReF6]·2H2O and Zn(viz)4[ReF6] (viz = 1-vinylimidazole) were shown to display slow relaxation of the magnetization with reported barriers of up to 29.6 K (20.6 cm−1).14 Notably, for all four systems, the observed barrier is significantly lower than the expected value, as determined from the spin-Hamiltonian parameters. We previously reported on (Bu4N)2[ReIVCl4(CN)2]·2DMA (1; Fig. 1a),15a and, subsequently, high-frequency and high-field electron paramagnetic resonance (HF-EPR) spectroscopic studies that allowed determination of its anisotropy parameters using the (zero-field) spin-Hamiltonian pertaining to S = 3/2 given in eqn (1): D/kB = 16 K (+11 cm−1) and |E|/kB = 4.6 K (3.2 cm−1).15b
| Ĥ = D(Ŝz2 − ⅓S(S + 1)) + E(Ŝx2 − Ŝy2) | (1) |
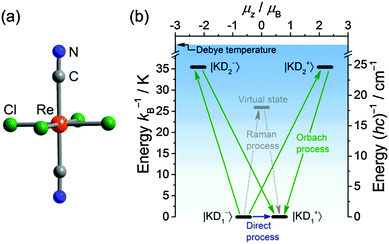 | ||
| Fig. 1 (a) Structure of the trans-[ReIVCl4(CN)2]2− complex obtained from single-crystal X-ray diffraction on 1 at 50 K. (b) Energy level diagram calculated from the previously determined D and E parameters.15b The blue shading is a schematic representation of the acoustic phonon density with the cut-off at the Debye temperature. The arrows indicate the direct (blue), Raman (grey), and Orbach (green) relaxation pathways from state |KD1−〉 to |KD1+〉 (the diagram is symmetrical for the reverse processes). The magnetic moment, μz, is defined as μz = geff,zμB/2, where geff is the g-factor treating the two doublets as effective spin 1/2. The virtual state, relevant for the Raman process, is placed pictorially at an arbitrary energy with an arbitrary magnetic moment. | ||
Using these EPR-derived parameters, the ground (KD1) and excited (KD2) state Kramers doublet compositions are as follows:
| |KD1〉 = −0.23|±3/2〉 + 0.97|∓1/2〉 |
| |KD2〉 = ±0.23|∓1/2〉 ± 0.97|±3/2〉 |
Despite the positive value of D and the predominant MS = ±1/2 character of the ground state Kramers doublet, KD1, we hereby report the observation of a slow relaxation of the magnetization for 1 that is reminiscent of SMM behavior, and discuss its possible origins. In 1, the rhenium(IV) center of the [ReCl4(CN)2]2− complex anion resides in an approximate D4h symmetric environment15a and close inspection of the X-ray crystal structures at 50 and 139 K reveals only small differences in the local coordination environment of the metal ion. As expected for typical thermal contraction, the bond distances shrink slightly with decreasing temperature. The Re–Cl distances are 2.351(1) and 2.341(1) Å at 139 K, while at 50 K, these distances are 2.321(2) and 2.334(2) Å, respectively. Selected interatomic distances and bond angles for 1 are listed in Table S1 (ESI†).
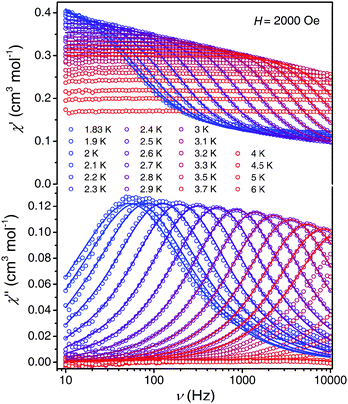
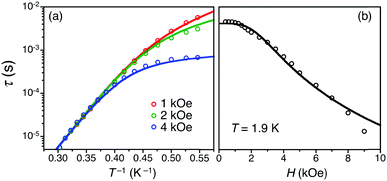
Alternating current (ac) magnetic susceptibility measurements were performed to detect any slow relaxation of the magnetization in a polycrystalline sample of 1. In the absence of a static magnetic field, the relaxation time is too short to be observed in the experimentally accessible time window. Upon applying small static fields, H, however, clear frequency- and temperature-dependent ac signals were observed (Fig. 2 and Fig. S1, ESI†). To elucidate the effect of the applied magnetic field on the slow relaxation of magnetization, the relaxation time was extracted from the variable-field and variable-temperature ac susceptibility data (τ(T,H); Fig. 3 and Fig. S1–S6, ESI†) by fitting the experimental data to the generalized Debye model. The relaxation time at 1.9 K monotonically decreases with increasing magnetic field (Fig. 3b). This behavior is expected for a direct process of relaxation, which involves only the two components of the lowest-lying Kramers doublet and is accelerated upon increasing magnetic field (in accordance with the first term in eqn (2)).13,16 In the low field regime (μBH ≪ kBT), other processes (Orbach, Raman, etc.) governing magnetization relaxation are only weakly field dependent and thus their contributions to the relaxation time have been assumed to be constant, k(T), as in eqn (2).12b,17,18
| τ(H)−1 = AH4T + k(T) | (2) |
Note that upon increasing the dc field, the probability of magnetization relaxation by quantum tunneling should be suppressed, thereby resulting in a slowing down of the relaxation. This effect is not experimentally observed for 1, and quantum tunnelling has thus not been considered in the modeling of its relaxation properties.16d,19,20
In order to reproduce the temperature dependence of the relaxation time, Orbach and/or Raman processes should be considered in addition to the direct process already evidenced in the field dependence (vide supra).17,21,22 Accordingly, two simple parameter-sparse models were considered, as given in eqn (3) and (4). These two approaches incorporate the contributions from both direct and Raman processes (eqn (3)) and both direct and Orbach processes (eqn (4)), with the fixed parameter A = 3.0 × 104 s−1 K−1 T−4 extracted from the τ(1.9 K,H) data.
| τ(T)−1 = AH4T + CTn | (3) |
| τ(T)−1 = AH4T + τ0−1exp[−Δeff/(kBT)] | (4) |
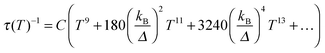 | (5) |
| τ(T,H)−1 = AH4T + CTn + τ0−1exp[−Δeff/(kBT)] | (6) |
Spectroscopy offers an opportunity to access the energy levels involved in the Orbach relaxation, but has rarely been employed in directly determining the energy level structure in mononuclear SMMs.9b,12d,14b In an attempt to confirm the energy level structure of 1 (Fig. 1b) in the absence of a perturbing magnetic field, inelastic neutron scattering (INS) spectroscopy was performed on a polycrystalline sample. We were, however, unable to observe the presence of any excited state in the energy window from ca. 10 to 50 cm−1 (14 to 72 K; Fig. S10 and S11, ESI†), possibly due to the high content of 1H that gives rise to an intense spectral background. Interestingly, analysis of the energy dependence of the phonon spectrum affords a Debye temperature of θD ≈ 139 K (Fig. S12, ESI†), suggesting the applicability of the Debye model in the studied temperature regime. To circumvent the problem of the strong spectral background, frequency-domain Fourier-transform THz-EPR (FD-FT THz-EPR) spectroscopy was employed in detecting EPR transitions in the energy range of ca. 10 to 30 cm−1 (14 to 43 K; Fig. S13 and S14, ESI†). Unfortunately, no magnetic excitations could be detected here either, preventing the confirmation of the energy level diagram derived from the preceding high-field EPR spectroscopic study.
The foregoing results demonstrate the necessity of invoking Orbach, direct, and Raman-type processes in explaining the multifaceted magnetization dynamics observed in the mononuclear complex salt (Bu4N)2[ReIVCl4(CN)2]·2DMA. Whilst this multi-parameter description of τ(T,H) was only satisfactory after introduction of the Orbach mechanism, the lack of observation of the relevant excited state by spectroscopic techniques brings justified doubts on the presence of an operative Orbach relaxation in 1. This original example highlights the complexity of describing paramagnetic relaxation in molecular systems. Even if these elaborate multi-mechanism models for describing the relaxation phenomena are now becoming fashionable in the field of molecular magnetism, the combination of a large number of parameters with relatively uncharacteristic τ(T,H) data in a narrow temperature interval may lead to questionable conclusions.
This work was supported by the National Science Foundation (NSF) under Grant CHE-1464841, the CNRS (PICS No. 06485), the University of Bordeaux, the Conseil Regional d'Aquitaine, the ANR, the French Embassy in the US (Chateaubriand fellowship for X. F.), the GdR MCM-2 and Sun Yat-Sen University (the International Program of Project 985 for J.-L. L.). K. S. P. thanks the Danish Research Council for Independent Research for a DFF-Sapere Aude Research Talent grant (4090-00201). The low temperature crystal structure was collected at the Stanford Nano Shared Facilities (SNSF), supported by the NSF under award ECCS-1542152. We thank also Philip C. Bunting for helpful discussions.
Notes and references
- G. Gatteschi, R. Sessoli and J. Villain, Molecular Nanomagnets, Oxford University Press, Oxford, 2006 Search PubMed.
- (a) R. Sessoli, H. L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 1804 CrossRef CAS; (b) R. Sessoli, D. Gatteschi, A. Caneschi and M. A. Novak, Nature, 1993, 365, 141 CrossRef CAS.
- C. J. Milios, A. Vinslava, W. Wernsdorfer, S. Moggach, S. Parsons, S. P. Perlepes, G. Christou and E. K. Brechin, J. Am. Chem. Soc., 2007, 129, 2754 CrossRef CAS PubMed.
- (a) F. Neese and E. I. Solomon, Inorg. Chem., 1998, 37, 6568 CrossRef CAS PubMed; (b) O. Waldmann, Inorg. Chem., 2007, 46, 10035 CrossRef CAS PubMed; (c) E. Ruiz, J. Cirera, J. Cano, S. Alvarez, C. Loose and J. Kortus, Chem. Commun., 2008, 52 RSC; (d) F. Neese and D. A. Pantazis, Faraday Discuss., 2011, 148, 229 RSC.
- (a) N. Ishikawa, M. Sugita, T. Ishikawa, S. Y. Koshihara and Y. Kaizu, J. Am. Chem. Soc., 2003, 125, 8694 CrossRef CAS PubMed; (b) F. Branzoli, P. Caretta, M. Filibian, G. Zoppellaro, M. J. Graf, J. R. Galan-Mascaros, O. Fuhr, S. Brink and M. Ruben, J. Am. Chem. Soc., 2009, 131, 4387 CrossRef CAS PubMed; (c) S.-D. Jiang, B.-W. Wang, H.-L. Sun, Z.-M. Wang and S. Gao, J. Am. Chem. Soc., 2011, 133, 4730 CrossRef CAS PubMed; (d) J.-L. Liu, Y.-C. Chen, Y.-Z. Zheng, W.-Q. Lin, L. Ungur, W. Wernsdorfer, L. F. Chibotaru and M.-L. Tong, Chem. Sci., 2013, 4, 3310 RSC; (e) J. D. Rinehart and J. R. Long, J. Am. Chem. Soc., 2009, 131, 12558 CrossRef CAS PubMed; (f) K. R. Meihaus, J. D. Rinehart and J. R. Long, Inorg. Chem., 2011, 50, 8484 CrossRef CAS PubMed; (g) M. Gregson, N. F. Chilton, A.-M. Ariciu, F. Tuna, I. F. Crowe, W. Lewis, A. J. Blake, D. Collison, E. J. L. McInnes, R. E. P. Winpenny and S. T. Liddle, Chem. Sci., 2016, 7, 155 RSC; (h) J. J. Baldoví, S. Cardona-Serra, J. M. Clemente-Juan, E. Coronado and A. Gaita-Arino, Chem. Sci., 2013, 4, 938 RSC; (i) L. Ungur, J. J. Le Roy, I. Korobkov, M. Murugesu and L. F. Chibotaru, Angew. Chem., Int. Ed., 2014, 53, 4413 CrossRef CAS PubMed.
- J. Liu, Y.-C. Chen, J.-L. Liu, V. Vieru, L. Ungur, J.-H. Jia, L. F. Chibotaru, Y. Lan, W. Wernsdorfer, S. Gao, X.-M. Chen and M.-L. Tong, J. Am. Chem. Soc., 2016, 138, 5441 CrossRef CAS PubMed.
- D. E. Freedman, W. H. Harman, T. D. Harris, G. J. Long, C. J. Chang and J. R. Long, J. Am. Chem. Soc., 2010, 132, 1224 CrossRef CAS PubMed.
- H. A. Kramers, Proc. R. Acad. Sci. Amsterdam, 1930, 33, 959 CAS.
- (a) G. A. Craig and M. Murrie, Chem. Soc. Rev., 2015, 44, 2135 RSC; (b) Y. Rechkemmer, F. D. Breitgoff, M. van der Meer, M. Atanasov, M. Hakl, M. Orlita, P. Neugebauer, F. Neese, B. Sarkar and J. van Slageren, Nat. Commun., 2016, 7, 10467 CrossRef CAS PubMed.
- (a) J. M. Zadrozny, D. J. Xiao, M. Atanasov, G. J. Long, F. Grandjean, F. Neese and J. R. Long, Nat. Chem., 2013, 5, 577 CrossRef CAS PubMed; (b) J. M. Zadrozny, D. M. Xiao, J. R. Long, M. Atanasov, F. Neese, F. Grandjean and G. J. Long, Inorg. Chem., 2013, 52, 13123 CrossRef CAS PubMed.
- (a) X.-Y. Wang, C. Avendaño and K. R. Dunbar, Chem. Soc. Rev., 2011, 40, 3213 RSC; (b) K. S. Pedersen, J. Bendix and R. Clérac, Chem. Commun., 2014, 50, 4396 RSC; (c) S. K. Singh and G. Rajaraman, Nat. Commun., 2016, 7, 10669 CrossRef CAS PubMed.
- (a) J. M. Zadrozny, J. Liu, N. A. Piro, C. J. Chang, S. Hill and J. R. Long, Chem. Commun., 2012, 48, 3927 RSC; (b) S. Gómez-Coca, A. Urtizberea, E. Cremades, P. J. Alonso, A. Camón, E. Ruiz and F. Luis, Nat. Commun., 2014, 5, 4300 Search PubMed; (c) S. Gómez-Coca, E. Cremades, N. Aliaga-Alcalde and E. Ruiz, J. Am. Chem. Soc., 2013, 135, 7010 CrossRef PubMed; (d) E. Colacio, J. Ruiz, E. Ruiz, E. Cremades, J. Krzystek, S. Carretta, J. Cano, T. Guidi, W. Wernsdorfer and E. K. Brechin, Angew. Chem., Int. Ed., 2013, 52, 9130 CrossRef CAS PubMed; (e) S. Vaidya, A. Upadhyay, S. K. Singh, T. Gupta, S. Tewary, S. K. Langley, J. P. S. Walsh, K. S. Murray, G. Rajaraman and M. Shanmugam, Chem. Commun., 2015, 51, 3739 RSC; (f) J. Vallejo, I. Castro, R. Ruiz-Garcia, J. Cano, M. Julvet, F. Lloret, G. De Munno, W. Wernsdorfer and E. Pardo, J. Am. Chem. Soc., 2012, 134, 15704 CrossRef CAS PubMed.
- R. Orbach, Proc. R. Soc. London, Ser. A, 1961, 264, 458 CrossRef CAS.
- (a) J. Martinez-Lillo, T. F. Mastropietro, E. Lhotel, C. Paulsen, J. Cano, G. Di Munno, J. Faus, F. Lloret, M. Julve, S. Nellutia and J. Krzystek, J. Am. Chem. Soc., 2013, 135, 13737 CrossRef CAS PubMed; (b) K. S. Pedersen, M. Sigrist, M. A. Sørensen, A.-L. Barra, T. Weyhermüller, S. Piligkos, C. Aa. Thuesen, M. G. Vinum, H. Mutka, H. Weihe, R. Clérac and J. Bendix, Angew. Chem., Int. Ed., 2014, 53, 1351 CrossRef CAS PubMed.
- (a) T. D. Harris, M. V. Bennet, R. Clérac and J. R. Long, J. Am. Chem. Soc., 2010, 132, 3980 CrossRef CAS PubMed; (b) X. Feng, J. Liu, T. D. Harris, S. Hill and J. R. Long, J. Am. Chem. Soc., 2012, 134, 7521 CrossRef CAS PubMed.
- (a) R. Orbach, Proc. R. Soc. London, Ser. A, 1961, 264, 485 CrossRef CAS; (b) T. L. Bohan and H. J. Stapleton, Phys. Rev., 1969, 182, 385 CrossRef CAS; (c) K. N. Shrivastava, Phys. Status Solidi B, 1983, 117, 437 CrossRef CAS; (d) J. M. Zadrozny, M. Atanasov, A. M. Bryan, C.-Y. Lin, B. D. Rekken, P. P. Power, F. Neese and J. R. Long, Chem. Sci., 2013, 4, 125 RSC.
- A. Abragam and B. Bleaney, Electron Paramagnetic Resonance of Transition Ions, Dover, New York, 1986 Search PubMed.
- See for example: (a) I. Chiorescu, W. Wernsdorfer, A. Müller, H. Bögge and B. Barbara, Phys. Rev. Lett., 2000, 84, 3454 CrossRef CAS PubMed; (b) K. S. Pedersen, J. Dreiser, H. Weihe, R. Sibille, H. V. Johannesen, M. A. Sørensen, B. E. Nielsen, M. Sigrist, H. Mutka, S. Rols, J. Bendix and S. Piligkos, Inorg. Chem., 2015, 54, 7600 CrossRef CAS PubMed; (c) L. Tesi, E. Lucaccini, I. Cimatti, M. Perfetti, M. Mannini, M. Atzori, E. Morra, M. Chiesa, A. Caneschi, L. Sorace and R. Sessoli, Chem. Sci., 2016, 7, 2074 RSC; (d) M. Atzori, L. Tesi, E. Morra, M. Chiesa, L. Sorace and R. Sessoli, J. Am. Chem. Soc., 2016, 138, 2154 CrossRef CAS PubMed.
- E. Lucaccini, L. Sorace, M. Perfetti, J.-P. Costes and R. Sessoli, Chem. Commun., 2014, 50, 1648 RSC.
- A. Fort, A. Rettori, J. Villain, D. Gatteschi and R. Sessoli, Phys. Rev. Lett., 1998, 80, 612 CrossRef CAS.
- (a) K. N. Shrivastava, Phys. Status Solidi B, 1972, 51, 377 CrossRef CAS; (b) A. Singh and K. N. Shrivastava, Phys. Status Solidi B, 1979, 95, 273 CrossRef CAS.
- C. B. P. Finn, R. Orbach and W. P. Wolf, Proc. Phys. Soc., London, 1961, 77, 261 CrossRef CAS.
- A. Kiel and W. B. Mims, Phys. Rev., 1967, 161, 386 CrossRef CAS.
- (a) S. S. Eaton and G. R. Eaton, Biol. Magn. Reson., 2000, 19, 29 CrossRef CAS; (b) S. T. Liddle and J. van Slageren, Chem. Soc. Rev., 2015, 44, 6655 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, crystallographic data, and magnetic and spectroscopic characterization. CCDC 1488917. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc05473k |
| ‡ Current address: Department of Chemistry, University of Washington, Seattle, Washington 98115, USA. |
| This journal is © The Royal Society of Chemistry 2016 |