 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile synthesis of one-dimensional organometallic–organic hybrid polymers based on a diphosphorus complex and flexible bipyridyl linkers†
M.
Elsayed Moussa
a,
B.
Attenberger
a,
E. V.
Peresypkina
abc,
M.
Fleischmann
a,
G.
Balázs
a and
M.
Scheer
 *a
*a
aInstitute für Anorganische Chemie der Universitöt Regensburg, 93040 Regensburg, Germany. E-mail: manfred.scheer@chemie.uni-r.de
bNikolaev Institute of Inorganic Chemistry, Siberian Division of RAS Acad.Lavrentyev prosp. 3, 630090 Novosibirsk, Russia
cNovosibirsk State University, ul. Pirogova, 2, 630090 Novosibirsk, Russia
First published on 19th July 2016
Abstract
The selective synthesis of a series of new “ladderlike” one-dimensional organometallic–organic hybrid polymers is shown. The polymers are obtained from the reaction of the diphosphorus ligand complex [Cp2Mo2(CO)4(η2-P2)] with the copper salt [Cu(CH3CN)4]BF4 in the presence of flexible organic bipyridyl linkers in high selectivity. This unique behaviour is supported by DFT calculations.
In recent years, coordination polymers have attracted significant attention due to their intriguing network topologies1,6 and unique properties.2,6 The coordination polymers are assembled via the coordination of organic ligands usually bearing N, O or S donor atoms to metal ions.3 Yet, the understanding of the supramolecular aggregation processes is still limited, as many factors influence the formation of the final products. Those factors can be external (solvent systems, pH values, temperatures, crystallisation techniques, and template involved)4,6 or internal (the geometry or the position of the donor atoms of the ligands and counteranions involved).5,6 One way to avoid the low selectivity of the assembling reactions and direct the synthesis towards targeted products is the use of pre-designed precursors.7 Another way is to utilise rigid linkers,8 as the use of flexible linkers often leads to unpredictable frameworks9 due to their conformational freedom.10 Recently, C. Lescop et al. succeeded in the selective synthesis of tetranuclear metallacycles based on mixed rigid and flexible linkers.7a In addition, several groups have synthesized dinuclear metallacycles using flexible ditopic linkers.11 In all these examples, the authors have benefited from the metal-directed self-assembly process to build their targeted inorganic–organic metallacycles. Therefore, the question arises whether it is possible to use flexible organic linkers to control a regular arrangement in the solid state. Obviously, the used interplay of linkers/nodes will then play a decisive role. In this field, our group has previously reported a new approach by using Pn ligand complexes as organometallic connectors between metal centers allowing the formation of one- and two-dimensional coordination polymers.12 Moreover, one of those Pn ligand complexes, the tetrahedrane complex [Cp2Mo2(CO)4(η2-P2)] (Cp = C5H5) (A), has been reacted with AgI and CuI salts in the presence of trans-1,2-di(pyridine-4-yl)ethene to give unprecedented organometallic–organic hybrid polymers.13 Herein, we present the reaction between the P2 ligand complex A, the [Cu(CH3CN)4]BF4 (B), and the flexible ditopic linkers 1–4 (Scheme 1). Due to the different possible arrangements of the flexible linkers10 and different coordination modes of the P2 ligand complex14 towards the CuI cation, a large number of combinations is expected resulting in a large variety of accessible networks. However, those reactions yielded selectively the novel one-dimensional organometallic–organic coordination polymers 5–8 (Scheme 1, Fig. 2), where, to the best of our knowledge, no general approach has yet been reported to synthesize selectively coordination polymers based on flexible organic linkers and organometallic nodes.
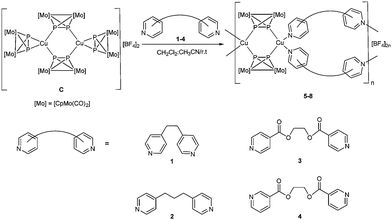 | ||
| Scheme 1 The reaction of the dimeric complex C with the flexible organic linkers 1–4, Synthesis of the one-dimensional organometallic–organic coordination polymers 5–8. | ||
Recently,15 we have shown that the reaction of the copper salt B and the P2 ligand complex A affords a compound C that possesses a dimeric [Cu2(A)4][BF4]2 structure in the solid state with two μ-η1:η1 bridging ligands A and two ligands A in η2 side-on coordination (Fig. 1, Scheme 1). In order to study the dimer C as a suitable precursor for the selective synthesis of organometallic–organic hybrid polymers, DFT calculations have been performed at the B3LYP/def2-TZVP level of theory. Accordingly, the substitution of the terminal ligands A in the preformed dimeric complex C by pyridine as a model ligand, leading to F, is exothermic (−104.6 kJ mol−1) in solution (Fig. 1). In contrast, the substitution by dichloromethane (Q) is endothermic by 87.4 kJ mol−1 (Fig. S22 in ESI†). Just like pyridine, acetonitrile can also substitute the terminal ligands A in C, but the reaction is less exothermic than in the case of pyridine (−76.2 kJ mol−1; Fig. 1). The substitution of the terminal ligands A in C leading to D and H is more favoured for pyridine than for acetonitrile. This indicates that, starting from the dimer C, it should be possible to synthesise organometallic–organic hybrid polymers by reacting it with pyridine-based multitopic ligands. In addition, the tuning of the final outcome of the reactions should also be attainable.
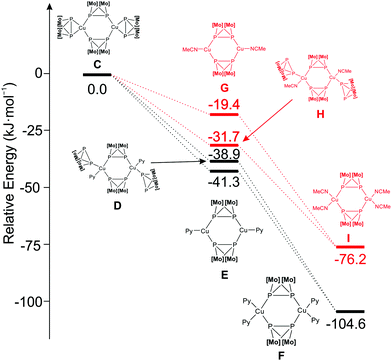 | ||
| Fig. 1 Energy diagram of the reaction of C with pyridine and CH3CN. The positive charges, ligands added or cleaved are not depicted. [Mo] = CpMo(CO)2. | ||
Based on this information, complex C was reacted with the 4,4′-bipyridine (1–3) or 3-3′-bipyridine (4) linkers bearing flexible (C2H4, 1), (C3H6, 2), (C4H4O4, 3 and 4) linkages in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry in a mixture of CH2Cl2 and CH3CN at room temperature resulting in the formation of the coordination polymers 5–8 as orange or red crystalline solids in good to high (65–94%) yields (Scheme 1). In either case, the same products could also be selectively isolated when all the components were mixed together using different stoichiometries (1 or 3 equivalents) of the organic linker, which suggest that the products 5–8 are thermodynamically favoured, even passing other possible intermediates.
2 stoichiometry in a mixture of CH2Cl2 and CH3CN at room temperature resulting in the formation of the coordination polymers 5–8 as orange or red crystalline solids in good to high (65–94%) yields (Scheme 1). In either case, the same products could also be selectively isolated when all the components were mixed together using different stoichiometries (1 or 3 equivalents) of the organic linker, which suggest that the products 5–8 are thermodynamically favoured, even passing other possible intermediates.
The derivatives 5–8 are slightly soluble in donor solvents like CH3CN, but insoluble in other common organic solvents like CH2Cl2, THF and n-pentane. The compounds 5–8 were crystallized at room temperature from pentane diffusion into CH2Cl2/CH3CN solutions of the crude reaction mixtures and characterised by single crystal X-ray structural analysis (Tables S1–S5, ESI†). Unexpectedly, despite the various lengths of spacers between the pyridyl fragments and the different positions of the donor atoms in the linkers 1–4, the assemblies 5–8 are 1D coordination polymers (Fig. 2). In the derivatives 5–8, each side-on coordinating ligand A present in the parent dimer C is substituted by the pyridine functions of two linker molecules. As a consequence, 1D polymers are formed with Cu2(A)2 repeating units, which are linked together via the organic connectors (Fig. 2). Within those repeating units, each copper ion comprises a distorted tetrahedral environment of two P and two N atoms. Interestingly, in the polymeric chains of 5 and 7, each of the Cu centers of the Cu2P4 six-membered rings participates in a different metallacyclic fragment, while they participate in similar metallacyclic repeating units in the derivatives 6 and 8. In other words, the derivatives 5 and 7 can be described as infinite dinuclear metallacyclic chains formed by Cu2(5)2 metallacycles connected to each other via Mo2P2 ligands, while the derivatives 6 and 8 are infinite tetranuclear metallacyclic chains where the Mo2P2 ligands are a part of the metallacycles. The P–P bond lengths in the derivatives 5–8 are in the range between 2.072(2) and 2.086(2) Å, comparable to those of the non-coordinated ligand A (2.079(6) Å) and slightly shortened compared to that of the complex C (2.094(2)–2.159(2) Å). The Cu–P bonds of the derivatives 5–8 vary in a wider range (2.261(1)–2.307(1) Å) and are also shortened as compared to those of the parent dimer C (2.260(2)–2.385(2) Å). The Cu2P4 six-membered rings in all derivatives 5–8 are nearly planar, with folding angles of (4.4–7.9°). In the assemblies 5–8, the central flexible linkages have an anti-“antiperiplanar” and gauche-“synperiplanar” and conformations for the linkers 1–3 and 4, respectively (Fig. 2). The coordination polymers possess virtual porosity, with cavities having “circular” (5–7) and “rectangular” (8) meshes of maximum diameters ranging between 1.02 and 1.47 nm and minimum diameters ranging between 0.32 and 0.73 nm.
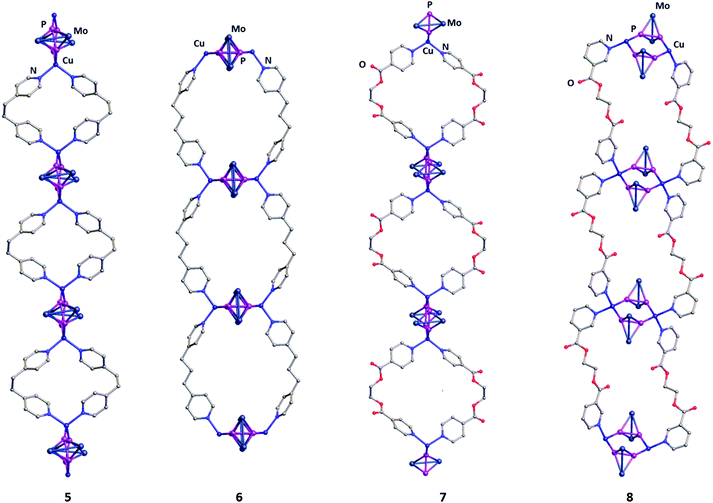 | ||
| Fig. 2 Sections of the 1D coordination polymers 5–8. Cp and CO ligands, hydrogen atoms as well as minor parts of the disordered fragments are omitted for clarity. | ||
The room temperature 31P NMR spectra in CD3CN of the complexes 5–8 show broad signals centered at −51.5 (5), −53.4 (6) and −48.2 (7 and 8) ppm, which are upfield shifted as compared to the free P2 ligand complex A (−43.2 ppm) and comparable to the signal of the dimer C (−49.1 ppm, Table S6, ESI†). Their room temperature 1H NMR spectra (Fig. S9–S12, ESI†) present simple sets of signals with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of A
1 ratio of A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L (L = 1–4), which suggest the formation of supramolecular assemblies with Cu2(A)2L2 repeating units. The 13C NMR spectra show also typical sets of signals corresponding to the bipyridine linkers and the A ligands (Fig. S13–S16, ESI†). The 19F NMR spectra of all complexes are featured by two signals centered at ca. −150.5, and −151.8 ppm, which correspond to the BF4 counteranions (Table S6, ESI†). The IR spectra exhibit two strong bands at ca. ν(CO) ≈ 1920 and 1980 cm−1, which can be attributed to the carbonyl groups of the A moieties.
L (L = 1–4), which suggest the formation of supramolecular assemblies with Cu2(A)2L2 repeating units. The 13C NMR spectra show also typical sets of signals corresponding to the bipyridine linkers and the A ligands (Fig. S13–S16, ESI†). The 19F NMR spectra of all complexes are featured by two signals centered at ca. −150.5, and −151.8 ppm, which correspond to the BF4 counteranions (Table S6, ESI†). The IR spectra exhibit two strong bands at ca. ν(CO) ≈ 1920 and 1980 cm−1, which can be attributed to the carbonyl groups of the A moieties.
The results obtained demonstrate a new approach for the selective synthesis of novel 1D organometallic–organic hybrid polymers 5–8 based on the reaction of the organometallic P2 ligand complex [Cp2Mo2(CO)4(η2-P2)] (A), the copper salt [Cu(CH3CN)4]BF4 (B) and the flexible pyridine-based linkers 1–4. This is possible due to the liability of the η2-coordinated P2 units, which could be easily substituted by the pyridine-based organic linkers 1–4. In these aggregation reactions, the flexibility of the linkers 1–4 plays a “positive role” instead of the usual “destructive role” in directing the synthesis towards selective product formation since those linkers can easily adopt their backbones to afford the thermodynamically favored polymers. Current investigations focus on the use of rigid linkers with defined lengths and functionalities. The reactions of those linkers with the P2-based dimer precursor C are expected to be directionally leading to selective products with the possibility of a fine tuning and control of the sizes of the cavities formed. In addition, the incorporated functionalities could play a role in the host–guest chemistry of defined host molecules.
The European Research Council via Grant ERC-2013-AdG 339072 is gratefully acknowledged for the comprehensive support of this work.
Notes and references
- (a) X.-Y. Dong, C.-D. Si, Y. Fan, D.-C. Hu, X.-Q. Yao, Y.-X. Yang and J.-C. Liu, Cryst. Growth Des., 2016, 16, 2062–2073 CrossRef CAS; (b) R. Haldar and T. K. Maji, Chem. Rev., 2014, 114, 7557–7580 CrossRef PubMed; (c) R. Haldar and T. K. Maji, Z. Anorg. Allg. Chem., 2014, 640, 1102–1108 CrossRef CAS; (d) W.-Q. Kan, J. Yang, Y.-Y. Liu and J.-F. Ma, CrystEngComm, 2012, 14, 6271–6281 RSC; (e) J. Guo, D. Sun, L. Zhang, Q. Yang, X. Zhao and D. Sun, Cryst. Growth Des., 2012, 12, 5649–5654 CrossRef CAS; (f) W. L. Leong and J. J. Vittal, Chem. Rev., 2011, 111, 688–764 CrossRef CAS PubMed.
- (a) S.-I. Noro, J. Mizutani, Y. Hijikata, R. Matsuda, H. Sato, S. Kitagawa, K. Sugimoto, Y. Inubushi, K. Kubo and T. Nakamura, Nat. Commun., 2015, 6, 5851 CrossRef CAS PubMed; (b) K. Banerjee, S. Roy, M. Kotal and K. Biradha, Cryst. Growth Des., 2015, 15, 5604–5613 CrossRef CAS; (c) Z. Zhou, C. He, J. Xiu, L. Yang and C. Duan, J. Am. Chem. Soc., 2015, 137, 15066–15069 CrossRef CAS PubMed; (d) X. Yan, T. R. Cook, J. B. Pollock, P. Wei, Y. Zhang, Y. Yu, F. Huang and P. J. Stang, J. Am. Chem. Soc., 2014, 136, 4460–4463 CrossRef CAS PubMed; (e) J. Heine and K.-M. Buschbaum, Chem. Soc. Rev., 2013, 42, 9232–9242 RSC.
- (a) T. R. Cook, Y.-R. Zheng and P. J. Stang, Chem. Rev., 2013, 113, 734–777 CrossRef CAS PubMed; (b) S. Park, S. Y. Lee, K.-M. Park and S. S. Lee, Acc. Chem. Res., 2012, 3, 391–403 CrossRef PubMed.
- (a) R. S. Patil, A. M. Drachnik, H. Kumari, C. L. Barnes, C. A. Deakyne and J. L. Atwood, Cryst. Growth Des., 2015, 15, 2781–2786 CrossRef CAS; (b) B. Liu, N. Li, W.-P. Wu, H. Miao, Y.-Y. Wang and Q.-Z. Shi, Cryst. Growth Des., 2014, 14, 1110–1124 CrossRef CAS; (c) K. P. Rao, M. Higuchi, J. Duan and S. Kitagawa, Cryst. Growth Des., 2013, 13, 981–985 CrossRef CAS; (d) C.-P. Li, J.-M. Wu and M. Du, Chem. – Eur. J., 2012, 18, 12437–12445 CrossRef CAS PubMed; (e) C.-P. Li, J.-M. Wu and M. Du, CrystEngComm, 2012, 14, 2748–2755 RSC.
- (a) K. Tripuramallu, P. Manna and S. K. Das, CrystEngComm, 2014, 16, 4816–4833 RSC; (b) R.-J. Wei, J. Tao, R.-B. Huang and L.-S. Zheng, Eur. J. Inorg. Chem., 2013, 916–926 CrossRef CAS; (c) H. T. Chifotides, I. D. Giles and K. R. Dunbar, J. Am. Chem. Soc., 2013, 135, 3039–3055 CrossRef CAS PubMed; (d) F.-J. Liu, D. Sun, H.-J. Hao, R.-B. Huang and L.-S. Zheng, Cryst. Growth Des., 2012, 12, 354–361 CrossRef CAS.
- W. L. Leong and J. J. Vittal, Chem. Rev., 2011, 111, 688–764 CrossRef CAS PubMed.
- (a) W. Shen, M. El Sayed Moussa, Y. Yao and C. Lescop, Chem. Commun., 2015, 51, 11560–11563 RSC; (b) S. Wang, T. Zhao, G. Li, L. Wojtas, Q. Huo, M. Eddaoudi and Y. Liu, J. Am. Chem. Soc., 2010, 132, 18038–18041 CrossRef CAS PubMed; (c) B. Nohra, Y. Yao, C. Lescop and R. Réau, Angew. Chem., Int. Ed., 2007, 46, 8242–8245 CrossRef CAS PubMed; (d) R. D. Sommer, A. L. Rheingold, A. J. Goshe and B. Bosnich, J. Am. Chem. Soc., 2001, 123, 3940–3952 CrossRef CAS PubMed.
- (a) H.-N. Wang, G.-G. Shan, H.-B. Li, X.-L. Wang, H.-T. Cao and Z.-M. Su, CrystEngComm, 2014, 16, 2754–2759 RSC; (b) A. N. Khlobystov, A. J. Blake, N. R. Champness, D. A. Lemenovskii, A. G. Majouqa, N. V. Zyk and M. Schröder, Coord. Chem. Rev., 2001, 222, 155–192 CrossRef CAS; (c) M. Fujita, K. Umemoto, M. Yoshizawa, N. Fujita, T. Kusukawa and K. Biradha, Chem. Commun., 2001, 509–518 RSC; (d) B. J. Holliday and C. A. Mirkin, Angew. Chem., Int. Ed., 2001, 40, 2022–2043 CrossRef CAS.
- (a) L. Liu, C. Huang, X. Xue, M. Li, H. Hou and Y. Fan, Cryst. Growth Des., 2015, 15, 4507–4517 CrossRef CAS; (b) L.-P. Xue, X.-H. Chang, L.-F. Ma and L.-Y. Wang, RSC Adv., 2014, 4, 60883–60890 RSC; (c) M.-M. Dong, L.-L. He, Y.-J. Fan, S.-Q. Zang, H.-W. Hou and T. C. W. Mak, Cryst. Growth Des., 2013, 13, 3353–3364 CrossRef CAS; (d) Q. Sun, Y.-Q. Wang, A.-L. Cheng, K. Wang and E.-Q. Gao, Cryst. Growth Des., 2012, 12, 2234–2241 CrossRef CAS; (e) T.-F. Liu, J. Lü and R. Cao, CrystEngComm, 2010, 12, 660–670 RSC.
- (a) Y. Wen, T. Sheng, Z. Xue, Z. Sun, Y. Wang, S. Hu, Y. Huang, J. Li and X. Wu, Cryst. Growth Des., 2014, 14, 6230–6238 CrossRef CAS; (b) D. Deng, L. Liu, B.-M. Ji, G. Yin and C. Du, Cryst. Growth Des., 2012, 12, 5338–5348 CrossRef CAS; (c) M. L. Hernández, M. K. Urtiaga, M. G. Barandika, R. Cortés, L. Lezama, N. de la Pinta, M. I. Arriortua and T. Rojo, J. Chem. Soc., Dalton Trans., 2001, 3010–3014 RSC; (d) M. L. Hernández, M. G. Barandika, M. K. Urtiaga, R. Cortés, L. Lezama and M. I. Arriortua, J. Chem. Soc., Dalton Trans., 2000, 79–84 RSC; (e) T. L. Hennigar, D. C. MacQuarrie, P. Losier, R. D. Rogers and M. J. Zaworotko, Angew. Chem., Int. Ed., 1997, 36, 972–973 CrossRef CAS.
- (a) S. Karthikeyan, R. Nagarajprakash, G. Satheesh, C. A. Kumar and B. Manimaran, Dalton Trans., 2015, 44, 17389–17398 RSC; (b) P. Crosshans, A. Jouaiti, V. Bulach, J.-M. Planeix, M. W. Hosseini and N. Kyritsakas, Eur. J. Inorg. Chem., 2004, 453–458 CrossRef; (c) B. Chatterjee, J. C. Noveron, M. J. E. Resendiz, J. Liu, T. Yamamoto, D. Parker, M. Cinke, C. V. Nguyen, A. M. Arif and P. J. Stang, J. Am. Chem. Soc., 2004, 126, 10645–10656 CrossRef CAS PubMed.
- (a) F. Dielmann, A. Schindler, S. Scheuermayer, J. Bai, R. Merkle, M. Zabel, A. V. Virovets, E. V. Peresypkina, G. Brunklaus, H. Eckert and M. Scheer, Chem. – Eur. J., 2012, 18, 1168–1179 CrossRef CAS PubMed; (b) M. Scheer, Dalton Trans., 2008, 4372–4386 RSC; (c) J. Bai, A. V. Virovets and M. Scheer, Angew. Chem., Int. Ed., 2012, 41, 1737–1740 CrossRef; (d) J. Bai, E. Leiner and M. Scheer, Angew. Chem., Int. Ed., 2002, 41, 783–786 CrossRef CAS.
- (a) B. Attenberger, E. V. Peresypkina and M. Scheer, Inorg. Chem., 2015, 54, 7021–7029 CrossRef CAS PubMed; (b) B. Attenberger, S. Welsch, M. Zabel, E. Peresypkina and M. Scheer, Angew. Chem., Int. Ed., 2011, 50, 11516–11519 CrossRef CAS PubMed.
- S. Welsch, C. Lescop, G. Balázs, R. Réau and M. Scheer, Chem. – Eur. J., 2011, 17, 9130–9141 CrossRef CAS PubMed.
- M. Fleischmann, S. Welsch, E. V. Peresypkina, A. V. Virovets and M. Scheer, Chem. – Eur. J., 2015, 21, 14332–14336 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, synthesis and characterisation and details of the DFT calculations. CCDC 1487111–1487114. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc05224j |
| This journal is © The Royal Society of Chemistry 2016 |
