Open Access Article
Open Access ArticleGlycosylation intermediates studied using low temperature 1H- and 19F-DOSY NMR: new insight into the activation of trichloroacetimidates†
Yan
Qiao
ab,
Wenzhi
Ge
a,
Lingyu
Jia
b,
Xianglin
Hou
b,
Yingxiong
Wang
*b and
Christian M.
Pedersen
 *c
*c
aAnalytical Instrumentation Center & State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, 27 South Taoyuan Road, Taiyuan 030001, People's Republic of China
bShanxi Engineering Research Center of Biorefinery, Institute of Coal Chemistry, Chinese Academy of Sciences, 27 South Taoyuan Road, Taiyuan 030001, People's Republic of China
cDepartment of Chemistry, University of Copenhagen, Universitetsparken 5, DK-2100 Copenhagen, Denmark. E-mail: cmp@chem.ku.dk
First published on 25th August 2016
Abstract
Low temperature 1H- and 19F-DOSY have been used for analyzing reactive intermediates in glycosylation reactions, where a glycosyl trichloroacetimidate donor has been activated using different catalysts. The DOSY protocols have been optimized for low temperature experiments and provided new insight into acid catalyzed glycosylation chemistry. From the study a new glycosylation intermediate was characterized.
The mechanism of the glycosylation reaction is central to carbohydrate chemistry.1 As the reactions often are complex mixtures containing excess amounts of promoters and additives, such as acid scavengers, drying agents and acceptors, intermediates are notoriously difficult to analyze and impossible to isolate.2 Half a century ago NMR spectroscopy became available and was immediately applied to study carbohydrates and soon also reactive intermediates at low temperature.3 The study of glycosylation reactions with low temperature NMR has recently experienced a renaissance, where especially the Crich group has used it extensively to study reactive intermediates, such as glycosyl triflates, and on this basis suggested mechanisms e.g. for β-mannosylation.4 The work by Crich and others3 in combination with better hardware and software has made this technology accessible and doable for most research groups. The culmination of this development has recently led to the first observation of a carbohydrate oxocarbenium ion by NMR.5 Despite the advances in NMR resolution one major obstacle remains, i.e. the highly complex spectra contain several carbohydrate derived compounds and it is therefore tedious if not impossible to analyze the data fully. To get more information about the intermediates formed and to separate them in order to analyze and to get information about both the relative amount and the number of intermediates we have studied the use of 1H- and 19F diffusion ordered NMR spectroscopy (DOSY) at low temperature.
Measurements of molecular diffusion by NMR have been carried out since the eighties, but only in recent years has it been part of the routine experiments provided by NMR-manufacturers. The diffusion is dependent on the molecular size, shape and whether the molecule is non-covalently interacting with other species.6 The relationship between molecular size and diffusion coefficient has been used by several groups to estimate the molecular weights of intermediates7 and complexes8 by the use of internal reference compounds. This approach has been applied mainly to 1H-DOSY, but also 19F-DOSY studying Brønsted acid–base complexes.9 We envisaged that a combination of 1H- and 19F-DOSY could provide information about the intermediates formed under the catalytic activation of trichloroacetimidate (TCA) donors. The most used catalysts, i.e. trimethylsilyl trifluorosulfonate (TMSOTf) or BF3·OEt2, contain fluorine, whereas the TCA donors do normally not. By using this double determination, i.e.1H- and 19F-DOSY, it should be possible to distinguish between intermediates having the catalyst bound or not.
Trichloroacetimidates (TCAs) are one of the most commonly used glycosyl donor types due to its simplicity and catalytic activation.10 It has therefore been studied using VT-NMR to elaborate the influence of additive,11 intermolecular participation12 and it has been used as a source of glycosyl triflates (as B in Fig. 1)13 by using TMSOTf or similar reagents in equivalent amounts. Despite its excessive use, the mechanism of the activation of TCA has only been sparsely investigated and only little is known about the reaction mechanism. It has commonly been illustrated that the Lewis (or Bronsted) acid reacts with the nitrogen atom in the trichloroacetimidate (like A in Fig. 1). This activates the TCA group and results in the formation of an oxocarbenium ion intermediate (C in Fig. 1) followed by the attack by the nucleophile (acceptor); commonly a hydroxyl group, i.e. an SN1 type of reaction. (Fig. 1, C).14 Recent studies by Peng and Schmidt have shown that such deduction might not always be the scenario, and that the catalyst in some cases could be activated by forming a nucleophile–catalyst complex (similar to A in Fig. 1).15
As a model donor for our NMR study, 2-O-benzyl-3,4,6-tri-O-acetyl α-D-glucopyranosyl trichloroacetimidate 116 was chosen. It has a commonly used protective group pattern consisting of benzyl and acetyl groups. The 2-O-benzyl group was required in order to avoid neighboring group participation, which would give a dioxolonium ion, which is well known and has been thoroughly studied for 2-O-acetyl groups.17 The remaining hydroxyl groups are acetylated, which reduces the donor reactivity and could thereby increase the lifetime of the glycosylation intermediate (not an oxocarbenium ion).18 Glycosylation reactions with trichloroacetimidates are most often catalyzed by TMSOTf or BF3·OEt2 and these catalysts were therefore the starting point for the study.19
As a standardized condition the donor was dissolved in CD2Cl2 (conc.: 0.04 M20) together with Tris-(trifluoromethyl)benzene as the internal 19F-reference compound. In the initial studies the decomposition temperature, i.e. when the reactive intermediates disappeared to give a stable product, was determined using the different catalysts. On this basis it was decided to initiate the reactions at −55 °C using different amounts of the catalyst first in sub-stoichiometric amounts, then in equivalent and finally in excess. The different conditions were analyzed using low temperature NMR. The catalysts were additionally analyzed under the same conditions but independently, i.e. without donor presence, to give reference spectra and to check whether they aggregated at low temperature.
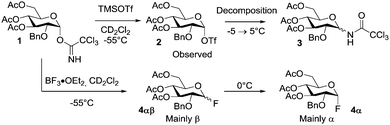
The first activation of the TCA-donor using TMSOTf was carried out, and α-triflate 2 was found to be the main product formed as expected from the examples in the literature (Scheme 1).13 The stereochemistry was confirmed by the small 3J1–2 (3 Hz) coupling constant, which is unambiguous for α-D-glucosides in the 4C1 conformation. When using 0.3 equiv. TMSOTf it was found that the donor reacted in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and hence did not undergo a catalytic transformation in the absence of an acceptor (see the ESI†). Due to the equal size of the donor and the product, these could not be clearly separated by DOSY, but from the comparison of 1H- and 19F-DOSY it was clear that the triflate group indeed was attached to the sugar moiety (19F-DOSY), whereas the TMS group was not (1H-DOSY). This confirms the formation of glucosyl triflate 2. Upon addition of equivalent amounts or excess of the catalyst full transformation to the α-triflate was observed. In time an additional product was formed under the reaction conditions at −55 °C. This product was assigned to be the corresponding trichloroacetamide 3. The ratio between triflate 2 and amide 3 remained stable until −5 °C. Between −5 °C and 5 °C the signals corresponding to the α-triflate disappeared completely. When a sub-stoichiometric amount (∼50%) of BF3·OEt2 was added to model donor 1 in CD2Cl2, the TCA was only consumed according to the amount of catalyst added (approx. 40%) and hence there was no catalytic transformation. Two new products appeared with anomeric signals between 5 and 6 ppm. From the unusual coupling pattern it could be confirmed that the α- and β-glucosyl fluorides 4αβ had been formed.2119F-NMR gave rise to three new major peaks, where 19F-DOSY confirmed that two of them had similar diffusion coefficients and were connected to the sugar moiety (similar diffusion coefficients based on 1H-DOSY and hence similar size). The 1H-DOSY confirms the existence of two new sugar species with very similar diffusion coefficients and that the signals of diethyl ether (from BF3·OEt2) are not associated with the sugar. Adding additionally 0.7 equiv. of BF3·OEt2 resulted in approx. 65% conversion of the TCA, which remained a stable ratio – the donor is not fully consumed even after adding more than 1 equiv. catalyst. The resulting mixture of glucosyl fluorides 4αβ and donor 1 remained stable for >7 h at −55 °C. Increasing the temperature stepwise (10 °C steps) resulted in a slow disappearance of the β-fluoride and at −15 °C mainly the α-anomer could be observed. Re-cooling did not change the composition of the sample.
1 ratio and hence did not undergo a catalytic transformation in the absence of an acceptor (see the ESI†). Due to the equal size of the donor and the product, these could not be clearly separated by DOSY, but from the comparison of 1H- and 19F-DOSY it was clear that the triflate group indeed was attached to the sugar moiety (19F-DOSY), whereas the TMS group was not (1H-DOSY). This confirms the formation of glucosyl triflate 2. Upon addition of equivalent amounts or excess of the catalyst full transformation to the α-triflate was observed. In time an additional product was formed under the reaction conditions at −55 °C. This product was assigned to be the corresponding trichloroacetamide 3. The ratio between triflate 2 and amide 3 remained stable until −5 °C. Between −5 °C and 5 °C the signals corresponding to the α-triflate disappeared completely. When a sub-stoichiometric amount (∼50%) of BF3·OEt2 was added to model donor 1 in CD2Cl2, the TCA was only consumed according to the amount of catalyst added (approx. 40%) and hence there was no catalytic transformation. Two new products appeared with anomeric signals between 5 and 6 ppm. From the unusual coupling pattern it could be confirmed that the α- and β-glucosyl fluorides 4αβ had been formed.2119F-NMR gave rise to three new major peaks, where 19F-DOSY confirmed that two of them had similar diffusion coefficients and were connected to the sugar moiety (similar diffusion coefficients based on 1H-DOSY and hence similar size). The 1H-DOSY confirms the existence of two new sugar species with very similar diffusion coefficients and that the signals of diethyl ether (from BF3·OEt2) are not associated with the sugar. Adding additionally 0.7 equiv. of BF3·OEt2 resulted in approx. 65% conversion of the TCA, which remained a stable ratio – the donor is not fully consumed even after adding more than 1 equiv. catalyst. The resulting mixture of glucosyl fluorides 4αβ and donor 1 remained stable for >7 h at −55 °C. Increasing the temperature stepwise (10 °C steps) resulted in a slow disappearance of the β-fluoride and at −15 °C mainly the α-anomer could be observed. Re-cooling did not change the composition of the sample.
From the results using TMSOTf or BF3·OEt2, it was clear that the activation mode of the trichloroacetimidate depends on the catalyst and in particular its counter ion. When this is a triflate ion a clean conversion to the α-glucosyl triflate 2 was observed; but what if the counter ion is less nucleophilic? Would it then be possible to avoid substitution of the activated trichloroacetimidate and instead observe an activated complex? To study this hypothesis TMSNTf222,23 as a catalyst was investigated, since it has been found to be more Lewis acidic than TMSOTf,24 but with a less nucleophilic counter ion.25 To our surprise the NMR, of the catalyst alone at −55 °C revealed 2 peaks in 1H- and 19F-NMR and therefore the existence of 2 compounds (Scheme 2). Upon increasing the temperature to 20 °C one broad signal was observed and hence the compounds are in a slow equilibrium; presumable as the mono- and dimer. This was further supported by increasing the concentration of the catalyst by a factor 5, which increases the amount of the proposed dimer in the sample (Scheme 2). Adding a sub-stoichiometric amount of MeOH (model acceptor) to the catalyst at −55 °C immediately resulted in the disappearance of the peak from the dimer (in 1H- and 19F-NMR) and the formation of a new peak assigned to [MeOH–TMS]+ appeared. The addition of more MeOH resulted in full conversion (Scheme 2). MeOH seems to catalyze the monomerization.
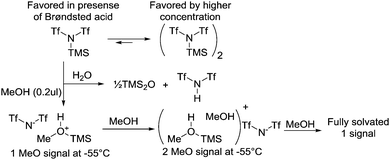 | ||
| Scheme 2 Reaction of Tf2NTMS with a model acceptor – MeOH. When present in sub-stoichiometric amounts different complexes are formed. | ||
When TMSNTf2 was added (0.3 equiv.) to model donor 1 at −55 °C no dimer was detected and the signal from trichloroacetimidate 1 disappeared rapidly (Scheme 3), which is in contrast to the same conditions using TMSOTf as the catalyst, where a one to one reaction took place. 1H-NMR showed a mixture of at least two compounds and 19F-NMR showed three peaks. 1H-DOSY of the mixture confirmed the presence of two sugar based compounds with a small difference in diffusion coefficient and hence size. From 1H-DOSY it is, however, clear that the TMS group from the catalyst is covalently attached to the sugar in contrast to when using TMSOTf. 19F-DOSY does not indicate that the sugar and −NTf2 are closely associated and a covalent bond between them can therefore be excluded. Upon the addition of equivalent amounts or excess of the catalyst the donor was immediately transformed and 4 broad peaks, between 5.5 and 6.5 ppm, appeared in 1H-NMR (presumable N-H). After 10 min, the mixture equilibrated into mainly one compound with no visible N-H signals. This compound was analyzed by 1H, 19F, COSY, HSQC Ed, 1H-DOSY, 19F-DOSY, NOESY and N-H-HSQC. The signals from the sugar-part could all be assigned and a chair conformation with an equatorial C1-substituent confirmed (3J are large (∼9 Hz), COSY, HSQC, NOESY). Two different TMS signals were observed – one attached to the sugar (DOSY) and one from TMS2O (from the side reaction with trace amounts of water). 19F-NMR showed 3 peaks, which were significantly different from the ones observed for the catalyst alone and its reaction with methanol, i.e. suggesting some interaction with the sugar, however not tightly associated (DOSY), but rather as an ion pair. TMS-activated TCA donor 6 was found to be more labile that the corresponding triflate 2 and even at −55 °C a slow degradation took place. Upon increasing the temperature to −20 °C resulted in full conversion into the Friedel–Crafts product 7 (determined from the crude NMR and confirmed by HRMS), but not trichloroacetamide 3, which is in contrast to when using TMSOTf. As far as it comes to our knowledge this is the first example of observing the activated complex of a trichloroacetimidate donor.26 The formation of this intermediate, when having a less nucleophile catalyst counter ion support that the triflate ion can act as a nucleophilic catalyst, whereas the triflylimide cannot to the same extent.28
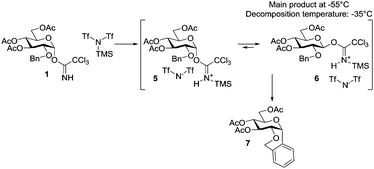 | ||
| Scheme 3 Activation of TCA donor 1 with Tf2NTMS. The counter ion Tf2N− is not nucleophilic enough to substitute the activated TCA, which instead anomerizes in time and eventually decompose to 7. | ||
The diffusion coefficients obtained are related to the size and shape of the molecules6 and as all compounds share the sugar moiety it was assumed that the main difference would be related to the molecular weight (MW) of the glycosyl donor and the intermediates. An estimation of MW by DOSY27 could shine light on the composition of the intermediates and other active species in the reaction mixture. Several methods have been developed for the determination of MW by DOSY. Normally these involve the use of a set of internal reference compounds. Due to the complexity and number of signals a recent published procedure involving external calibration curves and only one internal reference caught our interest.29 As the compounds in our study are larger and the solvent is CD2Cl2 a new calibration curve was prepared. A number of reference compounds with a similar three-dimensional structure were chosen and their diffusion coefficients determined in the presence of one internal reference – 1,3,5-tris(trifluoromethyl)benzene, which can be used as a reference in both 1H- and 19F-DOSY. For the 1H-DOSY calibration curve, carbohydrate based structures were preferred, and masses in the range from 74 to 1226 were included (6 compounds, see the ESI† for details). For 19F-DOSY the available carbohydrate based compounds were limited and only one, i.e. 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl fluoride, was part of the calibration curve (see the ESI† for details). With the calibration curves in hand the DOSY experiments were repeated to estimate the MW of the formed intermediates. Excess of the catalysts were used in order to ensure the formation of mainly one compound and the temperature was kept at −55 °C.
From the initial NMR studies and DOSY experiments good evidence for the structures of the intermediates formed had been obtained and hence their molecular weights (MW) could be predicted and compared with calculated values based on the calibration curve and the internal reference compound (Table 1). Estimation of MWs from the calibration curves gave a good estimation for the intermediates formed when using BF3·OEt2 and Tf2NTMS as the catalysts. The deviation is within 10% and the predicted MW is in-between the results from the two calibration curves. Both methods do however estimate larger MWs for the activation with TMSOTf and the 19F-DOSY deviates by 25%. One reason for the less precise MW prediction of this compound could be that the calibration curve is based on mainly non-carbohydrate based compounds, which have different three dimensional structures and hence a slightly different relation between diffusion constants and MWs. Generally the 1H-DOSY calibration curve, which is based on carbohydrate derivatives, gives the best correlation and hence the most accurate estimate. Most importantly the double determination of MWs of the new donor system using Tf2NTMS agrees with the predicted MWs and thereby strongly support that intermediate 6 containing the silylated trichloroacetimidate has been formed, which is a new activation pathway and could be a new way to improve the yield and selectivity in catalytic glycosylations.
In conclusion, we have used DOSY NMR for the first time to investigate a glycosylation reaction at low temperature. The interaction between three different catalysts with a glucosyl trichloroacetimidate was studied, and it was found that the reaction pathways and intermediates are different. The two common catalysts, BF3·OEt2 and TMSOTf, give glucosyl-fluorides and triflates, respectively, as intermediates. The donor is not transformed catalytically under these conditions, where an acceptor is not present. From this insight a new catalyst, Tf2NTMS was suggested and found to activate the trichloroacetimidate catalytically, but without cleavage of the glycosidic bond. The counter ion is not nucleophilic enough to substitute the leaving group and this is a novel promising concept for catalytic stereospecific glycosylations.
CMP acknowledges the CAS President's International Fellowship Initiative (2015VMB052).
Notes and references
- (a) B. Capon, Chem. Rev., 1969, 69, 407 CrossRef CAS; (b) L. K. Mydock and A. V. Demchenko, Org. Biomol. Chem., 2010, 8, 497 RSC; (c) S. C. Ranade and A. V. Demchenko, J. Carbohydr. Chem., 2013, 32, 1 CrossRef CAS; (d) L. Bohé and D. Crich, C. R. Chim., 2011, 14, 3 CrossRef.
- Examples of preactivation and intermediates see: (a) L. Yang, Q. Qin and X.-S. Ye, Asian J. Org. Chem., 2013, 2, 30 CrossRef CAS; (b) M. T. C. Walvoort, G. A. van der Marel, H. S. Overkleeft and J. D. C. Codée, Chem. Sci., 2013, 4, 897 RSC.
- T. G. Frihed, M. Bols and C. M. Pedersen, Chem. Rev., 2015, 115, 4963 CrossRef CAS PubMed.
- (a) D. Crich and S. Sun, J. Am. Chem. Soc., 1997, 119, 11217 CrossRef CAS; (b) D. Crich, Acc. Chem. Res., 2010, 43, 1144 CrossRef CAS PubMed.
- A. Martin, A. Arda, J. Désiré, A. Martin-Mingot, N. Probst, P. Sinaÿ, J. Jiménez-Barbero, S. Thibaudeau and Y. Blériot, Nat. Chem., 2016, 8, 186 CAS.
- (a) L. Avram and Y. Cohen, Chem. Soc. Rev., 2015, 44, 586 RSC; (b) Y. Cohen, L. Avram and L. Frish, Angew. Chem., Int. Ed., 2005, 44, 520 CrossRef CAS PubMed.
- D. Li, I. Keresztes, R. Hopson and P. G. Williard, Acc. Chem. Res., 2009, 42, 270 CrossRef CAS PubMed.
- Studies of the complexation of sugars by diffusion-ordered NMR spectroscopy: M. D. Diaz and S. Berger, Carbohydr. Res., 2000, 329, 1 CrossRef CAS PubMed.
- H. Subramanian, C. P. Jasperse and M. P. Sibi, Org. Lett., 2015, 17, 1429 CrossRef CAS PubMed.
- (a) R. R. Schmidt and J. Michel, Angew. Chem., Int. Ed., 1980, 19, 731–733 CrossRef; (b) Reviewed in: X. Zhu and R. R. Schmidt, in Handbook of Chemical Glycosylation, ed. A. V. Demchenko, Wiley-VCH, Weinheim, 2008, pp. 143–185 Search PubMed.
- (a) S. R. Lu, Y. H. Lai, J. H. Chen, C. Y. Liu and K. K. T. Mong, Angew. Chem., Int. Ed., 2011, 50, 7315 CrossRef CAS PubMed; (b) Y. H. Lin, B. Ghosh and K. K. T. Mong, Chem. Commun., 2012, 48, 10910 RSC; (c) A. B. Ingle, C. S. Chao, W. C. Hung and K. K. T. Mong, Org. Lett., 2013, 15, 5290 CrossRef CAS PubMed.
- (a) J. H. Kim, H. Yang, J. Park and G. J. Boons, J. Am. Chem. Soc., 2005, 127, 12090 CrossRef CAS PubMed; (b) T. J. Boltje, J. H. Kim, J. Park and G. J. Boons, Org. Lett., 2011, 13, 284 CrossRef CAS PubMed; (c) T. Fang, K. F. Mo and G. J. Boons, J. Am. Chem. Soc., 2012, 134, 7545 CrossRef CAS PubMed; (d) D. J. Cox, G. P. Singh, A. J. A. Watson and A. J. Fairbanks, Eur. J. Org. Chem., 2014, 4624 CrossRef CAS.
- (a) J. Y. Baek, B. Y. Lee, M. G. Jo and K. S. Kim, J. Am. Chem. Soc., 2009, 131, 17705 CrossRef CAS PubMed; (b) A. Rencurosi, L. Lay, G. Russo, E. Caneva and L. Poletti, Carbohydr. Res., 2006, 341, 903 CrossRef CAS PubMed; (c) J. Park, S. Kawatkar, J. H. Kim and G. J. Boons, Org. Lett., 2007, 9, 1959 CrossRef CAS PubMed.
- See for an example: G.-J. Boons and K. J. Hale, Organic Synthesis with Carbohydrates, Sheffield Academic Press Ltc, 2000, p. 108 Search PubMed.
- P. Peng and R. R. Schmidt, J. Am. Chem. Soc., 2015, 137, 12653 CrossRef CAS PubMed.
- (a) N. J. Davis and S. L. Flitsch, J. Chem. Soc., Perkin Trans. 1, 1994, 359–368 RSC; (b) N. C. R. van Straten, G. A. van der Marel and J. H. van Boom, Tetrahedron, 1997, 53, 6523 CrossRef CAS.
- (a) Generally: S. Winstein and R. E. Buckles, J. Am. Chem. Soc., 1942, 64, 2780 CrossRef CAS; (b) Early examples in carbohydrate chemistry have been summarized in: R. U. Lemieux, Adv. Carbohydr. Chem., 1954, 9, 1 CAS.
- (a) M. N. Kamat and A. V. Demchenko, Org. Lett., 2005, 7, 3215 CrossRef CAS PubMed ; A 2-O-acetyl can, besides controlling the selectivity also increase the reactivity, when anchimeric assistance is possible: ; (b) D. Crich and M. Li, Org. Lett., 2007, 9, 4115 CrossRef CAS PubMed; (c) M. Heuckendorff, C. M. Pedersen and M. Bols, Org. Lett., 2011, 13, 5956 CrossRef CAS PubMed.
- (a) R. R. Schmidt and K.-H. Jung, in Preparative Carbohydrate Chemistry, ed. S. Hanessian, Marcel Dekker, New York, 1997, pp. 283–312 Search PubMed; (b) X. Zhu and R. R. Schmidt, in Handbook of Chemical Glycosylation, ed. A. V. Demchenko, Wiley VCH, Weinheim, 2008, p. 143 Search PubMed.
- The concentration was optimized for the DOSY experiments.
- Glycosyl fluorides have earlier been observed in glycosylations using trichloroacetimidate donors in combination with BF3·OEt2. See: H. M. Christensen, S. Oscarson and H. H. Jensen, Carbohydr. Res., 2015, 408, 51 CrossRef CAS PubMed.
- TMSNTf2 has been used to catalyze the glycosylation using permethacrylated O-glycosyl trichloroacetimidates with simple acceptors. The yields were however low (up to 40%) and the selectivity controlled by neighboring group participation. See: C. Zandanel, L. Dehuyser, A. Wagner and R. Baati, Tetrahedron, 2010, 66, 3365 CrossRef CAS.
- (a) Tf2NTMS has previously been used for: Friedel-Craft alkylations e.g. A. Isgii, O. Kotera, T. Saeki and K. Mikami, Synlett, 1997, 1145 Search PubMed; (b) β-allylation of enones: N. Kuhnert, J. Peverly and J. Robertson, Tetrahedron Lett., 1998, 39, 3215 CrossRef CAS; (c) Mukayama aldol reaction: K. Ishihara, Y. Hiraiwa and H. Yamamoto, Synlett, 2001, 1851 CrossRef CAS; (d) Silylations: G. Simchen and S. Jonas, J. Prakt. Chem., 1998, 340, 506 CrossRef CAS; (e) See also M. B. Boxer in e-EROS Encyclopedia of Reagents for Organic Synthesis, 2008 (1,1,1-trifluoro-N-[(trifluoromethyl)sulfonyl]-N-(trimethylsilyl)methanesulfonamide).
- B. Mathieu and L. Ghosez, Tetrahedron Lett., 1997, 38, 5497 CrossRef CAS.
- TfOH has been found to be more Bronsted acidic and its conjugated base more nucleophilic compared to Tf2NH: J. Foropoulos and D. D. DesMarteau, Inorg. Chem., 1984, 23, 3720 CrossRef CAS; B. Matieu, L. Ghosez Tetrahedron, 2002, 58, 8219 CrossRef.
- The structurally related imidinium ion has been observed by VT-NMR: (a) V. Dourtoglou, J.-C. Ziegler and B. Gross, Tetrahedron Lett., 1979, 20, 4371 CrossRef; (b) Y. Shingu, A. Miyachi, Y. Miura, K. Kobayashi and Y. Nishida, Carbohydr. Res., 2005, 340, 2236 CrossRef CAS PubMed; (c) Y. Nishida, Y. Shingu, H. Dohi and K. Kobayashi, Org. Lett., 2003, 5, 2377 CrossRef CAS PubMed . See also ref. 11.
- Molecular weight determination of oligo- and poly-saccharides by DOSY: (a) O. Assemat, M.-A. Coutouly, R. Hajjar and M.-A. Delsuc, C. R. Chim., 2010, 13, 412–415 CrossRef CAS; (b) S. Viel, D. Capitani, L. Mannina and A. Segre, Biomacromolecules, 2003, 4, 1843 CrossRef CAS PubMed.
- Influence of the counter ion in glycosylation has recently been studied by VT-NMR: Y. Zhu and B. Yu, Chem. – Eur. J., 2015, 21, 8771 CrossRef CAS PubMed.
- R. Neufeld and D. Stalke, Chem. Sci., 2015, 6, 3354 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc05272j |
| This journal is © The Royal Society of Chemistry 2016 |